MAGNETIC BEADS RETENTION DEVICE FOR ONCHIP SANDWICH IMMUNOASSAY
MAGNETIC BEADS RETENTION DEVICE FOR ON-CHIP SANDWICH IMMUNO-ASSAY F. Lacharme 1, C. Vandevyver 2 and M. A. M. Gijs 1 Institute of Microelectronics and Microsystems Research Commission EPFL-SNF Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland Reporter:Yen-Po Lin (林諺伯) Date: December 30 , 2008

Outline l Introduction l Abstract l Design l Fabrication l Principle l Results l Conclusion l Reference

Introduction l Enzyme-Linked Immuno-sorbent Assay l What is magnetic micro-bead? l How can fix micro-bead? l Examples of this topic
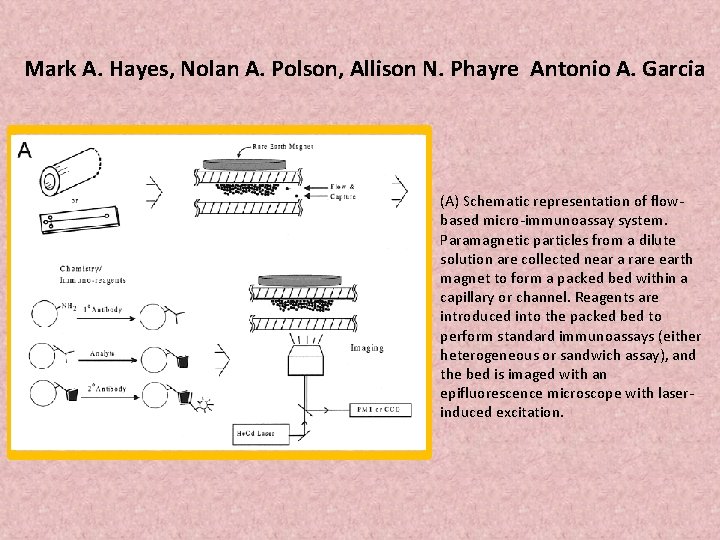
Mark A. Hayes, Nolan A. Polson, Allison N. Phayre Antonio A. Garcia (A) Schematic representation of flowbased micro-immunoassay system. Paramagnetic particles from a dilute solution are collected near a rare earth magnet to form a packed bed within a capillary or channel. Reagents are introduced into the packed bed to perform standard immunoassays (either heterogeneous or sandwich assay), and the bed is imaged with an epifluorescence microscope with laserinduced excitation.

Jin-Woo Choi Schematic diagram of a generic micro-fluidic system for biochemical detection. Analytical concept based on sandwich immunoassay and electrochemical detection. Conceptual illustration of bio-sampling and immunoassay procedure: (a) injection of magnetic beads; (b) separation and (holding of beads: (c) flowing samples: (d) immobilization of target antigen; (e) flowing labeled antibody; @ electrochemical detection; and (g) washing out magnetic beads and ready for another immunoassay.

