Magma Rocks Classification Textures MAGMA MAGMA Larutan silikat
















- Slides: 54

Magma, Rocks Classification & Textures

MAGMA

MAGMA • Larutan silikat yang sangat panas • Mengandung oksida, sulfida serta volatiles (CO 2, sulfur, chlorine, fluorin, boron dll) • Temperatur antara 600°C (magma asam) sampai 1250°C (magma basa)

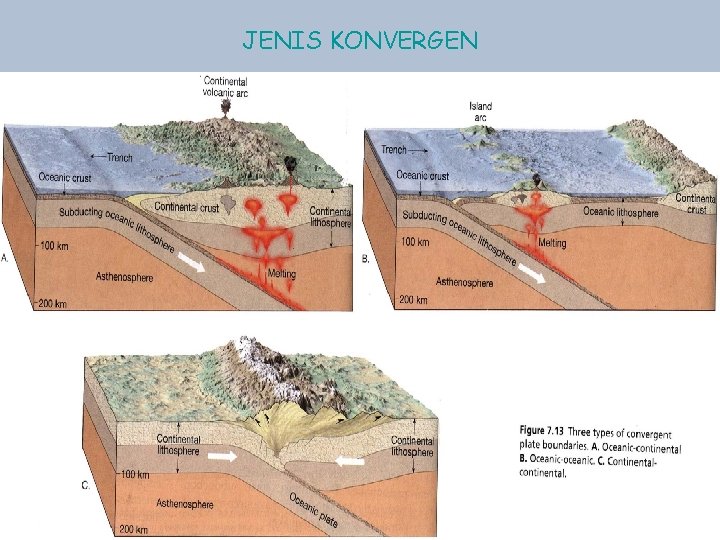
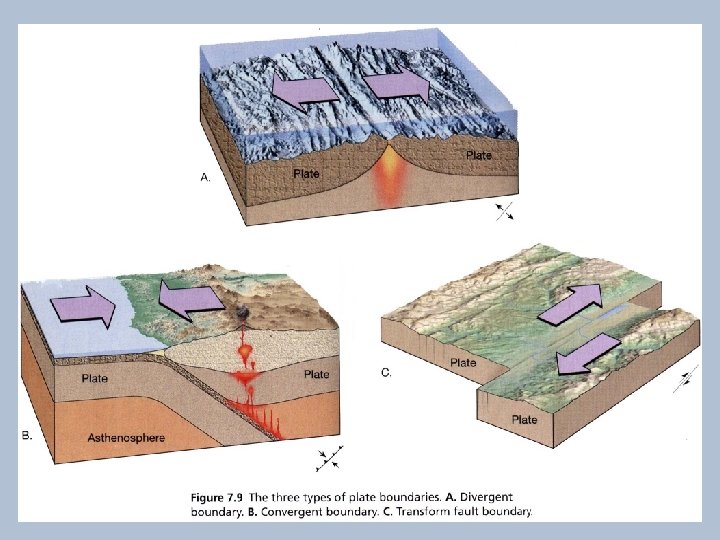
JENIS KONVERGEN


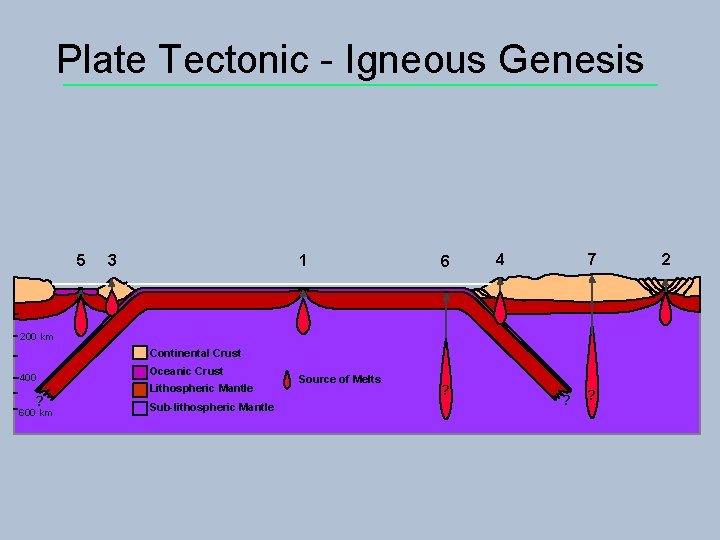
Plate Tectonic - Igneous Genesis 5 3 1 6 7 4 200 km Continental Crust 400 ? 600 km Oceanic Crust Lithospheric Mantle Sub-lithospheric Mantle Source of Melts ? ? ? 2

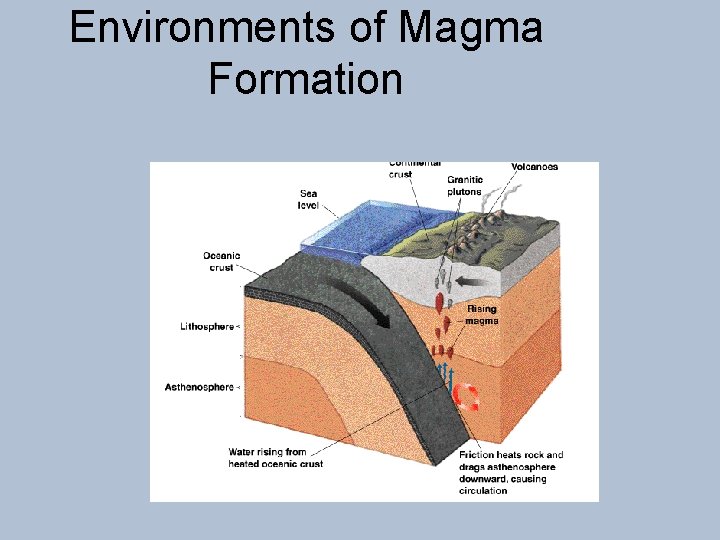
Environments of Magma Formation

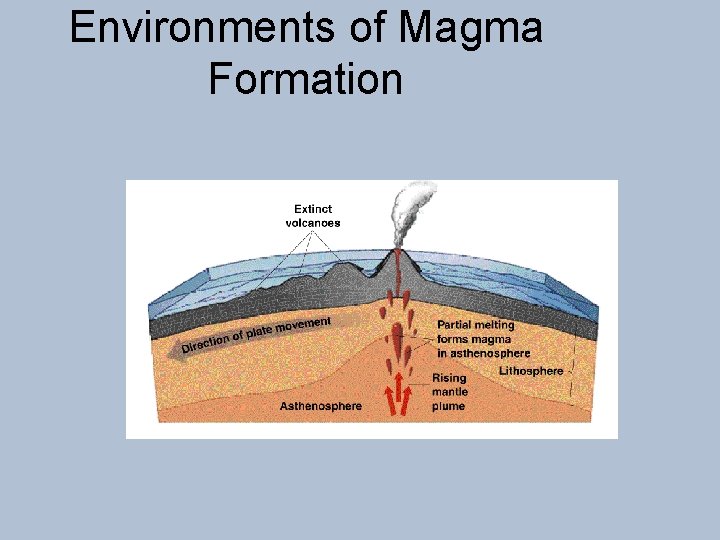
Environments of Magma Formation

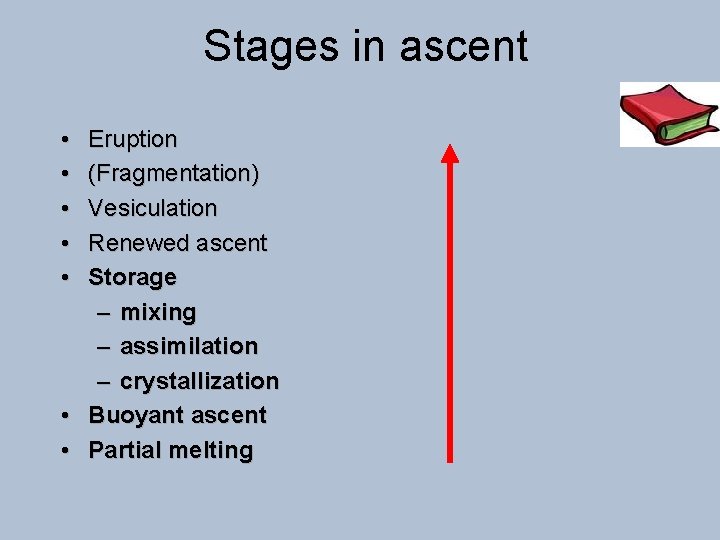
Stages in ascent • • • Eruption (Fragmentation) Vesiculation Renewed ascent Storage – mixing – assimilation – crystallization • Buoyant ascent • Partial melting

