Magic Iceland Treatment of Myeloma Recent advances And























- Slides: 49

Magic Iceland

Treatment of Myeloma Recent advances And Future hopes The IFM experience Jean Luc Harousseau

Venetoclax in MM with t(11; 14) The first targeted therapy in MM ?

Multiple Myeloma Survival Improving With New Drugs AA 1960 -65 1965 -70 1970 -75 1975 -80 1980 -85 1985 -90 1990 -95 1995 -00 2000 -05 2005 -10 Adapted from Kumar et al Leukemia 2014 4

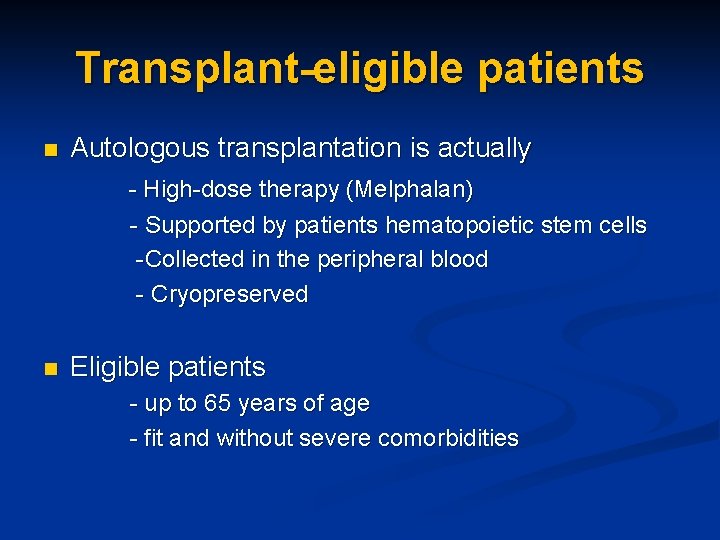
Transplant-eligible patients Autologous transplantation is actually - High-dose therapy (Melphalan) n - Supported by patients hematopoietic stem cells -Collected in the peripheral blood - Cryopreserved n Eligible patients - up to 65 years of age - fit and without severe comorbidities

First-generation New Agents n n n Thalidomide (immunomodulator) Lenalidomide/Revlimid (immunomodulator) Bortezomib/Velcade (Proteasome-inhibitor)

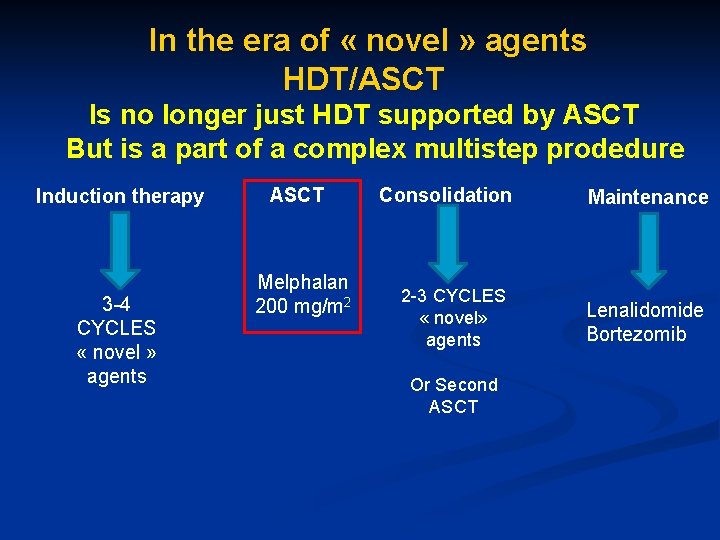
In the era of « novel » agents HDT/ASCT Is no longer just HDT supported by ASCT But is a part of a complex multistep prodedure Induction therapy 3 -4 CYCLES « novel » agents ASCT Consolidation Melphalan 200 mg/m 2 2 -3 CYCLES « novel» agents Or Second ASCT Maintenance Lenalidomide Bortezomib

Induction therapy with novel agents Triple Combination > Double Combination - VTD > TD Cavo M Lancet 2010; 376: 2075 n Rosinol L Blood 2012; 120: 1589 - v. TD > VD Moreau P Blood 2011; 118: 5752 n Triplets should contain 1 IMId and 1 PI - VTD >VCD Moreau P (Blood 2016; 127: 2569 -2574) n VTD (Velcade/Thalidomide/Dexamethasone) is the standard induction regimen n Should Revlimid replace Thalidomide (RVD) ?