Photograph of the fabricated bio-filter and immuno-sensor. Micro-fluidic biochemical analysis system that includes a surface-mounted bio-filter and immuno-sensor on a glass micro-fluidic motherboard. Integrated micro-fluidic biochemical detection system has been successfully developed and fully tested for fast and low Photograph of the fabricated micro-fluidic biochemical detection system for magnetic bead-based immunoassay
Abstract • Geometrical retention of self-assembled magnetic microbeads in a structured micro-channel. • Brought in a homogeneous magnetic field, a solution of 500 nm diameter magnetic micro-beads self-assembles in magnetic chains in the large sections of a periodically enlarge micro-channel • Device is able to perform full on-chip direct and sandwich immuno-assays, in a total assay time of less than 30 min
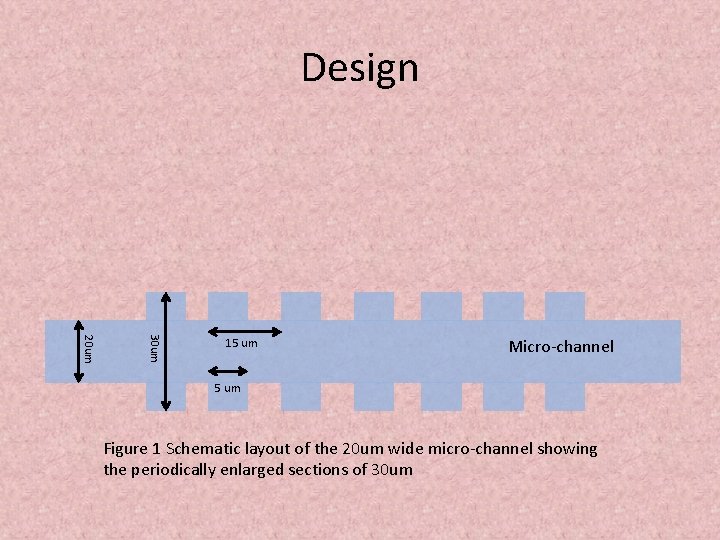
Design 30 um 20 um 15 um Micro-channel 5 um Figure 1 Schematic layout of the 20 um wide micro-channel showing the periodically enlarged sections of 30 um
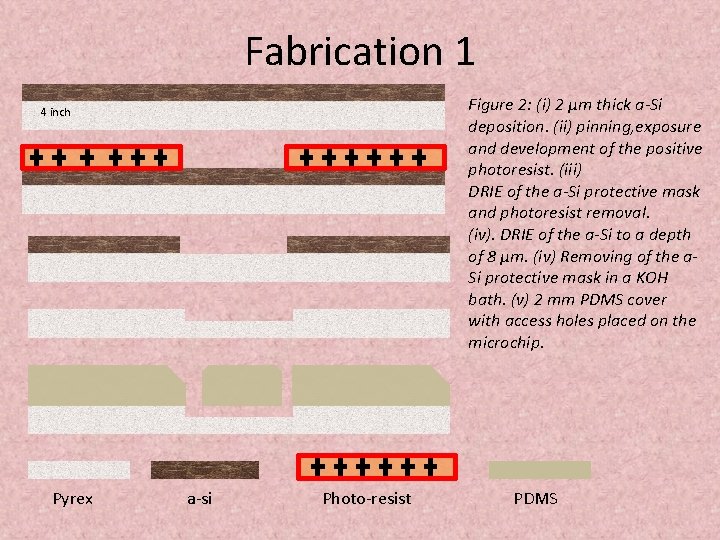
Fabrication 1 Figure 2: (i) 2 μm thick a-Si deposition. (ii) pinning, exposure and development of the positive photoresist. (iii) DRIE of the a-Si protective mask and photoresist removal. (iv). DRIE of the a-Si to a depth of 8 μm. (iv) Removing of the a. Si protective mask in a KOH bath. (v) 2 mm PDMS cover with access holes placed on the microchip. 4 inch Pyrex a-si Photo-resist PDMS
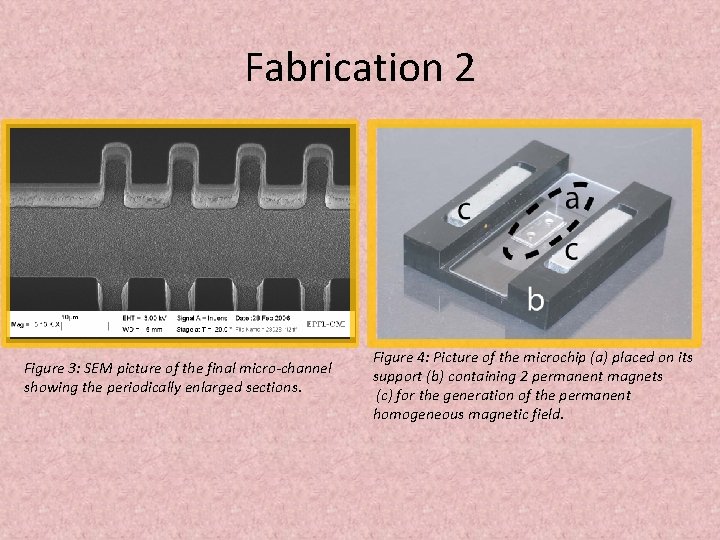
Fabrication 2 Figure 3: SEM picture of the final micro-channel showing the periodically enlarged sections. Figure 4: Picture of the microchip (a) placed on its support (b) containing 2 permanent magnets (c) for the generation of the permanent homogeneous magnetic field.