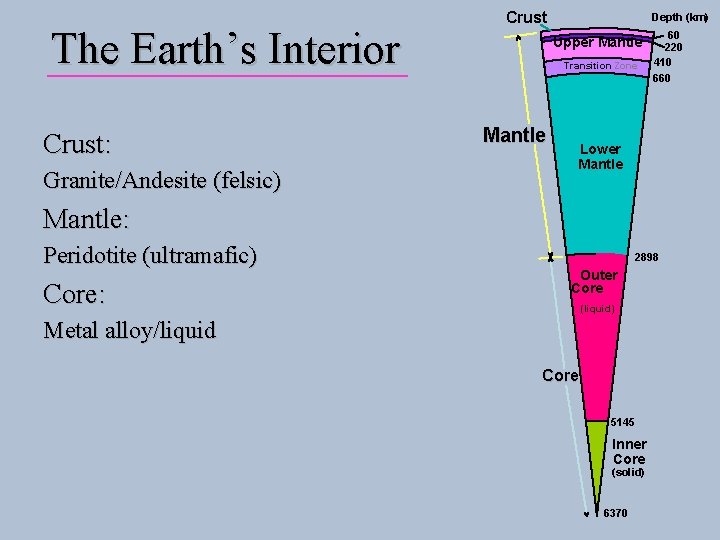
The Earth’s Interior Crust: Granite/Andesite (felsic) Crust Depth (km) Upper Mantle Transition Zone Mantle 60 220 410 660 Lower Mantle: Peridotite (ultramafic) Core: 2898 Outer Core (liquid) Metal alloy/liquid Core 5145 Inner Core (solid) 6370

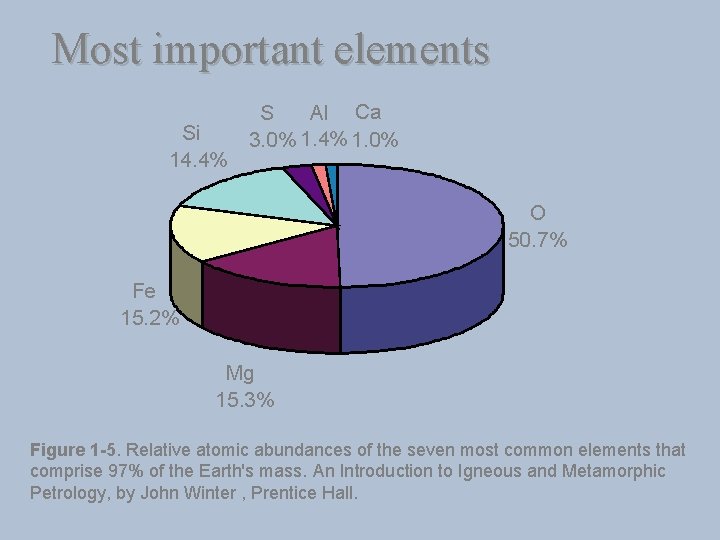
Most important elements Si 14. 4% Al Ca S 3. 0% 1. 4% 1. 0% O 50. 7% Fe 15. 2% Mg 15. 3% Figure 1 -5. Relative atomic abundances of the seven most common elements that comprise 97% of the Earth's mass. An Introduction to Igneous and Metamorphic Petrology, by John Winter , Prentice Hall.

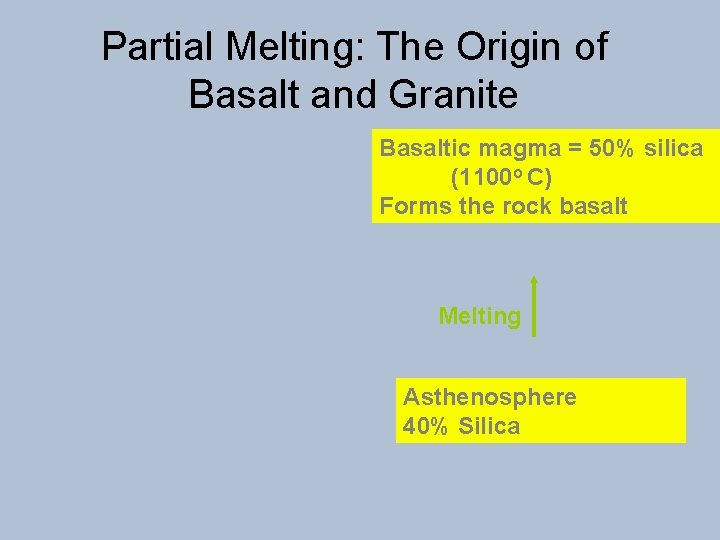
Partial Melting: The Origin of Basalt and Granite Basaltic magma = 50% silica (1100 o C) Forms the rock basalt Melting Asthenosphere 40% Silica

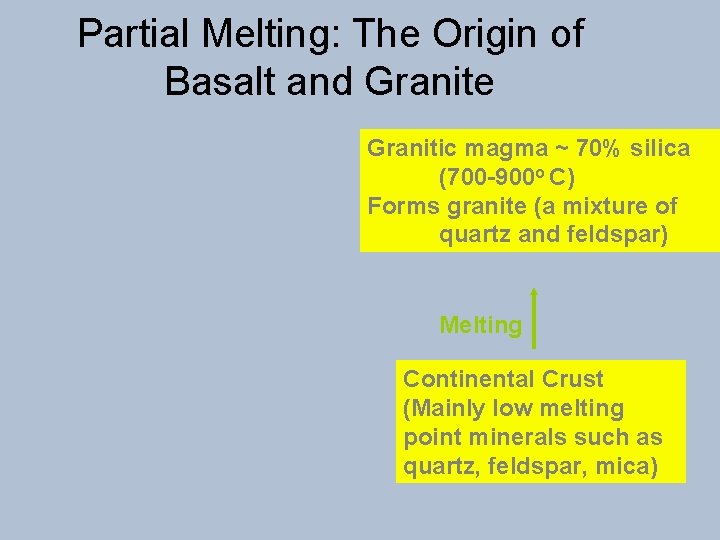
Partial Melting: The Origin of Basalt and Granite Granitic magma ~ 70% silica (700 -900 o C) Forms granite (a mixture of quartz and feldspar) Melting Continental Crust (Mainly low melting point minerals such as quartz, feldspar, mica)

Urutan pembekuan magma • Pada pembekuan magma, pada awalnya mineral yang terbentuk adalah yang anhydrous (tidak mengandung air) tidak mengandung gugus OH, disebut mineral pyrogenetik. • Cairan selanjutnya akan lebih banyak mengandung komponen gas dan terbentuk mineral-mineral yang mengandung gugusan hydroksil (OH), disebut mineral hydratogenetik.

