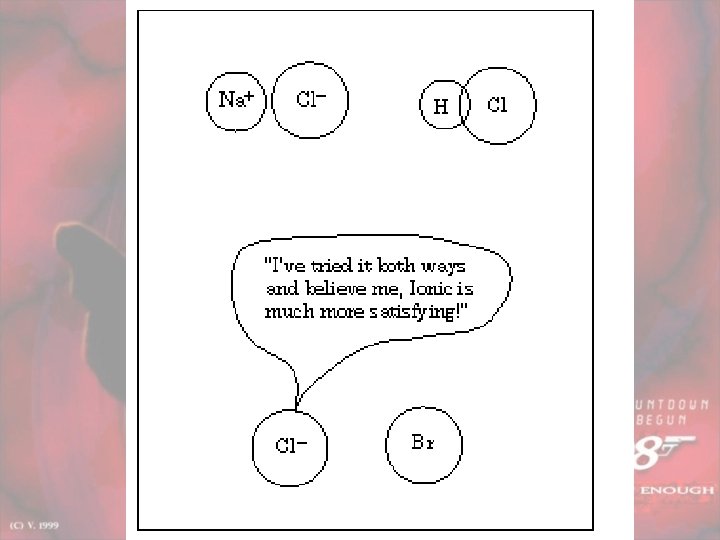
Major Bond Types IONIC COVALENT e are transferred


Major Bond Types IONIC COVALENT e- are transferred from one atom to another e- are shared between 2 atoms

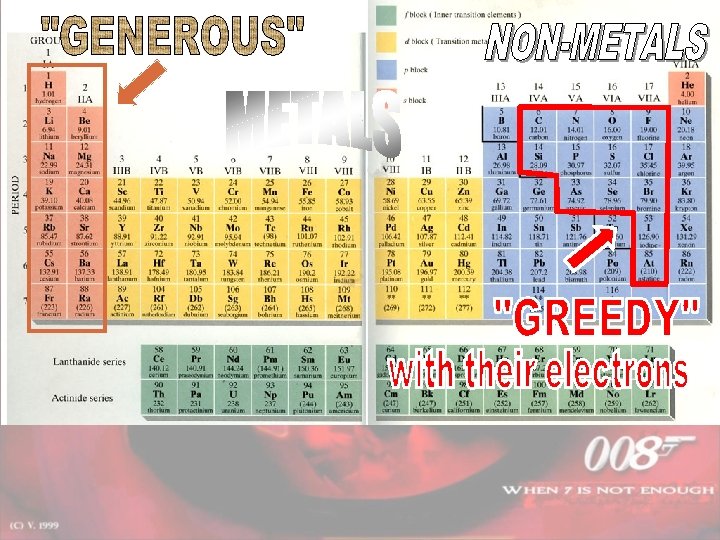
When does it occur? IONIC Formed between a metal and non-metal COVALENT Formed between 2 nonmetals

How does it hold atoms together? IONIC Electromagnetic forces; cation/ anion (opposites attract) COVALENT Shared attraction for same e- (+nucleus pulls on e-) Polar: doesn’t share equally Nonpolar: shares equally
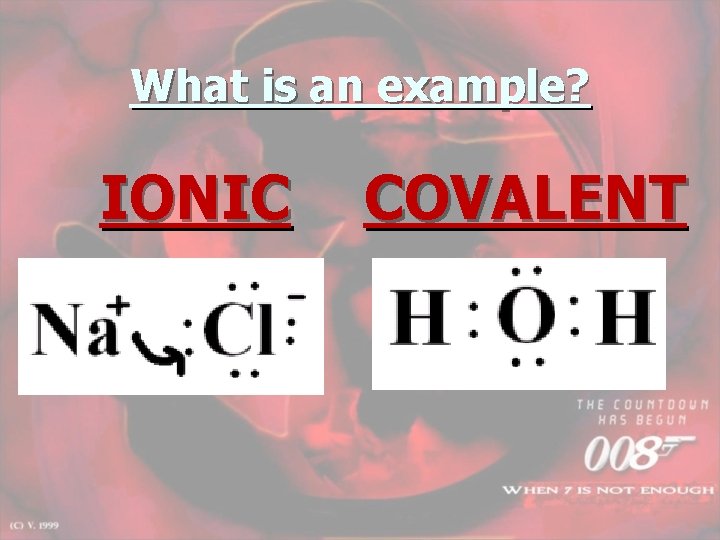
What is an example? IONIC COVALENT
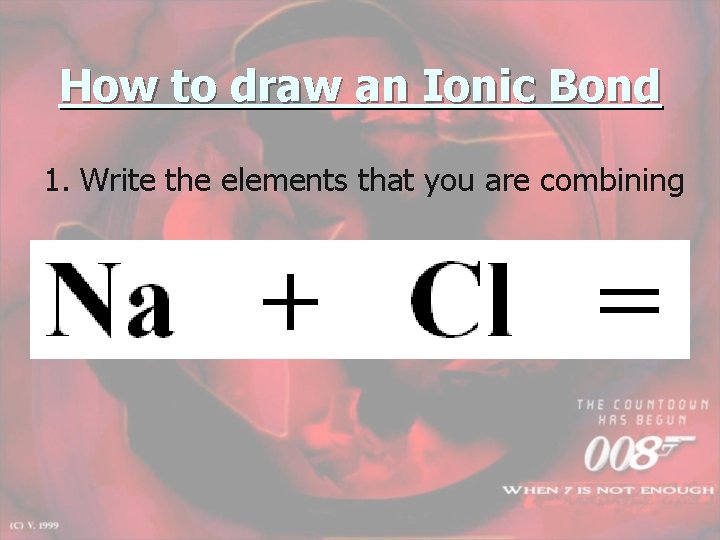
How to draw an Ionic Bond 1. Write the elements that you are combining