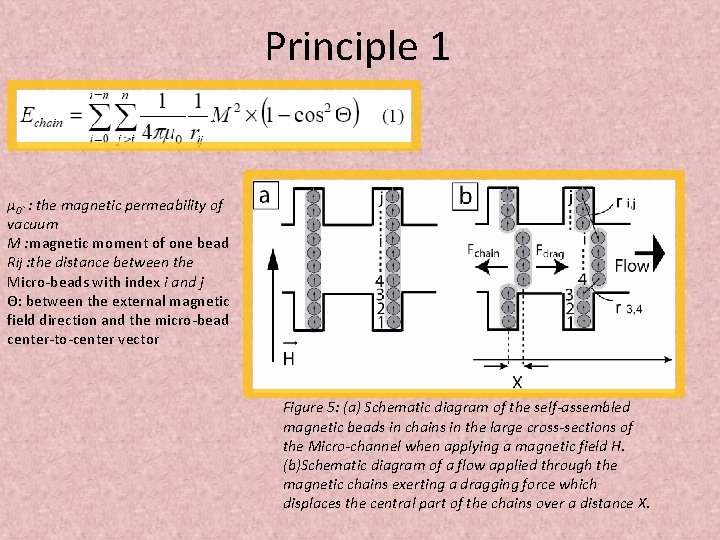
Principle 1 μ 0` : the magnetic permeability of vacuum M : magnetic moment of one bead Rij : the distance between the Micro-beads with index i and j Θ: between the external magnetic field direction and the micro-bead center-to-center vector Figure 5: (a) Schematic diagram of the self-assembled magnetic beads in chains in the large cross-sections of the Micro-channel when applying a magnetic field H. (b)Schematic diagram of a flow applied through the magnetic chains exerting a dragging force which displaces the central part of the chains over a distance X.
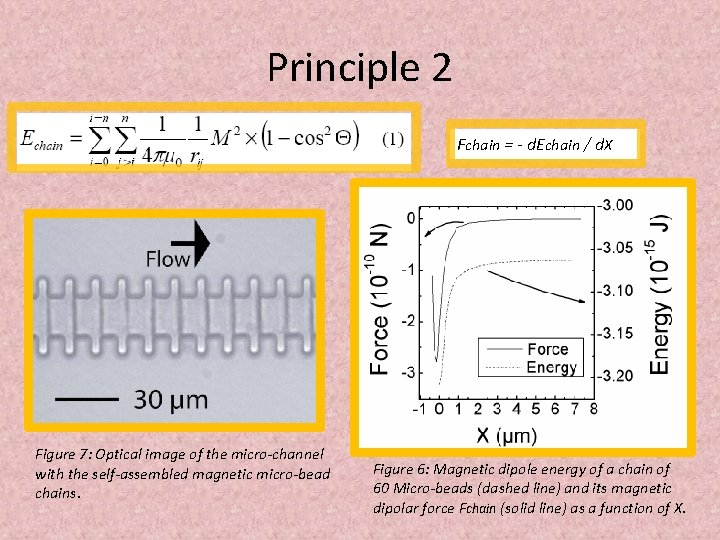
Principle 2 Fchain = - d. Echain / d. X Figure 7: Optical image of the micro-channel with the self-assembled magnetic micro-bead chains. Figure 6: Magnetic dipole energy of a chain of 60 Micro-beads (dashed line) and its magnetic dipolar force Fchain (solid line) as a function of X.