Diferensiasi Magma • Proses diferensiasi meliputi semua kegiatan yang mengakibatkan suatu jenis magma induk yang semula relatif homogen terpecah-pecah menjadi beberapa bagian atau fraksi dengan komposisi yang berbeda-beda. Hal ini disebabkan karena migrasi ion atau molekul dalam larutan magma karena adanya perubahan temperatur dan tekanan. Yang pada akhirnya akan membentuk berbagai jenis batuan beku dengan komposisi yang berbeda-beda pula.

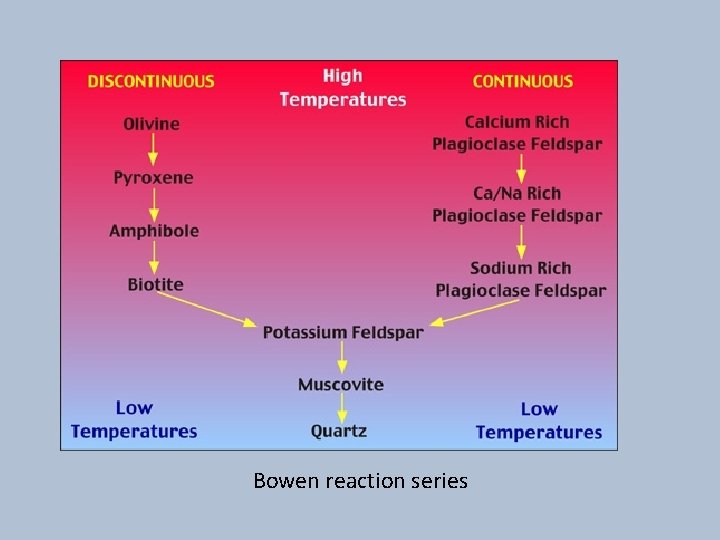
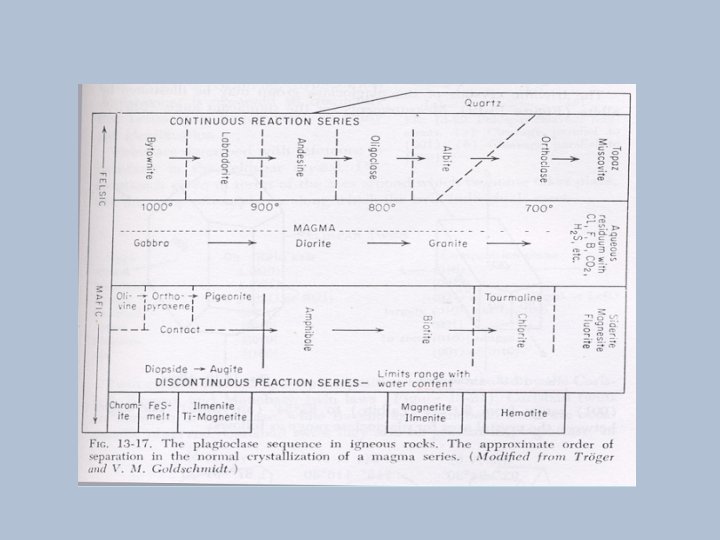
Bowen reaction series


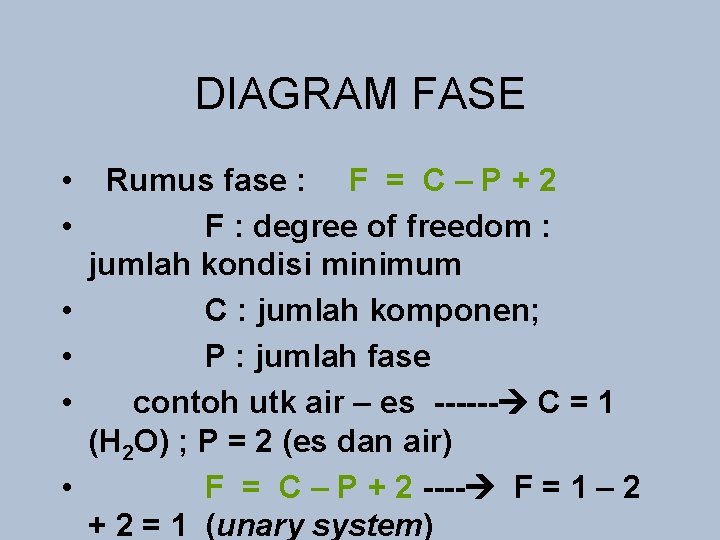
DIAGRAM FASE • Fase : padat, cair, gas • Diagram fase : menggambarkan kondisi magma pada kondisi P & T tertentu • Parameter penting dalam sistem magma : fase, komponen, variabel intensif

DIAGRAM f. ASE • fase : padat, cair • komponen : komponen terkecil yang diperlukan utk pembentukan fase-fase • dalam sistem (OH, H 2 O, Mg. O, Na. Al. Si 3 O 8, dll) • • variabel intensif : temperatur dan tekanan, jumlah komponen

DIAGRAM FASE • • • Rumus fase : F = C – P + 2 F : degree of freedom : jumlah kondisi minimum C : jumlah komponen; P : jumlah fase contoh utk air – es ------ C = 1 (H 2 O) ; P = 2 (es dan air) F = C – P + 2 ---- F = 1 – 2 + 2 = 1 (unary system)

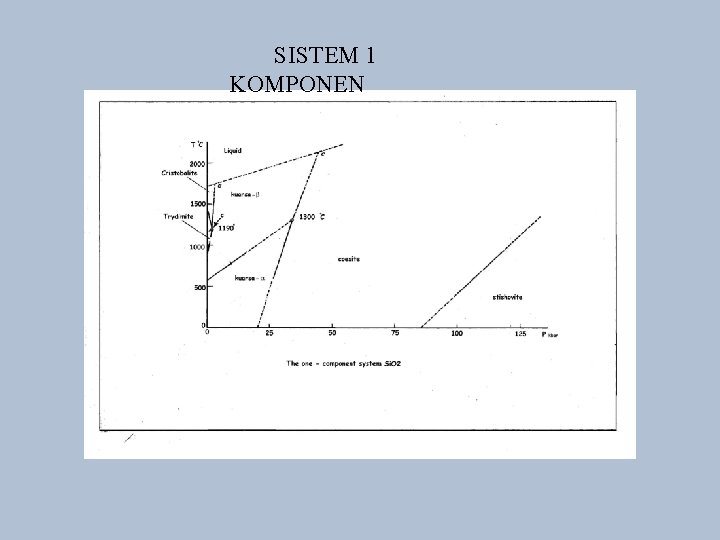
SISTEM 1 KOMPONEN

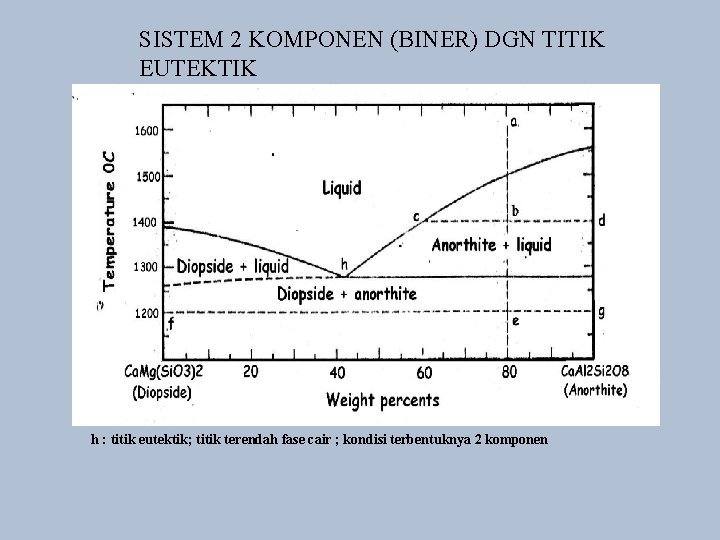
SISTEM 2 KOMPONEN (BINER) DGN TITIK EUTEKTIK h : titik eutektik; titik terendah fase cair ; kondisi terbentuknya 2 komponen