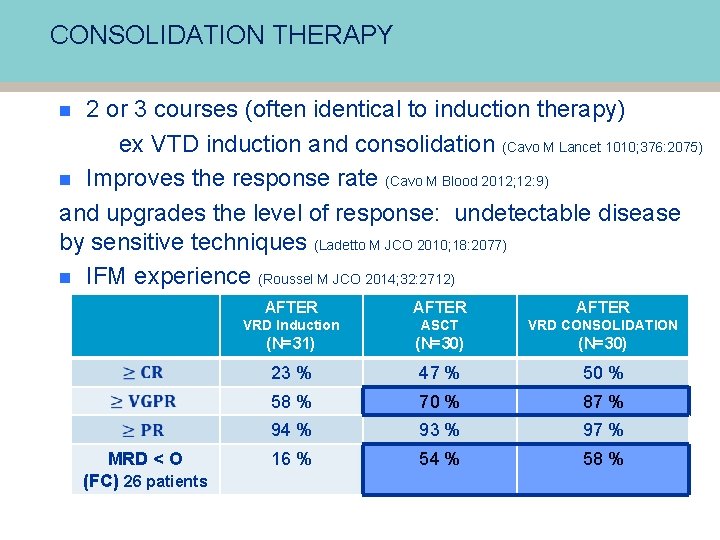
CONSOLIDATION THERAPY 2 or 3 courses (often identical to induction therapy) ex VTD induction and consolidation (Cavo M Lancet 1010; 376: 2075) n Improves the response rate (Cavo M Blood 2012; 12: 9) and upgrades the level of response: undetectable disease by sensitive techniques (Ladetto M JCO 2010; 18: 2077) n IFM experience (Roussel M JCO 2014; 32: 2712) n AFTER MRD < O (FC) 26 patients AFTER VRD Induction ASCT VRD CONSOLIDATION (N=31) (N=30) 23 % 47 % 50 % 58 % 70 % 87 % 94 % 93 % 97 % 16 % 54 % 58 %

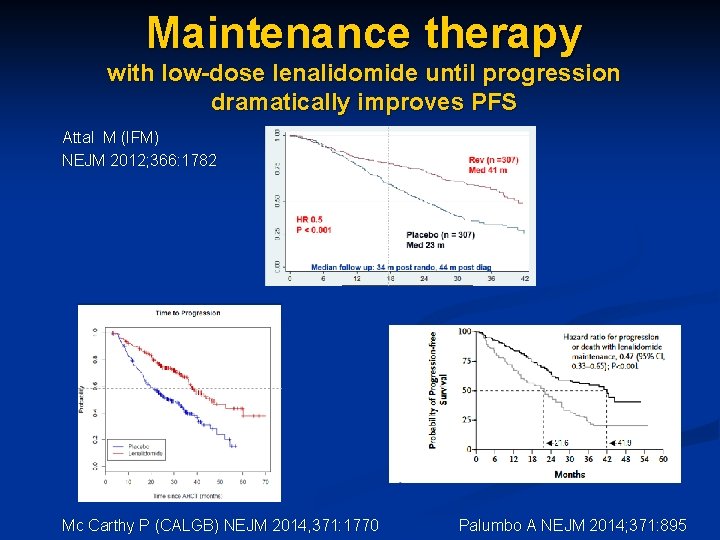
Maintenance therapy with low-dose lenalidomide until progression dramatically improves PFS Attal M (IFM) NEJM 2012; 366: 1782 Palumbo A NEJM 2014; 371: 895 Mc Carthy P (CALGB) NEJM 2014, 371: 1770

