MAIN GROUP III ELEMENTS Chapter 12 13 The






![Dodecaborane [B 12 H 12]2 - Dodecaborane [B 12 H 12]2 -](https://slidetodoc.com/presentation_image/339f261740cd0432f5775e0d1d48fe0e/image-11.jpg)

























- Slides: 43

MAIN GROUP III ELEMENTS Chapter 12, 13

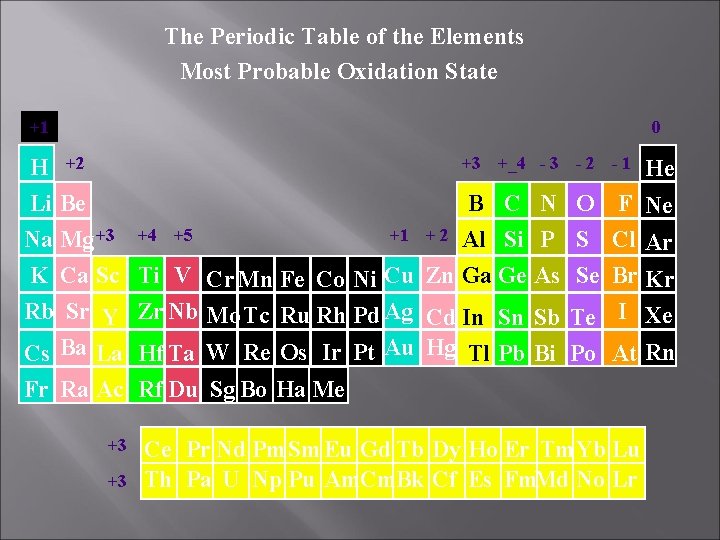
The Periodic Table of the Elements Most Probable Oxidation State +1 0 +3 +_4 - 3 H +2 Li Be B C N +1 + 2 Al Si P Na Mg +3 +4 +5 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Fr Ra Ac Rf Du Sg Bo Ha Me +3 +3 -2 -1 He O F Ne S Cl Ar Se Br Kr Te I Xe Po At Rn Ce Pr Nd Pm. Sm Eu Gd Tb Dy Ho Er Tm. Yb Lu Th Pa U Np Pu Am. Cm. Bk Cf Es Fm. Md No Lr

Boron � � Boron: In nature it is found as Borates: � Ulexite : {Na. Ca[B 5 O 6(OH)6]. 5 H 20} � Borax : {Na 2[B 405(OH)4]. 8 H 20} � Colemanite: {Ca 2[B 304(OH)3]2. 2 H 20)} � Kernite: {Na 2[B 4 O 5(OH)4]. 2 H 20} � Borates do have complex structures, but common to all is that Boron is contained as trigonal BO 3 or tetragonal BO 4 units.

Boron � � � The cations in these minerals are typically alkali or alkaline earth cations. The largest source of Boron is in the form of Borax found in the mojave desert in california No ionic compounds involving simple B 3+ cations are formed because the ionization enthalpies for boron are so high that lattice energies or hydration enthalpies cannot offset the energy required formation of a cation.

Boron � � Boron is sp 2 hybridized in trigonal planes. All BX 3 planes compounds are strong lewis acids interaction with Lewis bases (molecules or ions) gives tetrahedral adducts such as BF 3. O(C 2 H 5)2 , BF 4 -, and B(C 6 H 5)-4. The formation of such Lewis acid-base adducts requires a change to Sp 3 hybridization for boron.