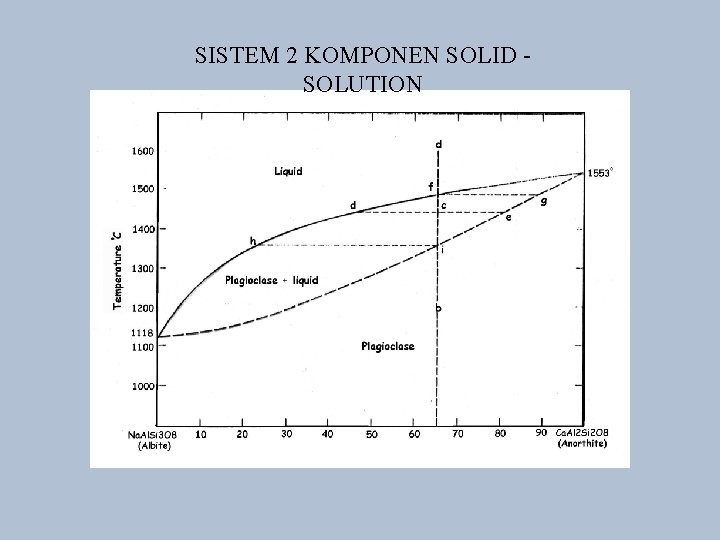
SISTEM 2 KOMPONEN SOLID - SOLUTION

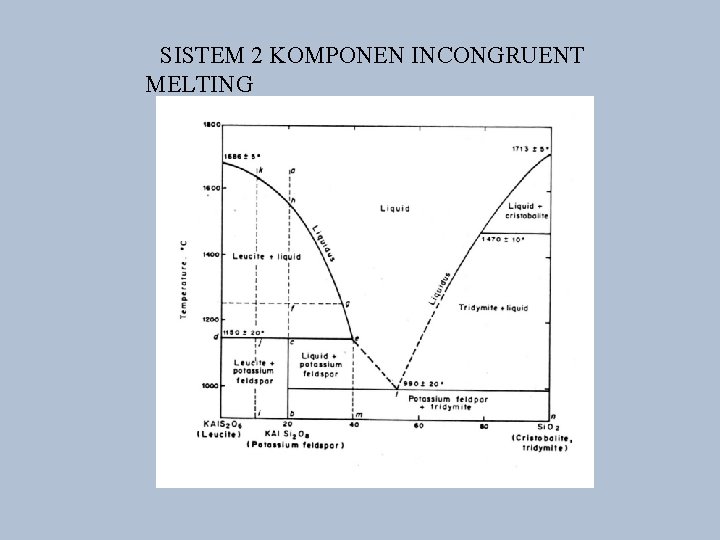
SISTEM 2 KOMPONEN INCONGRUENT MELTING

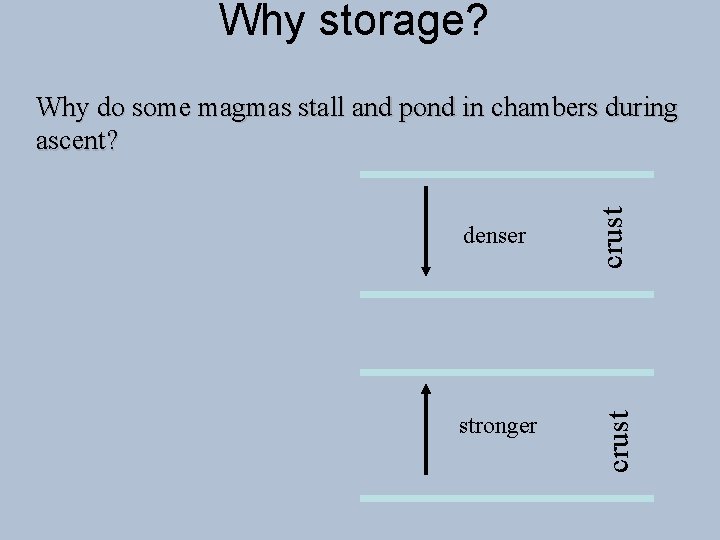
Why storage? stronger crust denser crust Why do some magmas stall and pond in chambers during ascent?

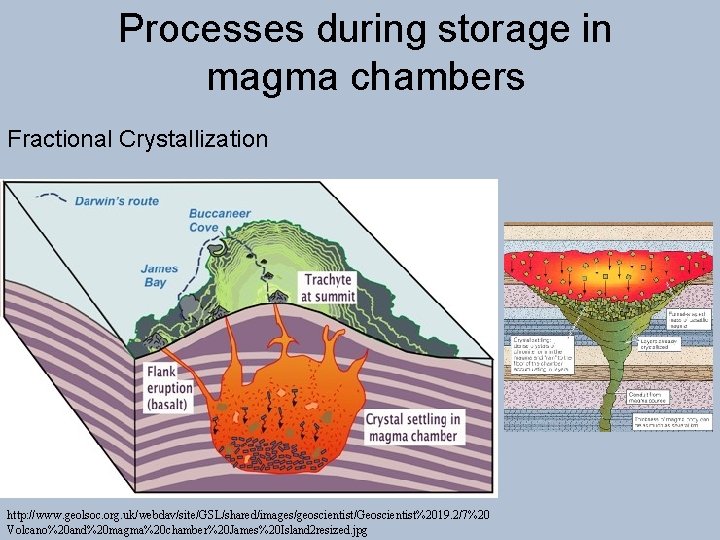
Processes during storage in magma chambers Fractional Crystallization http: //www. geolsoc. org. uk/webdav/site/GSL/shared/images/geoscientist/Geoscientist%2019. 2/7%20 Volcano%20 and%20 magma%20 chamber%20 James%20 Island 2 resized. jpg

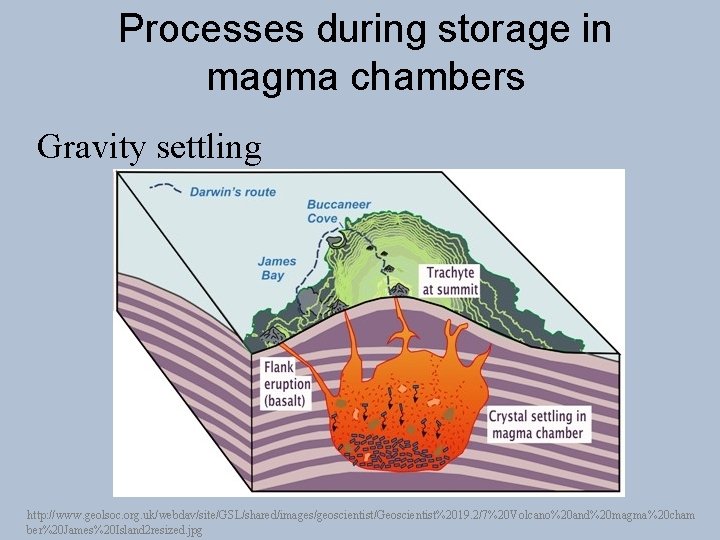
Processes during storage in magma chambers Gravity settling http: //www. geolsoc. org. uk/webdav/site/GSL/shared/images/geoscientist/Geoscientist%2019. 2/7%20 Volcano%20 and%20 magma%20 cham ber%20 James%20 Island 2 resized. jpg