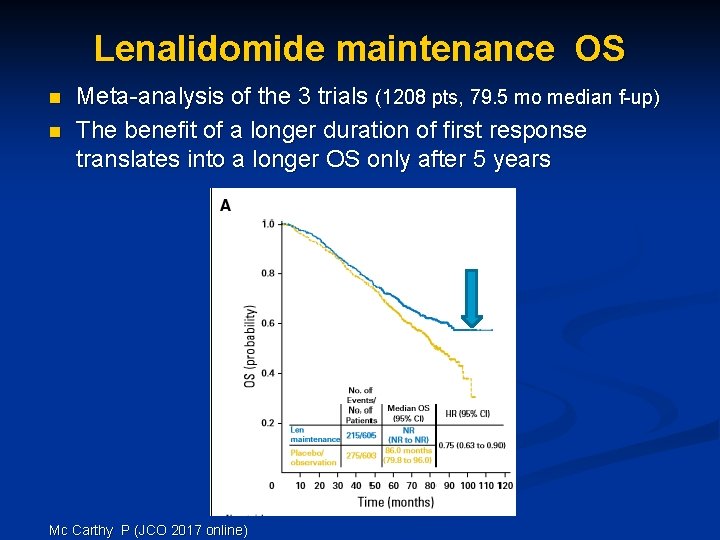
Lenalidomide maintenance OS n n Meta-analysis of the 3 trials (1208 pts, 79. 5 mo median f-up) The benefit of a longer duration of first response translates into a longer OS only after 5 years Mc Carthy P (JCO 2017 online)

LENALIDOMIDE MAINTENANCE REMAINING QUESTIONS Lenalidomide maintenance after ASCT is now approved by FDA and EMA n However less convincing results in high-risk MM (ISS 3, poor-risk cytogenetics) Bortezomib ? n Toxicity of long-term treatment - 29% of AE treatment discontinuation - 4. 3% SPM vs 1% n Cost (unaffordable in some countries) n Optimal duration still unknown n

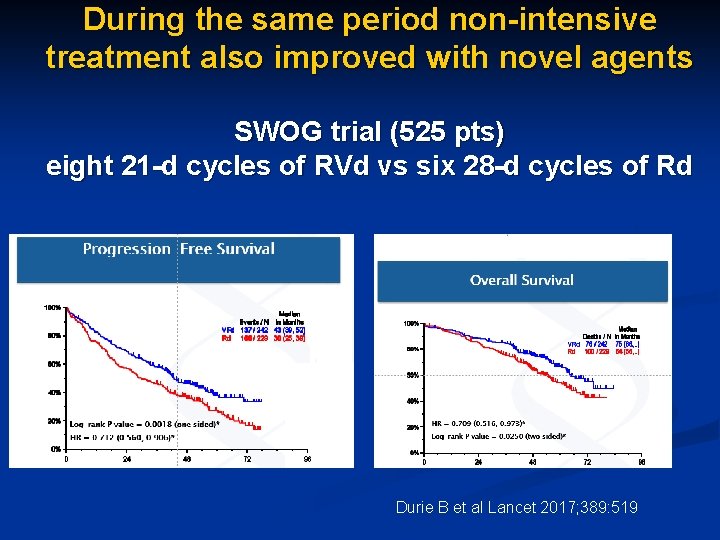
During the same period non-intensive treatment also improved with novel agents SWOG trial (525 pts) eight 21 -d cycles of RVd vs six 28 -d cycles of Rd Durie B et al Lancet 2017; 389: 519

IFM/DFCI 2009 Study Early vs Late ASCT in newly diagnosed MM pts up to 65 years Randomize RVDx 3 CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg Melphalan 200 mg/m 2* + ASCT RVDx 3 CY (3 g/m 2) MOBILIZATION Goal: 5 x 106 cells/kg RVD x 5 RVD x 2 Revlimid SCT at relapse MEL 200 mg/m 2 if <65 yrs , >65 yrs 140 mg/m 2

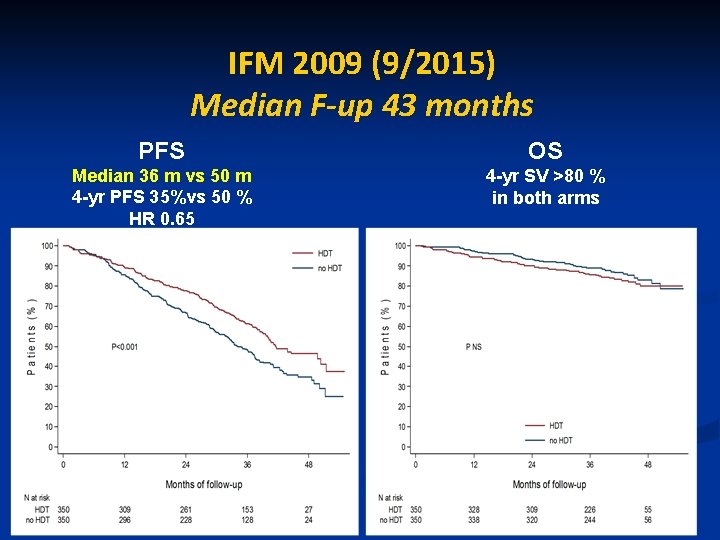
IFM 2009 (9/2015) Median F-up 43 months PFS OS Median 36 m vs 50 m 4 -yr PFS 35%vs 50 % HR 0. 65 4 -yr SV >80 % in both arms

