MAIN FEATURES OF DOMINANT MECHANISM FOR DEHYDRATIONE 1

- Slides: 4

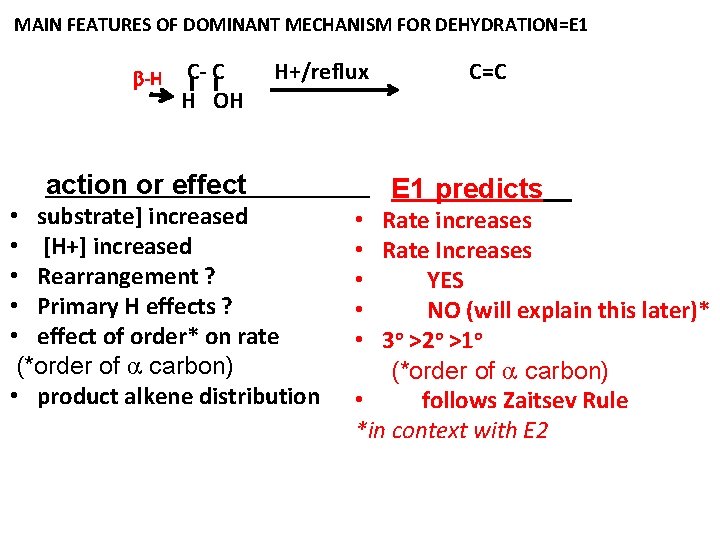
MAIN FEATURES OF DOMINANT MECHANISM FOR DEHYDRATION=E 1 -H C- C H OH H+/reflux action or effect • substrate] increased • [H+] increased • Rearrangement ? • Primary H effects ? • effect of order* on rate (*order of carbon) • product alkene distribution C=C E 1 predicts • Rate increases • Rate Increases • YES • NO (will explain this later)* • 3 o >2 o >1 o (*order of carbon) • follows Zaitsev Rule *in context with E 2

More E 1 factoids Is H+ catalytic or consumed ? catalytic Is a -H always needed ? No…methyl shift will allow alkene to form How can more than one product alkene form from E 1 ? Via rearrangement from lower higher degree What key intermediate is the signature of E 1 ? ? carbocation When will dehydration run E 2 ? ? ? For 1 o alcohol w/conditions favoring E 2 (non-polar solvent)

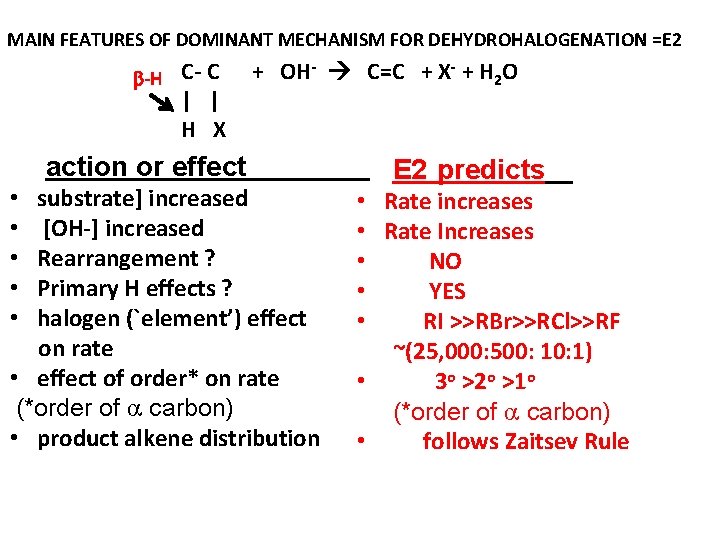
MAIN FEATURES OF DOMINANT MECHANISM FOR DEHYDROHALOGENATION =E 2 -H C- C | | H X + OH- C=C + X- + H 2 O action or effect substrate] increased [OH-] increased Rearrangement ? Primary H effects ? halogen (`element’) effect on rate • effect of order* on rate (*order of carbon) • product alkene distribution • • • E 2 predicts • Rate increases • Rate Increases • NO • YES • RI >>RBr>>RCl>>RF ~(25, 000: 500: 1) • 3 o >2 o >1 o (*order of carbon) • follows Zaitsev Rule

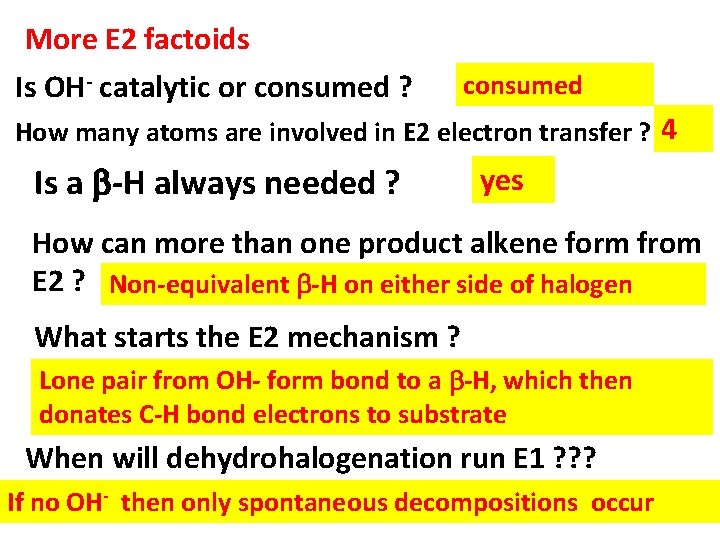
More E 2 factoids Is OH- catalytic or consumed ? consumed How many atoms are involved in E 2 electron transfer ? 4 Is a -H always needed ? yes How can more than one product alkene form from E 2 ? Non-equivalent -H on either side of halogen What starts the E 2 mechanism ? Lone pair from OH- form bond to a -H, which then donates C-H bond electrons to substrate When will dehydrohalogenation run E 1 ? ? ? If no OH- then only spontaneous decompositions occur