Gravity settling and cumulates http: //www. geol. lsu. edu/henry/Geology 3041/lectures/12 Layered. Mafic/Fig 12 -15. jpg

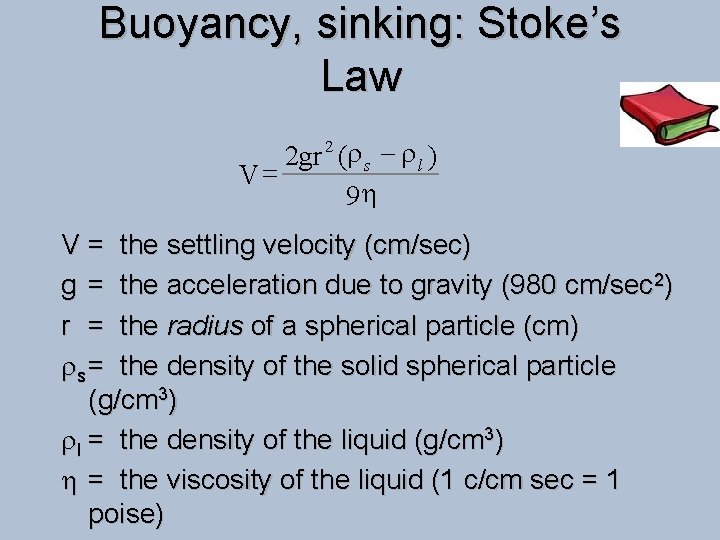
Buoyancy, sinking: Stoke’s Law 2 gr (r s - r l ) V= 9 h 2 V = the settling velocity (cm/sec) g = the acceleration due to gravity (980 cm/sec 2) r = the radius of a spherical particle (cm) rs = the density of the solid spherical particle (g/cm 3) rl = the density of the liquid (g/cm 3) h = the viscosity of the liquid (1 c/cm sec = 1 poise)

Sinking olivine in basalt Olivine in basalt F Olivine (rs = 3. 3 g/cm 3, r = 0. 1 cm) F Basaltic liquid (rl = 2. 65 g/cm 3, h = 1000 poise) F V = 2· 980· 0. 12 (3. 3 -2. 65)/9· 1000 = 0. 0013 cm/sec that’s ~1 m per day

Sinking x’tal in rhyolite Rhyolitic melt F F F h = 107 poise and rl = 2. 3 g/cm 3 hornblende crystal (rs = 3. 2 g/cm 3, r = 0. 1 cm) -7 s V = 2 x 10 cm/sec, or 6 cm/year feldspars (rl = 2. 7 g/cm 3) s V = 2 cm/year 4 s = 200 m in the 10 years that a stock might cool s If 0. 5 cm in radius (1 cm diameter) settle at 0. 65 meters/year, or 6. 5 km in 104 year cooling of stock

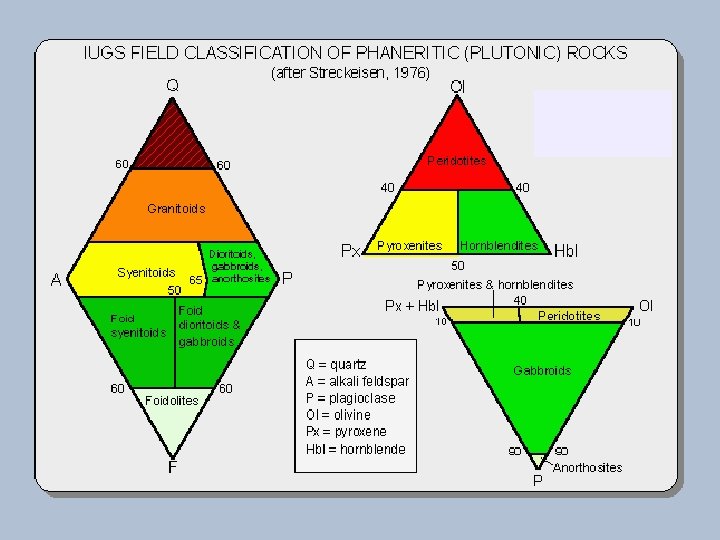
IGNEOUS ROCKS CLASSIFICATION

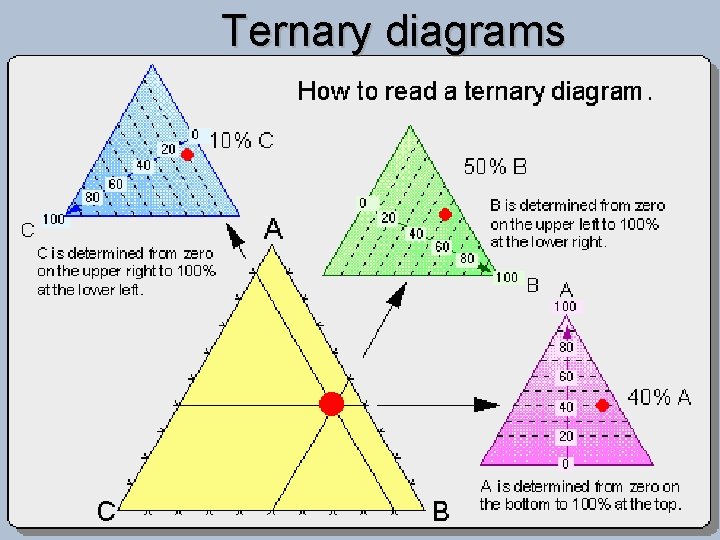
Ternary diagrams

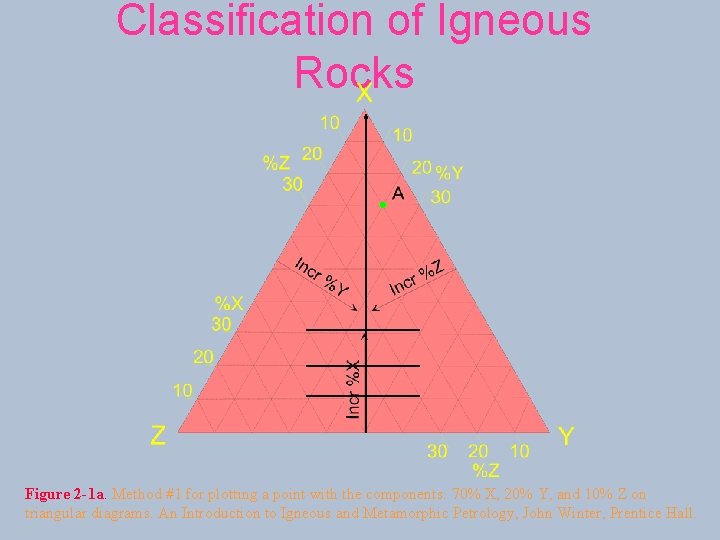
Classification of Igneous Rocks Figure 2 -1 a. Method #1 for plotting a point with the components: 70% X, 20% Y, and 10% Z on triangular diagrams. An Introduction to Igneous and Metamorphic Petrology, John Winter, Prentice Hall.