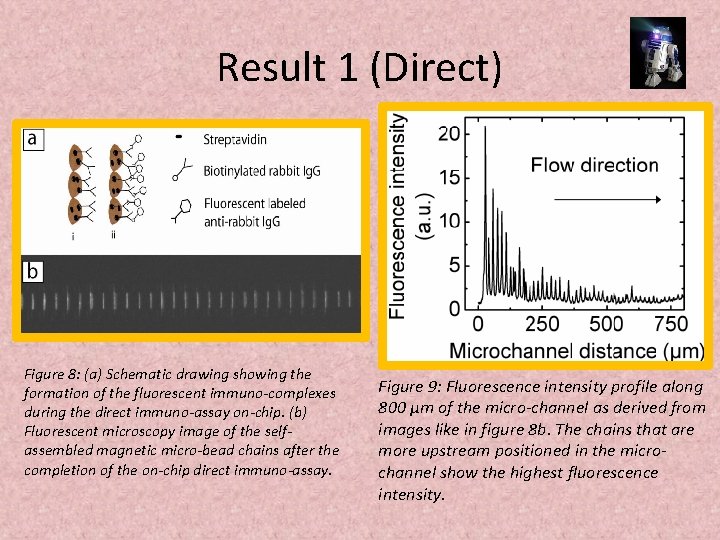
Result 1 (Direct) Figure 8: (a) Schematic drawing showing the formation of the fluorescent immuno-complexes during the direct immuno-assay on-chip. (b) Fluorescent microscopy image of the selfassembled magnetic micro-bead chains after the completion of the on-chip direct immuno-assay. Figure 9: Fluorescence intensity profile along 800 μm of the micro-channel as derived from images like in figure 8 b. The chains that are more upstream positioned in the microchannel show the highest fluorescence intensity.
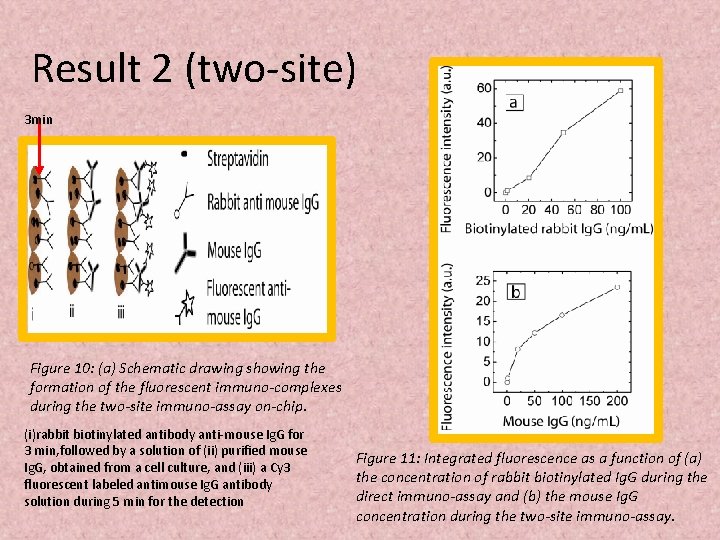
Result 2 (two-site) 3 min Figure 10: (a) Schematic drawing showing the formation of the fluorescent immuno-complexes during the two-site immuno-assay on-chip. (i)rabbit biotinylated antibody anti-mouse Ig. G for 3 min, followed by a solution of (ii) purified mouse Ig. G, obtained from a cell culture, and (iii) a Cy 3 fluorescent labeled antimouse Ig. G antibody solution during 5 min for the detection Figure 11: Integrated fluorescence as a function of (a) the concentration of rabbit biotinylated Ig. G during the direct immuno-assay and (b) the mouse Ig. G concentration during the two-site immuno-assay.