IFM 2009 : PFS 16

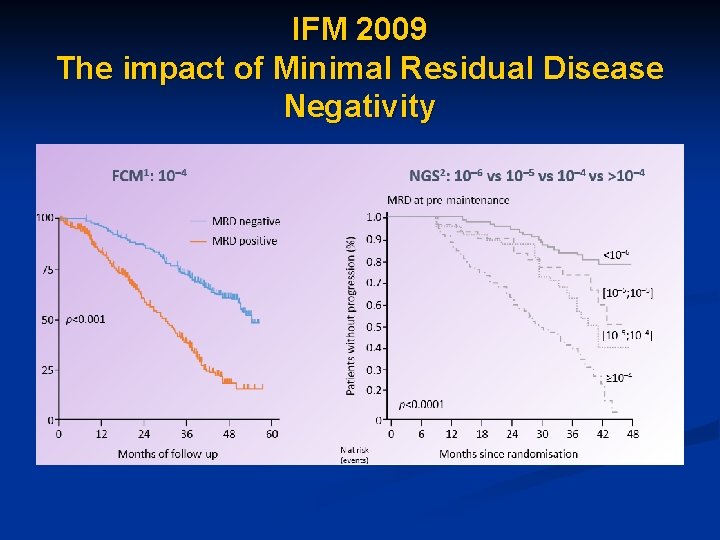
IFM 2009 The impact of Minimal Residual Disease Negativity

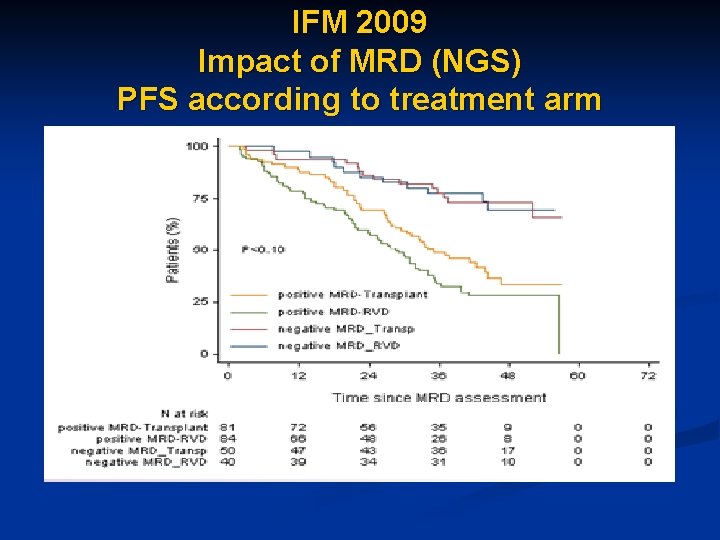
IFM 2009 Impact of MRD (NGS) PFS according to treatment arm

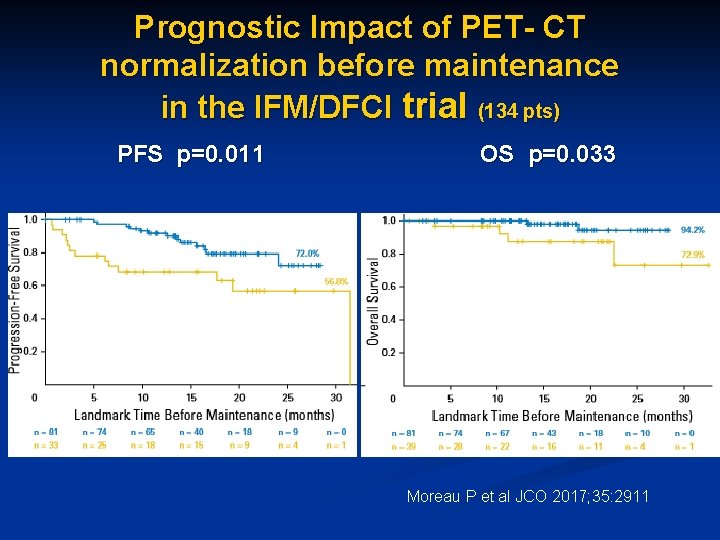
Prognostic Impact of PET- CT normalization before maintenance in the IFM/DFCI trial (134 pts) PFS p=0. 011 OS p=0. 033 Moreau P et al JCO 2017; 35: 2911