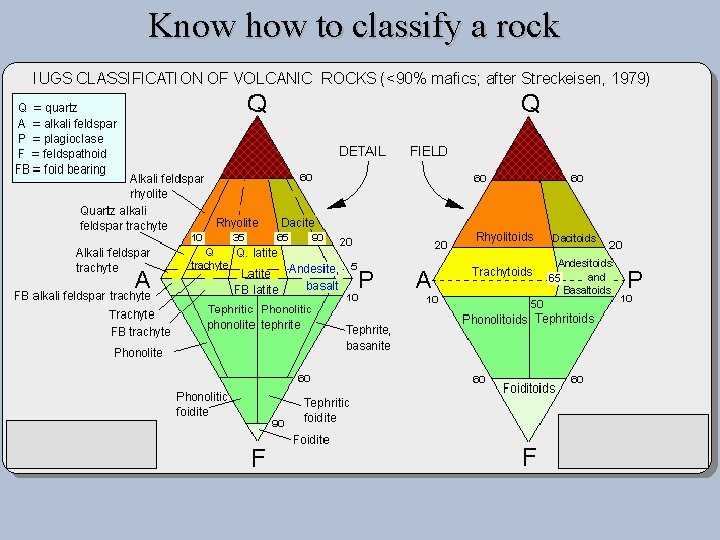
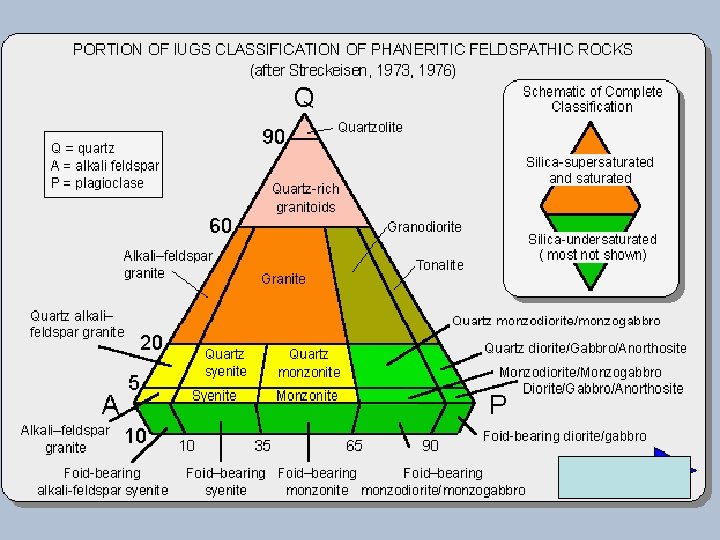
Know how to classify a rock



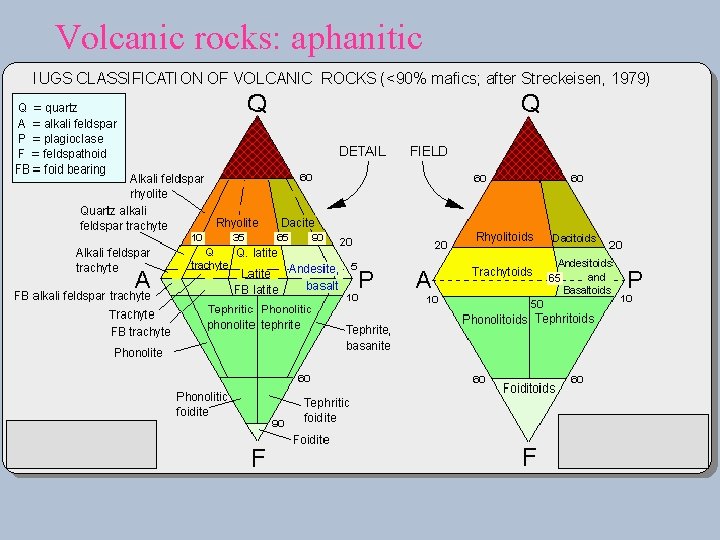
Volcanic rocks: aphanitic

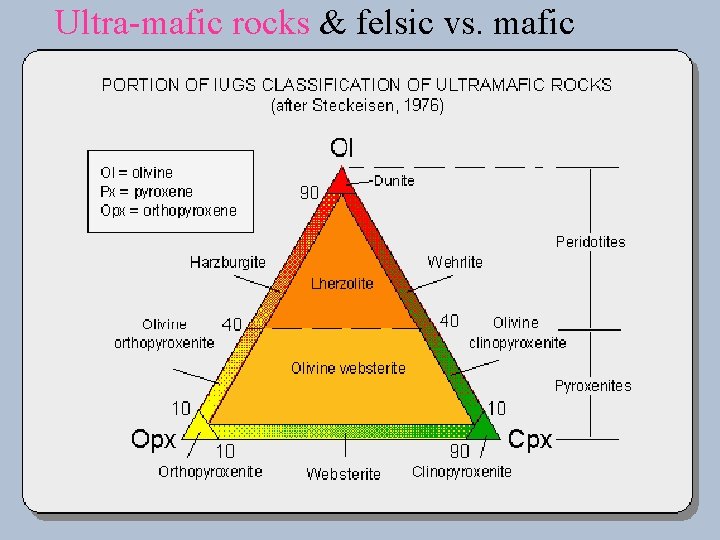
Ultra-mafic rocks & felsic vs. mafic

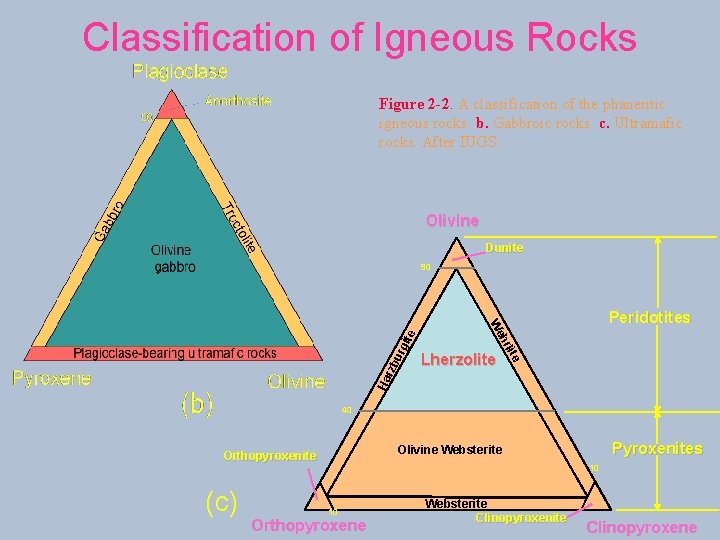
Classification of Igneous Rocks Figure 2 -2. A classification of the phaneritic igneous rocks. b. Gabbroic rocks. c. Ultramafic rocks. After IUGS. Olivine Dunite urg Ha Lherzolite hr rzb Peridotites We ite 90 40 (c) Pyroxenites Olivine Websterite Orthopyroxenite 10 10 Orthopyroxene Websterite Clinopyroxene

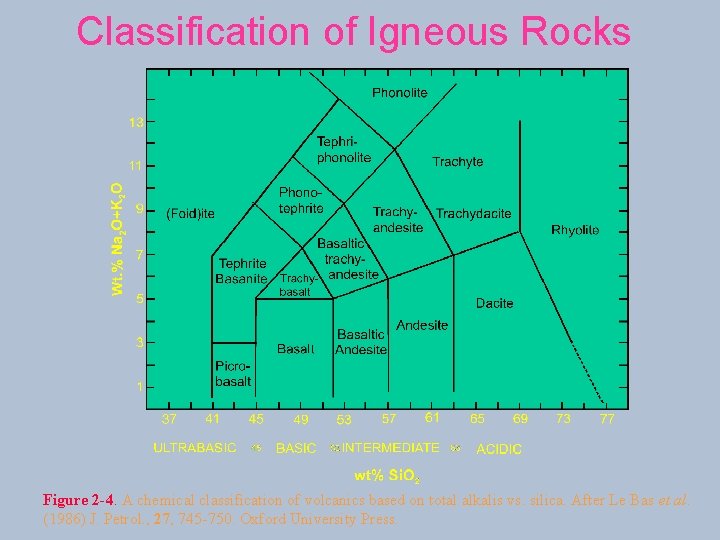
Classification of Igneous Rocks Figure 2 -4. A chemical classification of volcanics based on total alkalis vs. silica. After Le Bas et al. (1986) J. Petrol. , 27, 745 -750. Oxford University Press.