2. Do a Lewis Dot Diagram for each atom

3. Draw the arrow from the e- that are moving to their new location. Add the final molecule on the right side (don’t forget the charges)
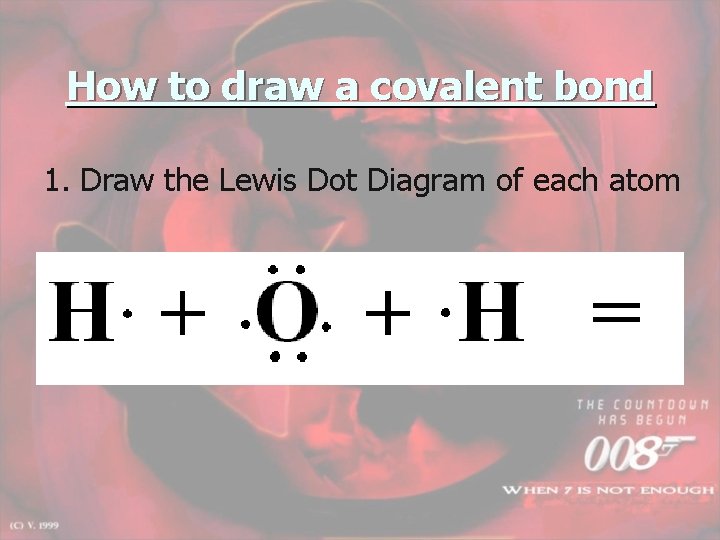
How to draw a covalent bond 1. Draw the Lewis Dot Diagram of each atom
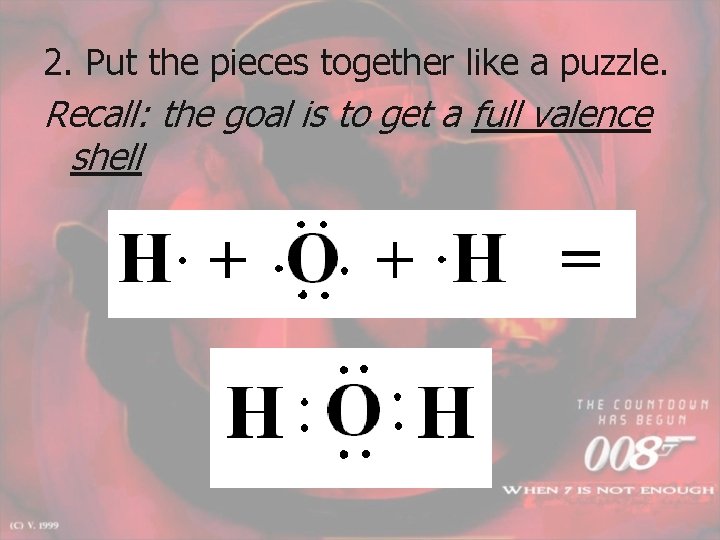
2. Put the pieces together like a puzzle. Recall: the goal is to get a full valence shell

Ionic Compounds (salts) n Made up of n positive and negative ions n cations and anions n a metal and a nonmetal n Smallest repeating unit- formula unit

Properties of Ionic Compounds n n All are Crystalline solids They have a regular repeating arrangement of ions in a rigid 3 -D structure Ionic bonds are the strongest bonds, so these compounds have high melting points