Summary of IFM 2009 results n. Compared to the best non-intensive treatment to-date ( 65 % <0 MRD, 36 months PFS) UPFRONT ASCT n. Longer PFS in all prognostic subgroups n. More patients with negative MRD BUT n. No difference in OS…. n. Due to excellent results of RVD and to more possibilities at time of relapse including ASCT in 2/3 of cases n. To show an OS benefit more F-up is needed

Intensive versus non-intensive upfront treatment n Four randomized studies Palumbo A et al NEJM 2014; 371: 895 Gay P Lancet Oncol 2015; 16: 1617 Attal M et al NEJM 2017; 376: 1311 Cavo P et al ASH 2016 n n Patients could receive HDT/ASCT at relapse in the non-intensive arm All 4 studies show a significant benefit in terms of PFS in the intensive arm Autotransplantation remains he standard of care But non intensive treatment with RVD is a valuable alternative

Overall Survival FOLLOW-UP OF IFM S 9321 TT PROTOCOLS IFM 2009 IFM 2005 IFM 99 IFM 94 TT 3: all risk, age <75 TT 2: all risk, age <75 IFM 99 -02: low risk, age <65 TT 1: all risk, age <75 IFM 90: all risk, age <65 IFM 94: all risk, age <65 IFM 99 -04: high risk, age <65 S 9321: all risk, age <70 IFM 90 Barlogie, Attal et al.

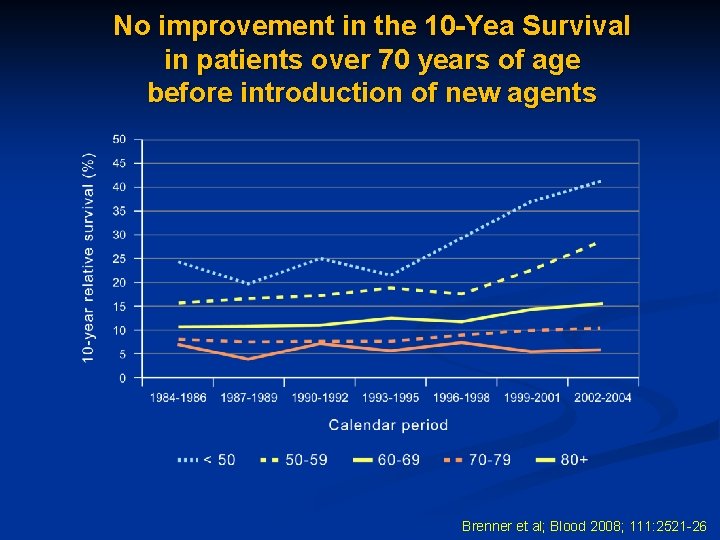
No improvement in the 10 -Yea Survival in patients over 70 years of age before introduction of new agents Brenner et al; Blood 2008; 111: 2521 -26

MPT Becomes a Standard of Care Facon T, et al. Lancet. 2007; 370: 1209 -18. Fayers PM, et al. Blood. 2011; 118: 123947.