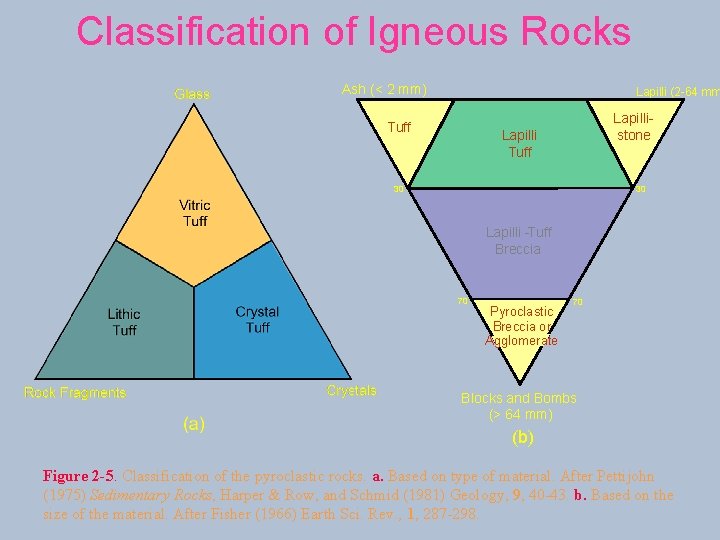
Classification of Igneous Rocks Ash (< 2 mm) Lapilli (2 -64 mm Tuff Lapillistone Lapilli Tuff 30 30 Lapilli -Tuff Breccia 70 Pyroclastic Breccia or Agglomerate 70 Blocks and Bombs (> 64 mm) (b) Figure 2 -5. Classification of the pyroclastic rocks. a. Based on type of material. After Pettijohn (1975) Sedimentary Rocks, Harper & Row, and Schmid (1981) Geology, 9, 40 -43. b. Based on the size of the material. After Fisher (1966) Earth Sci. Rev. , 1, 287 -298.

