Making Compounds Making Compounds Test Burning magnesium Heating

Making Compounds

Making Compounds Test Burning magnesium Heating Copper carbonate Magnesium + hydrochloric acid Potassium iodide + lead nitrate Observations Before After

Making Compounds Burning Magnesium. Burn a small piece of magnesium ribbon in a blue Bunsen flame.

Making Compounds Heating Copper carbonate. Add 2 spatulas of copper carbonate to a boiling tube. Heat strongly over a Bunsen.

Making Compounds Potassium iodide mixed with lead nitrate. Place 5 ml of one solution into a test tube, and 5 ml of the other solution into another test tube. Mix, then filter the resulting mixture.

Making Compounds Magnesium and hydrochloric acid Add 10 ml of hydrochloric acid to a boiling tube, then drop in a piece of magnesium ribbon.

Making a Compound: Combustion Joining an element with oxygen is called combustion. How do you know a chemical reaction has taken place?
Naming compounds When elements react they produce a compound What are elements?
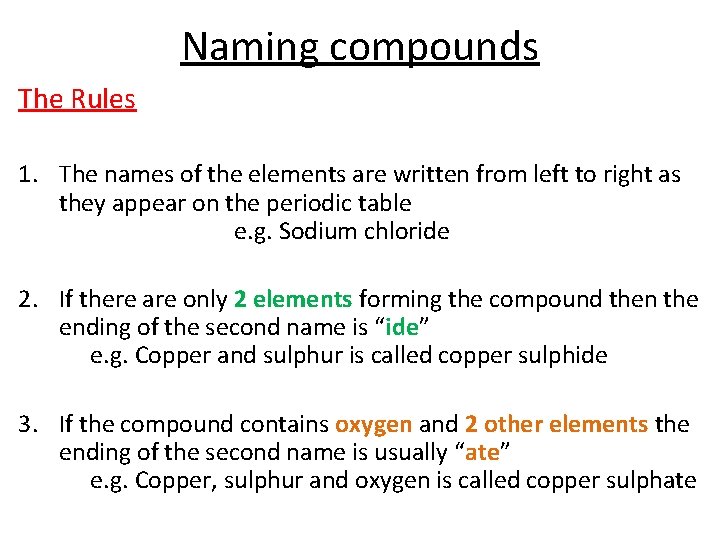
Naming compounds The Rules 1. The names of the elements are written from left to right as they appear on the periodic table e. g. Sodium chloride 2. If there are only 2 elements forming the compound then the ending of the second name is “ide” e. g. Copper and sulphur is called copper sulphide 3. If the compound contains oxygen and 2 other elements the ending of the second name is usually “ate” e. g. Copper, sulphur and oxygen is called copper sulphate
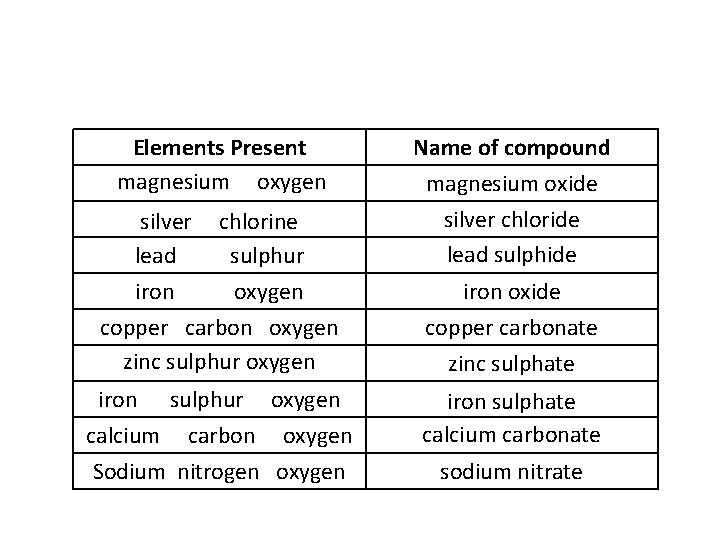
Elements Present magnesium oxygen silver lead iron chlorine sulphur oxygen Name of compound magnesium oxide silver chloride lead sulphide iron oxide copper carbon oxygen zinc sulphur oxygen copper carbonate zinc sulphate iron sulphur oxygen calcium carbon oxygen Sodium nitrogen oxygen iron sulphate calcium carbonate sodium nitrate

In pairs… Write the answer to the following questions on your show me board… Everyone hold up their board on the count of 3.

What elements make up… magnesium oxide

What elements make up… silver chloride

What elements make up… copper carbonate
Name the compound… iron + oxygen
Name the compound… calcium + carbon + oxygen
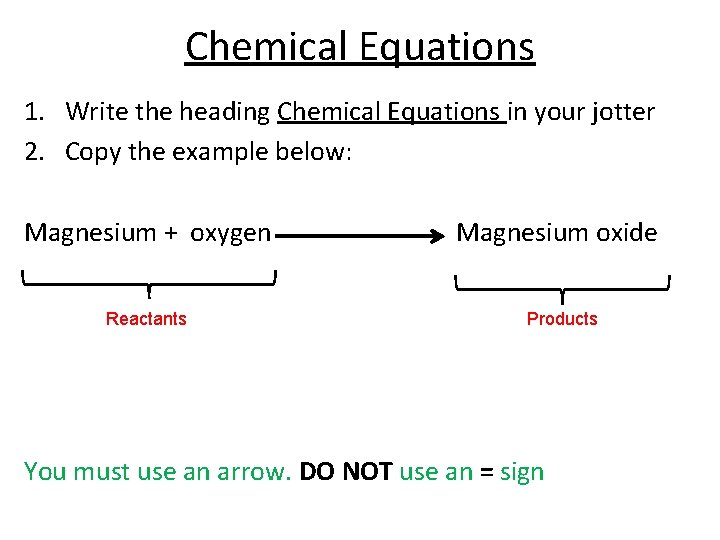
Chemical Equations 1. Write the heading Chemical Equations in your jotter 2. Copy the example below: Magnesium + oxygen Reactants Magnesium oxide Products You must use an arrow. DO NOT use an = sign
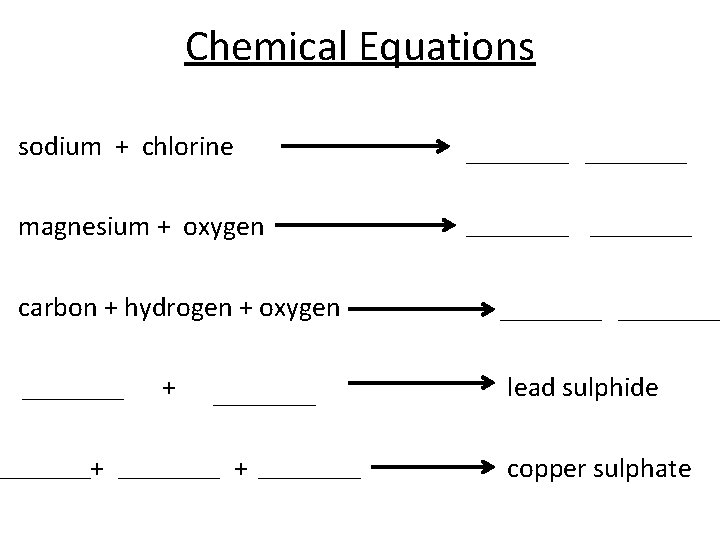
Chemical Equations sodium + chlorine magnesium + oxygen carbon + hydrogen + oxygen + + lead sulphide + copper sulphate
- Slides: 18