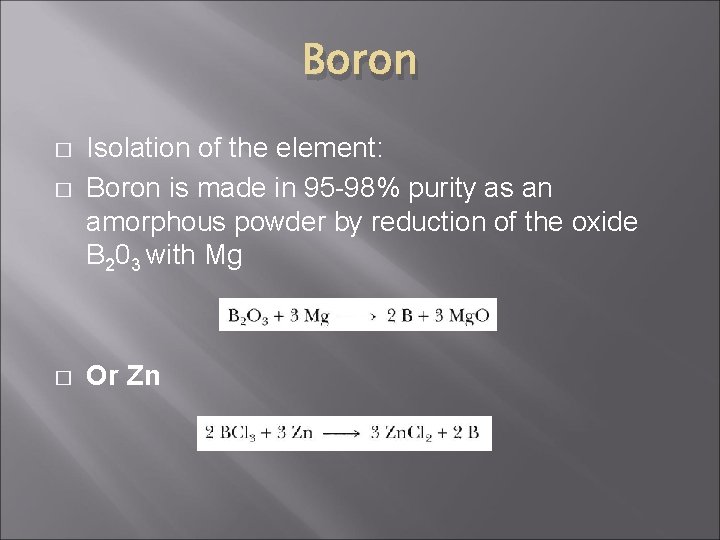
Boron � � � Isolation of the element: Boron is made in 95 -98% purity as an amorphous powder by reduction of the oxide B 203 with Mg Or Zn

Uses of Boron Borosilicate glass-pyrex Detergents Flame retardants Ceramics Pyrotechnics Used in production of impact resistant steels Control rods in nuclear reactors

Common Bonds in Boranes � � 2 c-2 e 3 c-2 e- B-H-B B-B-B

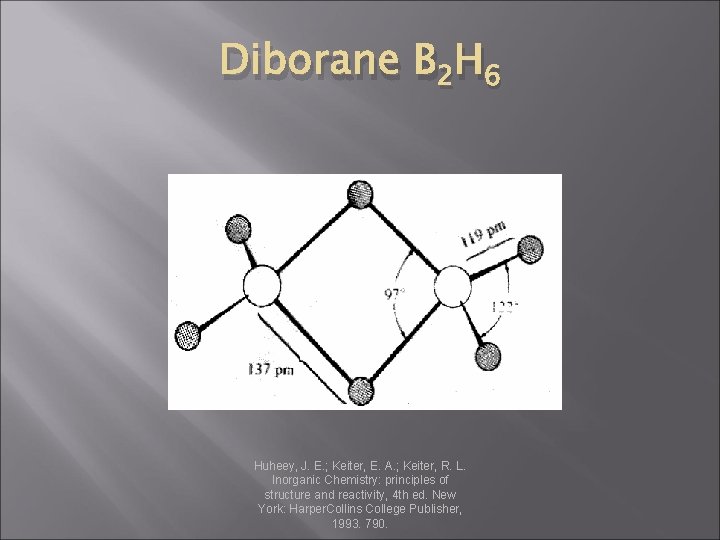
Diborane B 2 H 6 Huheey, J. E. ; Keiter, E. A. ; Keiter, R. L. Inorganic Chemistry: principles of structure and reactivity, 4 th ed. New York: Harper. Collins College Publisher, 1993. 790.

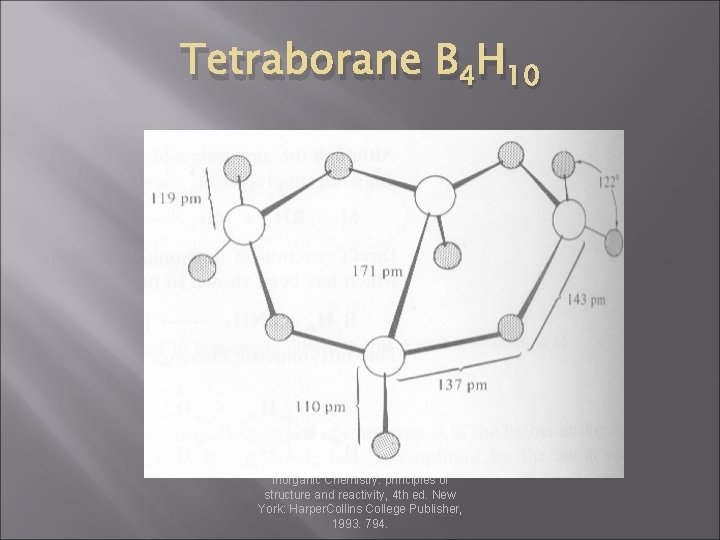
Tetraborane B 4 H 10 Huheey, J. E. ; Keiter, E. A. ; Keiter, R. L. Inorganic Chemistry: principles of structure and reactivity, 4 th ed. New York: Harper. Collins College Publisher, 1993. 794.
![Dodecaborane B 12 H 122 Dodecaborane [B 12 H 12]2 -](https://slidetodoc.com/presentation_image/339f261740cd0432f5775e0d1d48fe0e/image-11.jpg)
Dodecaborane [B 12 H 12]2 -

a- rhombohdral b-rhombohedral, B 12(B 12)12, (B 12)(B 60) Housecroft, C. E. ; Sharpe, A. G. inorganic Chemistry. New York: Pearson Education Limited, 2001. 251 -2.