Conclusion • Micro-beads specifically capture and detect a low number of target analyte molecules (less than 105 ) in a very small sample volume of ~31 n. L in an total assay time of less than 30 min. • The magnetic chains gradually deplete the target antigen concentration from the flow • Our method permits to position a well-controlled and small amount of micro-beads in the micro-fluidic system • The way for fast, precise and extremely cheap detection of specific molecules from a complex matrix
![Reference [1]A. Manz, N. Graber, and H. M. Widmer, "Miniaturized Total Chemical-Analysis Systems -a Reference [1]A. Manz, N. Graber, and H. M. Widmer, "Miniaturized Total Chemical-Analysis Systems -a](http://slidetodoc.com/presentation_image_h/0842e5955410bff5c07ca91ff67a5e78/image-16.jpg)
Reference [1]A. Manz, N. Graber, and H. M. Widmer, "Miniaturized Total Chemical-Analysis Systems -a Novel Concept for Chemical Sensing, " Sens. Actuators A, vol. 10, pp. 244 -248, 1990. [2] K. Sato, M. Tokeshi, H. Kimura, and T. Kitamori, "Determination of carcinoembryonic antigen inhuman sera by integrated bead bed immunoasay in a microchip for cancer diagnosis, " Analytical Chemistry, vol. 73, pp. 1213 -1218, 2001 [3] M. A. M. Gijs, "Magnetic bead handling on-chip: new opportunities for analytical applications, ”Micro-fluidics and Nano-fluidics, vol. 1, pp. 22 -40, 2004. [4] M. A. Hayes, N. A. Polson, A. N. Phayre, and A. A. Garcia, "Flow-based micro-immunoassay, " Anal. Chem. , vol. 73, pp. 5896 -5902, 2001. [5] J. W. Choi, K. W. Oh, J. H. Thomas, W. R. Heineman, H. B. Halsall, J. H. Nevin, A. J. Helmicki, H. T. Henderson, and C. H. Ahn, "An integrated micro-fluidic biochemical detection system for protein analysis with magnetic bead based sampling capabilities, " Lab on a Chip, vol. 2, pp. 27 -30, 2002. [6] A. C. Siegel, S. S. Shevkoplyas, D. B. Weibel, D. A. Bruzewicz, A. W. Martinez, and G. M. Whitesides, "Cofabrication of electromagnets and microfluldic systems in poly(dimethylsiloxane), “ Angewandte Chemie-International Edition, vol. 45, pp. 6877 -6882, 2006.
![Reference [7] T. Deng, M. Prentiss, and G. M. Whitesides, "Fabrication of magnetic microfiltration Reference [7] T. Deng, M. Prentiss, and G. M. Whitesides, "Fabrication of magnetic microfiltration](http://slidetodoc.com/presentation_image_h/0842e5955410bff5c07ca91ff67a5e78/image-17.jpg)
Reference [7] T. Deng, M. Prentiss, and G. M. Whitesides, "Fabrication of magnetic microfiltration systems using soft lithography, " Applied Physics Letters, vol. 80, pp. 461 -463, 2002. [8] A. Rida and M. A. M. Gijs, "Manipulation of selfassembled structures of magnetic beads for microfluidic mixing and assaying, " Analytical Chemistry, vol. 76, pp. 6239 -6246, 2004 [9] M. Herrmann, "Microfluidic ELISA on nonpassivated PDMS chip using magnetic bead transfer inside dual networks of channels, " Lab Chip, pp. DOI: 10. 1039/b 707883 h, 2007. [10] A. Rida, V. Fernandez, and M. A. M. Gijs, "Longrange transport of magnetic microbeads using simple planar coils placed in a uniform magnetostatic field, " Applied Physics Letters, vol. 83, pp. 2396 -2398, 2003. [11] P. S. Doyle, J. Bibette, A. Bancaud, and J. L. Viovy, "Self-assembled magnetic matrices for DNA separation chips, " Science, vol. 295, pp. 2237 -2237, 2002. [12] L. E. Helseth, "Self-assembly of colloidal pyramids in magnetic fields, " Langmuir, vol. 21, pp. 7276 -7279, 2005.
- Slides: 17