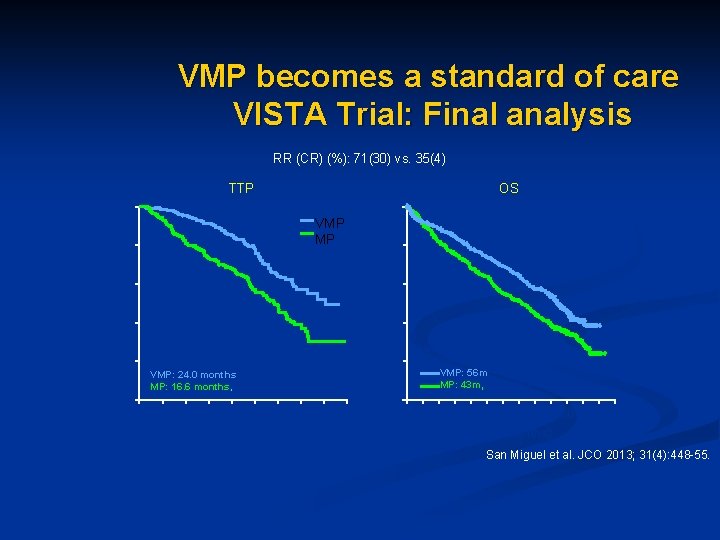
VMP becomes a standard of care VISTA Trial: Final analysis RR (CR) (%): 71(30) vs. 35(4) TTP VMP MP 80 60 40 20 0 VMP: 24. 0 months MP: 16. 6 months, P < 0. 000001 0 3 6 9 12 15 18 21 24 27 Time (months) 100 Patients without event (%) 100 OS 80 60 40 20 0 Median follow-up 60 months Median OS: VMP: 56 m MP: 43 m, P = 0. 0008 0 6 12 18 24 30 36 42 48 54 60 66 72 78 Time (months) San Miguel et al. JCO 2013; 31(4): 448 -55.

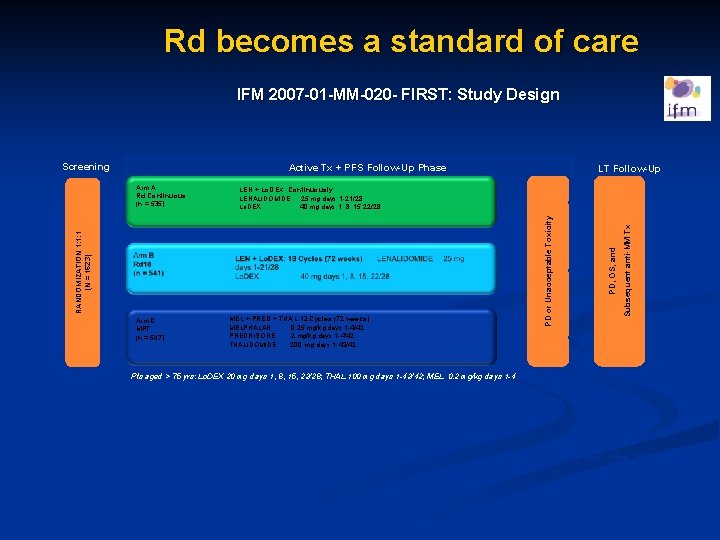
Rd becomes a standard of care IFM 2007 -01 -MM-020 - FIRST: Study Design Active Tx + PFS Follow-Up Phase Arm C MPT (n = 547) MEL + PRED + THAL 12 Cycles (72 weeks) MELPHALAN 0. 25 mg/kg days 1 -4/42 PREDNISONE 2 mg/kg days 1 -4/42 THALIDOMIDE 200 mg days 1 -42/42 Subsequent anti-MM Tx PD or Unacceptable Toxicity LEN + Lo. DEX: Continuously LENALIDOMIDE 25 mg days 1 -21/28 Lo. DEX 40 mg days 1, 8, 15, 22/28 RANDOMIZATION 1: 1: 1 (N = 1623) Arm A Rd Continuous (n = 535) LT Follow-Up PD, OS, and Screening Pts aged > 75 yrs: Lo. DEX 20 mg days 1, 8, 15, 22/28; THAL 100 mg days 1 -42/42; MEL 0. 2 mg/kg days 1 -4 FIRST, Frontline Investigation of Revlimid and Dexamethasone versus Standard Thalidomide; ISS, International Staging System; Lo. Dex, low-dose dexamethasone; LT, long-term; MM, multiple myeloma; OS, overall survival; PD, progressive disease; PFS, progression-free survival; pts, patients; Tx, treatment. Benboubker L, et al. N Engl J Med. 2014; 371: 906 -17.