Housecroft, C. E. ; Sharpe, A. G. inorganic Chemistry. New York: Pearson Education Limited, 2001. 275.

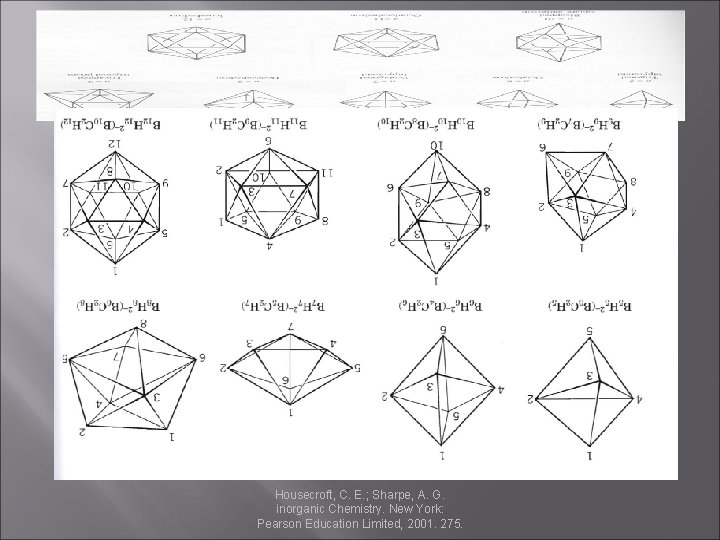
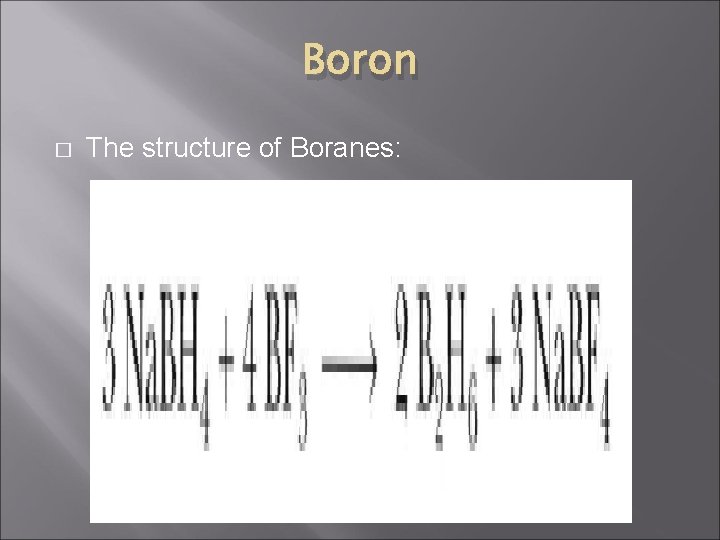
Boron � The structure of Boranes:

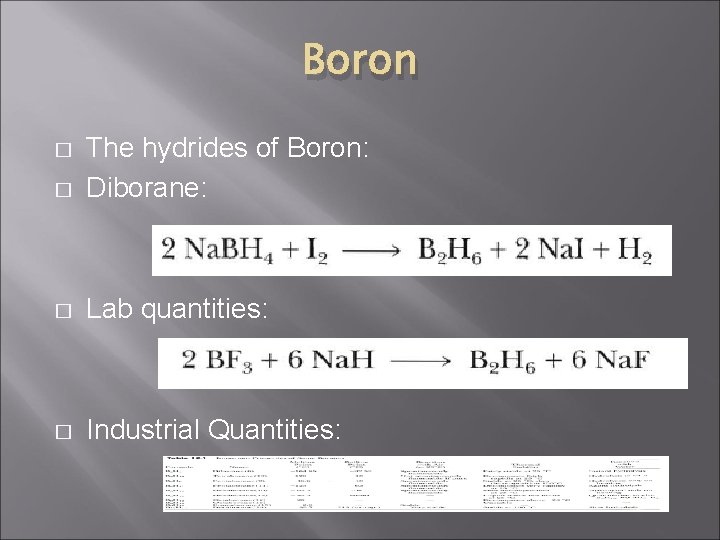
Boron � The hydrides of Boron: Diborane: � Lab quantities: � Industrial Quantities: �

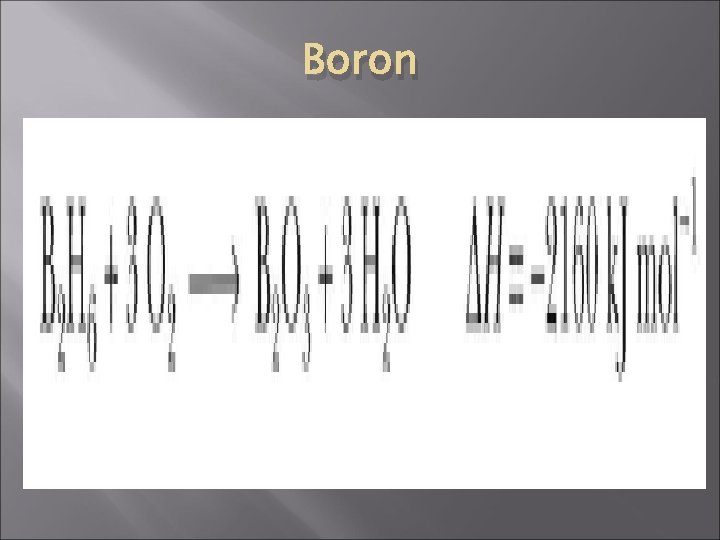
Boron

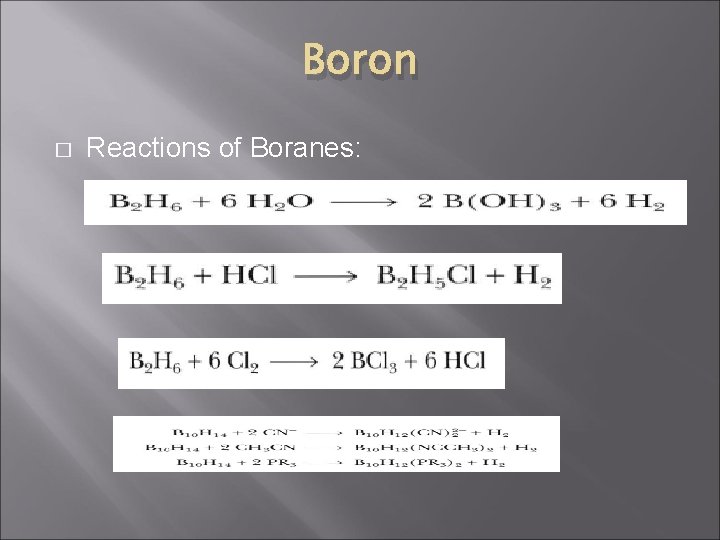
Boron � Reactions of Boranes:

Boron � Deca boranes:

Boron � Borohydrides of many metals have been made and some representative syntheses are:

Housecroft, C. E. ; Sharpe, A. G. inorganic Chemistry. New York: Pearson Education Limited, 2001. 272.

Huheey, J. E. ; Keiter, E. A. ; Keiter, R. L. Inorganic Chemistry: principles of structure and reactivity, 4 th ed. New York: Harper. Collins College Publishing, 1993. 799.

� � n = number of B atoms in parent closodeltahedron Always n+1 bonding e- pairs and n+1 bonding MOs � nido has n-1 vertices � arachno has n-2 vertices � hypho has n-3 vertices

� Find total available bonding e-s: � � Find parent closo-deltahedron � � Each B-H unit gives 2 e-s Each additional H gives 1 e. Overall charge n+1 bonding e- pairs Is it closo, nido, arachno, hypho? Lose highest connectivity B first then lose adjacent sites Determine number of remaining hydrogen atoms � � Each vertex has a H “sew up” hole with H atoms Bridging H atoms Low connectivity B atoms can get another 2 c-2 e- B-H bond Try to keep it as symmetrical as possible

Huheey, J. E. ; Keiter, E. A. ; Keiter, R. L. Inorganic Chemistry: principles of structure and reactivity, 4 th ed. New York: Harper. Collins College Publisher, 1993. 798.