Properties of Ionic Compounds n This orderly arrangement of component ions produces the beauty of crystalline solids.
More properties of ionic compounds n Conducting electricity is allowing charges (electrons) to move. n In a crystalline solid, the ions are locked in place so they can’t conduct electricity n When melted or dissolved in water, the ions are free to move around and, therefore, conduct electricity Brittle solids at room temperature
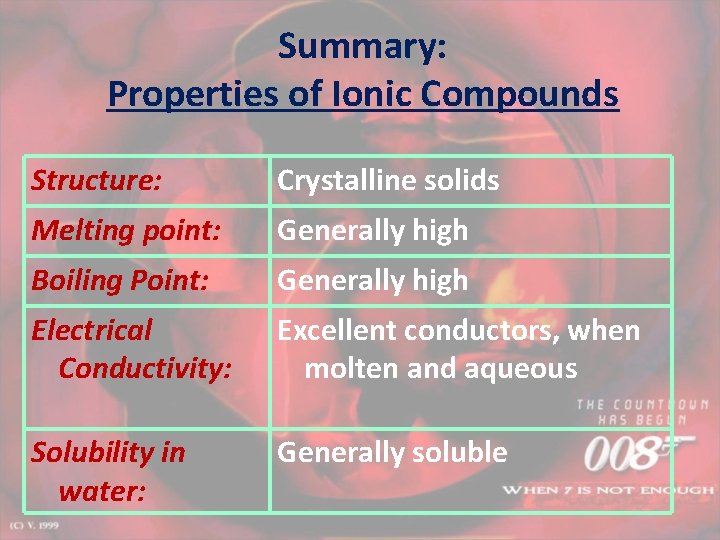
Summary: Properties of Ionic Compounds Structure: Crystalline solids Melting point: Generally high Boiling Point: Generally high Electrical Conductivity: Excellent conductors, when molten and aqueous Solubility in water: Generally soluble
Properties of Molecular (Covalent) Compounds Tend to have low melting and boiling points n Poor or nonconducting n Have a molecular formula which shows type and number of atoms in a molecule n n Not necessarily the lowest ratio! Ex. C 6 H 12 O 6 n (Formula doesn’t tell you about how atoms are arranged) n
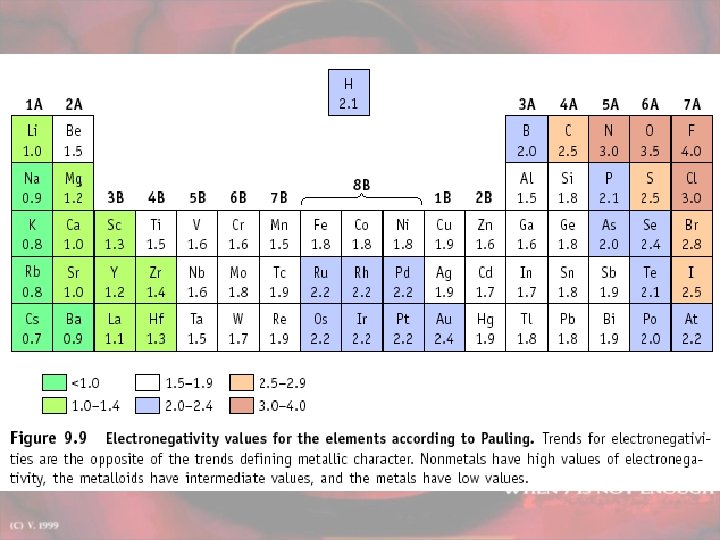
Bond type by electronegativity Electronegativities are listed on pg 151 Difference < 0. 4 between 0. 4 and 2. 0 > 2. 0 Bond Type nonpolar covalent ionic
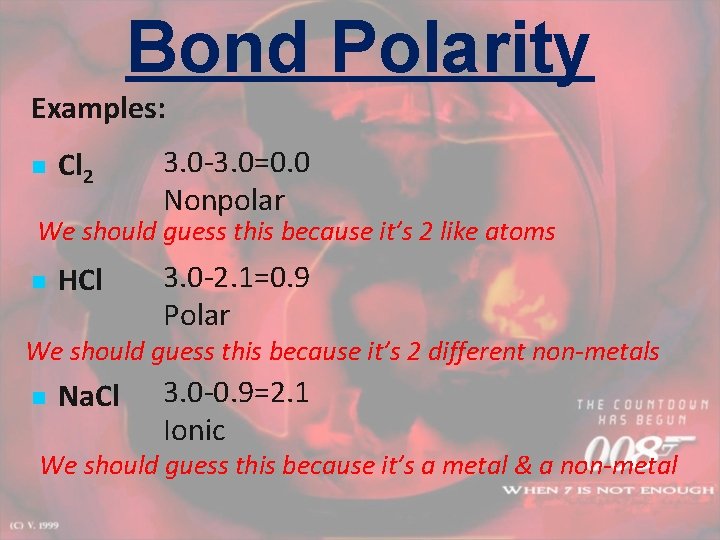
Bond Polarity Examples: n Cl 2 3. 0 -3. 0=0. 0 Nonpolar We should guess this because it’s 2 like atoms n HCl 3. 0 -2. 1=0. 9 Polar We should guess this because it’s 2 different non-metals n Na. Cl 3. 0 -0. 9=2. 1 Ionic We should guess this because it’s a metal & a non-metal

- Slides: 21