TEXTURES IN IGNEOUS ROCKS

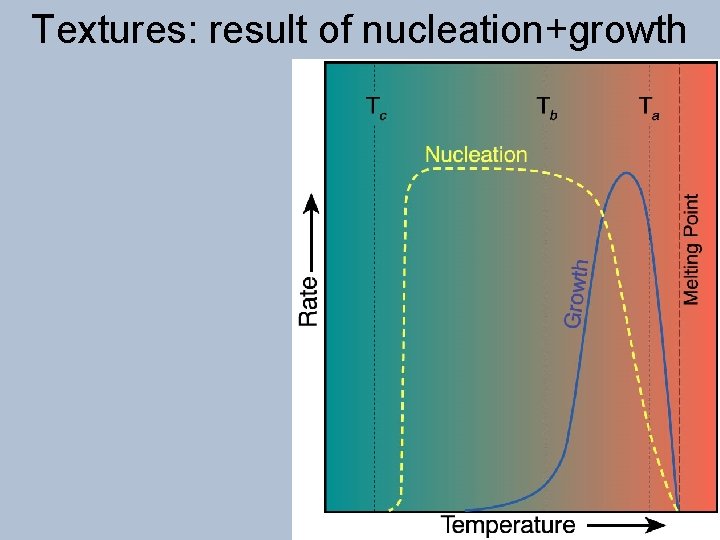
Textures: result of nucleation+growth

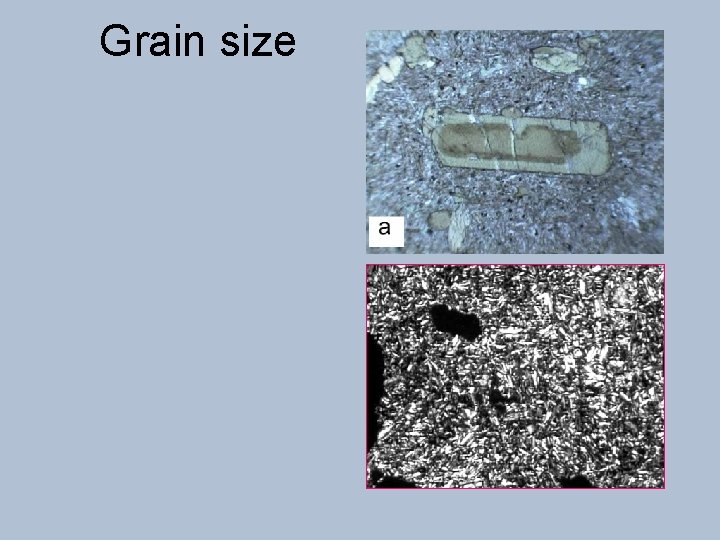
Grain size

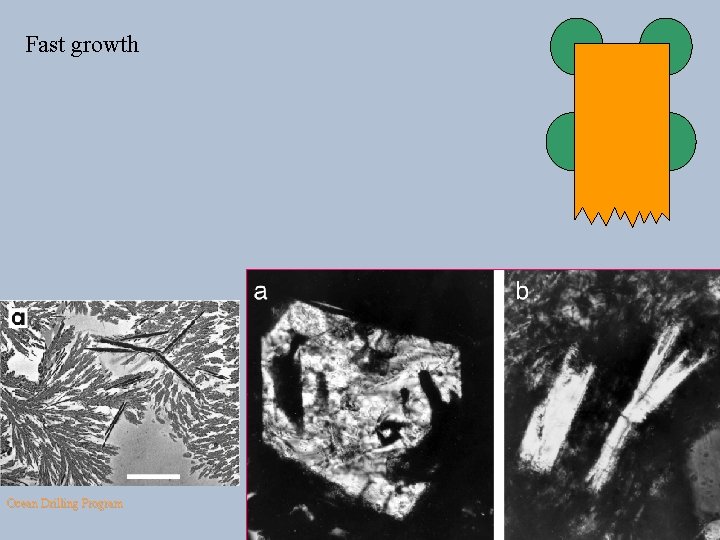
Fast growth a Ocean Drilling Program

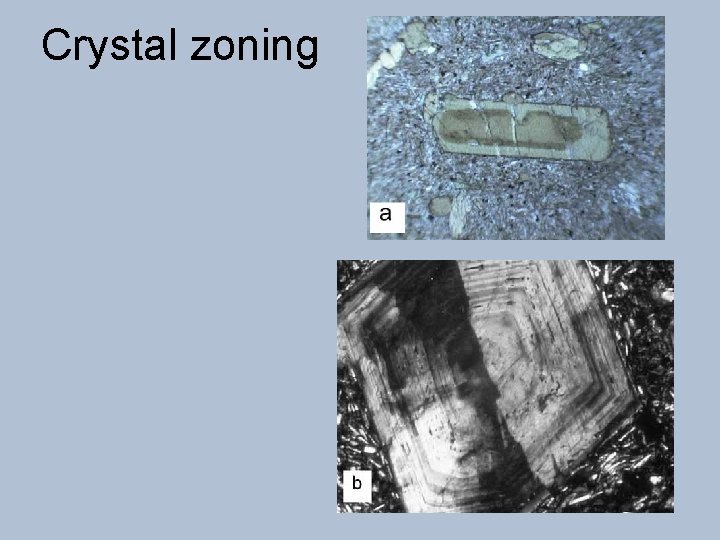
Crystal zoning

Crystal shape

Growth order

Quartz - feldspar intergrowth

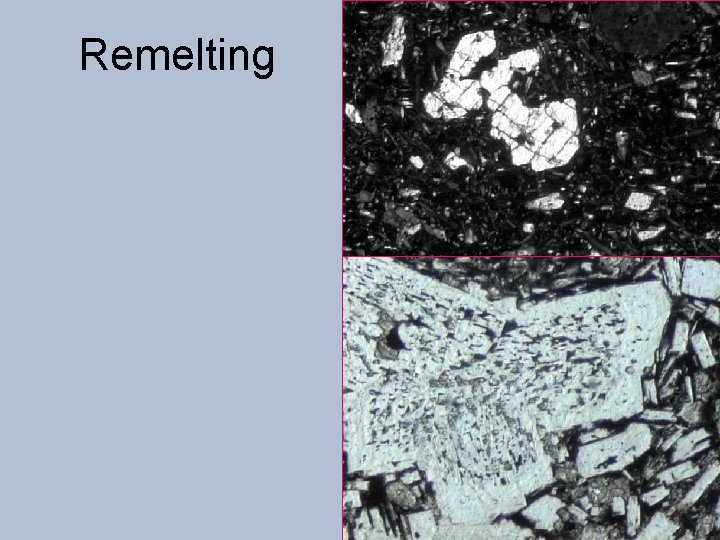
Remelting

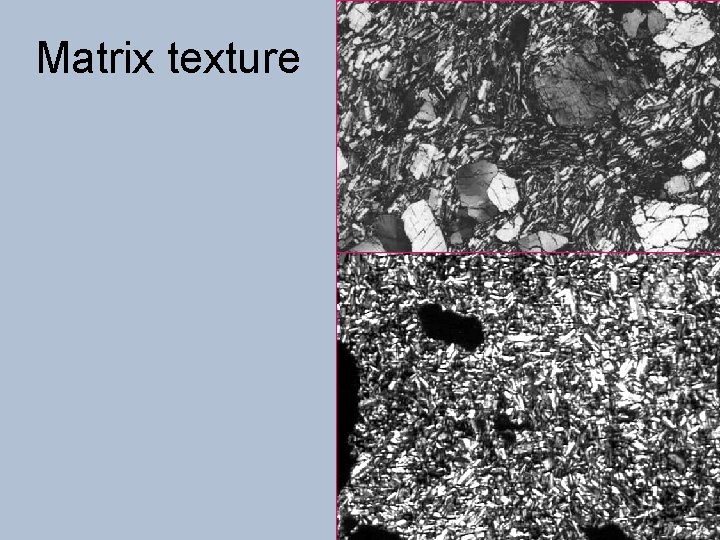
Matrix texture

Twinning

Replacements North Carolina State University Smith College
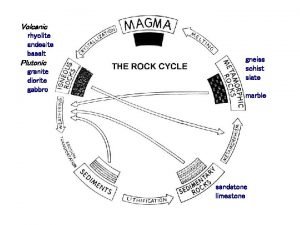
 Sedimentary rocks turn into metamorphic
Sedimentary rocks turn into metamorphic Igneous metamorphic sedimentary
Igneous metamorphic sedimentary Phazir
Phazir Na silikat
Na silikat Campuran berikut bersifat penyangga, kecuali...
Campuran berikut bersifat penyangga, kecuali... Larutan penyangga
Larutan penyangga Larutan penyangga dapat dibuat dengan mencampurkan larutan
Larutan penyangga dapat dibuat dengan mencampurkan larutan Hardened magma squeezed into vertical spaces between rocks
Hardened magma squeezed into vertical spaces between rocks Intrusive vs extrusive igneous rocks
Intrusive vs extrusive igneous rocks Andesite vs basalt
Andesite vs basalt Simulated texture in art
Simulated texture in art Textures drape fabric
Textures drape fabric The line of a dress or the garment's overall shape
The line of a dress or the garment's overall shape Partially resident textures
Partially resident textures Rock textures chart
Rock textures chart Invented texture in art
Invented texture in art Dynamic textures
Dynamic textures Rendering fur with three dimensional textures
Rendering fur with three dimensional textures Metamorphic textures
Metamorphic textures Metamorphic textures
Metamorphic textures Cuda texture object example
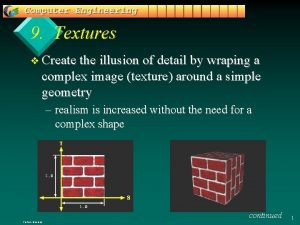
Cuda texture object example What are textures
What are textures The illusion of detail
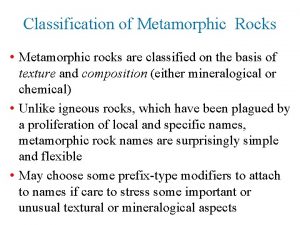
The illusion of detail Classification of metamorphic rocks
Classification of metamorphic rocks Rock
Rock Classification of metamorphic rocks
Classification of metamorphic rocks Metamorphic rocks form from
Metamorphic rocks form from Folk classification of carbonate rocks
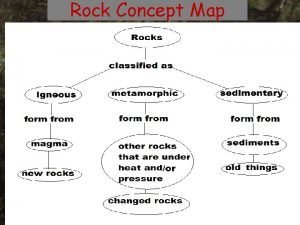
Folk classification of carbonate rocks Sedimentary rocks concept map
Sedimentary rocks concept map Iugs classification of igneous rocks
Iugs classification of igneous rocks Iugs classification of igneous rocks
Iugs classification of igneous rocks Iugs classification of igneous rocks
Iugs classification of igneous rocks Sublimisasi
Sublimisasi Keuntungan dan kerugian emulsi
Keuntungan dan kerugian emulsi Eter
Eter Ppt sifat koligatif larutan
Ppt sifat koligatif larutan Amfotir
Amfotir Macam macam larutan penyangga
Macam macam larutan penyangga Buffer adalah
Buffer adalah Campuran homogen tersusun dari
Campuran homogen tersusun dari Larutan adalah campuran yang … *
Larutan adalah campuran yang … * Contoh larutan standar primer
Contoh larutan standar primer Campuran berikut yang menghasilkan garam asam adalah
Campuran berikut yang menghasilkan garam asam adalah Gambar larutan jenuh
Gambar larutan jenuh Tekanan zat gas adalah
Tekanan zat gas adalah Rumus molaritas
Rumus molaritas Reaksi kimia dalam larutan air
Reaksi kimia dalam larutan air Susunan suatu larutan begitu seragam
Susunan suatu larutan begitu seragam Tekanan uap larutan
Tekanan uap larutan Pengertian konsentrasi larutan
Pengertian konsentrasi larutan 30 gram asam asetat (bm=60) dilarutkan dalam 45 gram air
30 gram asam asetat (bm=60) dilarutkan dalam 45 gram air Bahan yang bersifat alkali
Bahan yang bersifat alkali Ciri materi esensial
Ciri materi esensial Contoh soal keformalan
Contoh soal keformalan Materi kimia kelas 12 semester 1 sifat koligatif larutan
Materi kimia kelas 12 semester 1 sifat koligatif larutan