� � � 10 B has large cross-section for neutron capture 10 B + a + 7 Li Products can kill cells Cancer treatment Cages - need high [10 B] in cell

Boron � The main resemblances to silicon and differences from the more metallic aluminum are as follows: � 1. The oxide B 20 3 and B(OHh are acidic. The compound Al(OH)3 is a basic hydroxide, although it shows weak amphoteric properties by dissolving in strong Na. OH. � 2. Borates and silicates are built on similar structural principles with sharing of oxygen atoms so that complicated chain, ring, or other structures result.

Boron � 3. The halides of Band Si (except BF 3) are readily hydrolyzed. The AI halides are solids and only partly hydrolyzed by water. All act as Lewis acids. � 4. The hydrides of B and Si are volatile, spontaneously flammable, and readily hydrolyzed. Aluminum hydride is a polymer, (Al. H 3)n

Boron � Crystalline boron is very inert and is attacked only by hot concentrated oxidizing agents. Amorphous boron is more reactive. With ammonia for instance, amorphous boron at white heat gives (BN)x a slippery white solid with a layer structure resembling that of graphite, but with hexagonal rings of alternating B and N atoms.

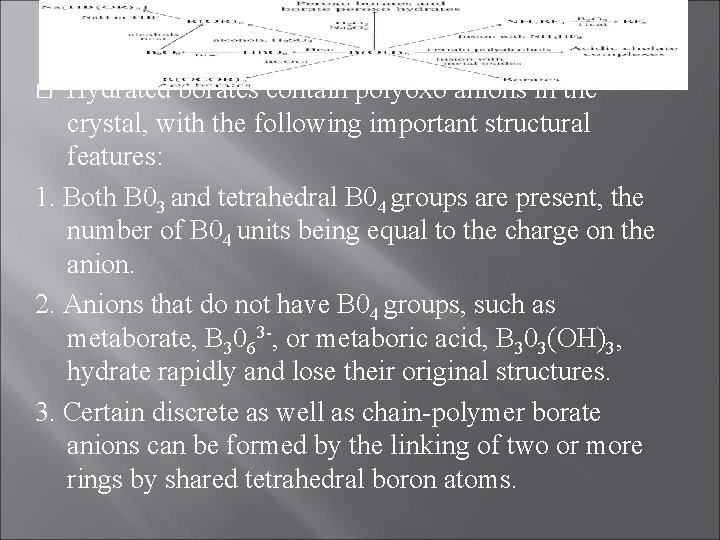
Hydrated borates contain polyoxo anions in the crystal, with the following important structural features: 1. Both B 03 and tetrahedral B 04 groups are present, the number of B 04 units being equal to the charge on the anion. 2. Anions that do not have B 04 groups, such as metaborate, B 3063 -, or metaboric acid, B 303(OH)3, hydrate rapidly and lose their original structures. 3. Certain discrete as well as chain-polymer borate anions can be formed by the linking of two or more rings by shared tetrahedral boron atoms. �

Boron � � � Boric acid: The acid B(OH)3 can be obtained as white needles either from borates, or by hydrolysis of boron trihalides. When heated, boric acid loses water stepwise to form one of three forms of metaboric acid, HB 02. If B(OH)3 is heated below 130°C, the so-called form. III is obtained, which has a layer structure in which B 303 rings are joined by hydrogen bonding. On continued heating of form-III of HB 02, between 130 and 150°C, HB 02 -II is formed.

Boron

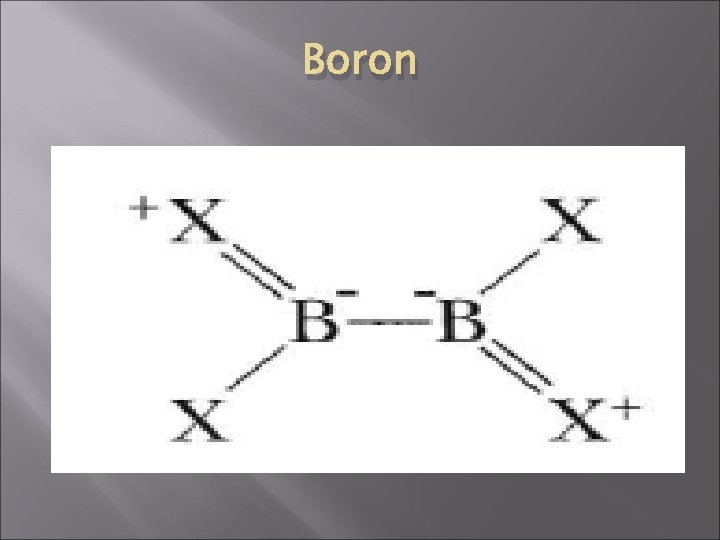
Boron � � � Halides: Boron trihalide is a gas (bp -101 deg C) Boron trihalides are the strongest lewis acids. They react with Lewis bases B-X bonds are somewhat shorter than is expected from the sum of the single-bond covalent radii. This suggests a delocalized πbond system

� � � Al is the most common of the elements It is produced in pure form by electrolysis, and is the most dirty of the industrial processes. Costs a lot of energy. Main source is Bauxite, a hydrous Al –oxide Al is attacked by diluted acids, but passivated by strong acids. Al oxides are used to protect metals (anodized)

� � � They are made from their salts by electrolysis. Ga is used mainly in semiconductors with Group V elements. (Ga. As). Tl is a trace element and is very toxic. � Main use to get rid of spies.

� � � Al has only one oxide formed Al 2 O 3 There is an alpha and a gamma oxide. Difference is the process and the temperature to get alpha or gamma oxide. Mixed Al oxides are ruby (Cr 3+)and sapphire (Fe 2+, Fe 3+, Ti 4+)

� � � Halides are formed of all elements, the only one that is special is Tl. I 3. Tl and I 2 form rather a Tl 1+ and I 3 - compound All halides readily dissolve in benzene



� � � The most important hydride is Li. Al. H 4 It is a strong reducing agent and is mainly used in organic chemistry It is used e. g. to hydrate double bonds

� 1. Boron � (a) Forms no simple B 3 +cation. � (b) Forms covalent compounds almost exclusively, and all polyatomic ions have covalent bonds. � (c) Obeys the octet rule, the maximum covalence being four. � (d) Forms trivalent compounds that readily serve as Lewis acids.

� (e) Frequently forms polyhedral structures: boranes and borates. � (f) Forms an oxide, B 203, and a hydroxide, B(OH)3 both of which are acidic. � (g) Forms covalent halides that are readily hydrolyzed. � (h) Forms numerous covalent hydrides, all of which are volatile, flammable, and readily hydrolyzed. � (i) Forms a stable and important hydride anion, BH 4 -.

� 2. Aluminum � (a) Readily forms an important 3+ ion, because it is electropositive. (b) Is much more metallic than boron, and forms a greater number and variety of ionic substances. (c) Forms both molecular and ionic substances, with coordination numbers of six and higher. (d) Forms two oxides, only one of which is acidic. (e) Forms a hydroxide that is weakly amphoteric, although mostly basic. (f) Forms solid halides that are only partially hydrolyzable. (g) Forms a polymeric hydride. (h) Forms an anionic hydride (Al. R-) that is more reactive than BH 4 -. � � � �

� 3. Gallium, Indium, and Thallium � (a) Readily give the M 3 + ion in solution, and have a rich coordination chemistry typical of metals. � (b) Form increasingly stable lower valent compounds, especially TI+. � (c) Increasinglyformweakercovalent bondsondeseentofthegroup, enhancing the formation of monovalent compounds. � (d) Form MX 3 halides that are increasingly aggregated in the solid state (through halide ion bridges) to give coordination numbers of four, six, and higher. � (e) Do not form important EH 4 - anions, except perhaps Ga. H 4 -.