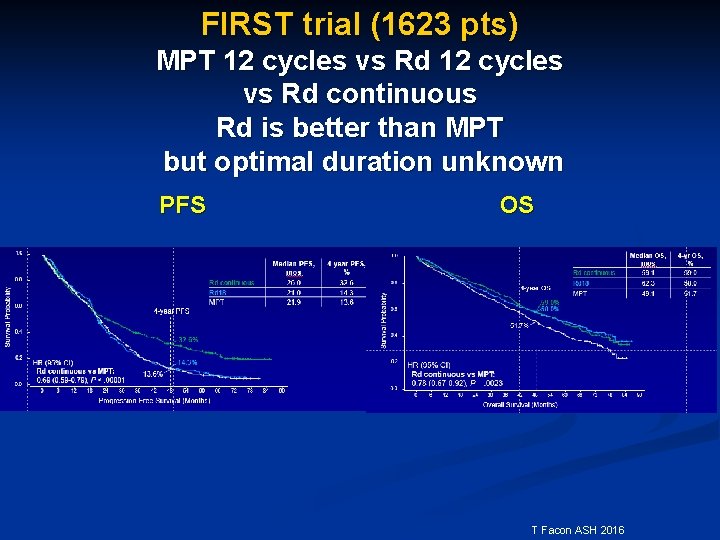
FIRST trial (1623 pts) MPT 12 cycles vs Rd continuous Rd is better than MPT but optimal duration unknown PFS OS T Facon ASH 2016

Other current approaches Combine IMId (thalidomide or lenalidomide)and PI (bortezomib) n Induction plus Maintenance with Bortezomib or Lenalidomide Ex : MRC XI trial n

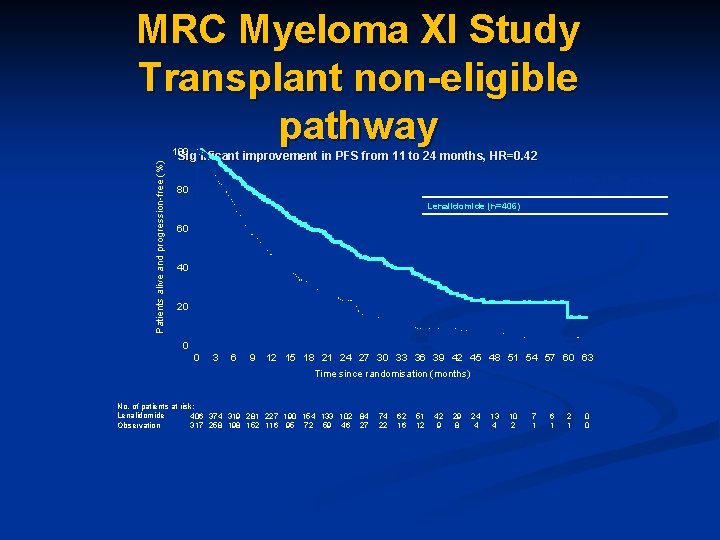
MRC Myeloma XI Study Transplant non-eligible pathway Patients alive and progression-free (%) 100 Significant improvement in PFS from 11 to 24 months, HR=0. 42 Median PFS, months [95% CI] 80 60 Lenalidomide (n=406) 24 [21, 30] Observation (n=317) 11 [9, 13] 40 20 0 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 Time since randomisation (months) No. of patients at risk: Lenalidomide 406 374 319 281 227 190 154 133 102 84 Observation 317 258 198 152 116 95 72 59 46 27 74 22 62 16 51 12 42 9 29 8 24 4 13 4 10 2 7 1 6 1 2 1 0 0

Conclusions Introduction of « novel » agents was actually the first improvement of MM treatment in the elderly n Use of one or two « novel » agents increased response rate, PFS and OS n Prolonged treatment is important but optimal duration is unknown n Assessment of fitness/frailty is necessary for optimal treatment selection n

Myeloma Drug Development Durv. Atezolizumab* alumab* Nivolumab* Pembrolizumab* Melflufen* Selinexor* Venetoclax* Nelfinavir*

Continuing evolution of myeloma treatment: 1 st Generation novel agents Lenalidomide 2 nd Generation novel therapies/Immunotherapy Carfilzomib Bortezomib Vaccines* Ixazomib Thalidomide Pomalidomide Bortezomib + Doxil Durv. Atezolizumab* alumab* Daratumumab Elotuzumab Marizomib* Oprozomib* Nivolumab* Pembrolizumab* Panobinostat 3 rd Generation IMi. Ds* AC-241/1215* CAR-T* Isatuximab* 2003 Proteasome inhibitor Targeted Therapy 2006 2007 2012 IMi. D Adoptive T cell therapy 2013 HDAC inhibitor Melflufen* Selinexor* Venetoclax* Nelfinavir* 2017+ 2015 Monoclonal antibody Checkpoint inhibitors Vaccines Adapted from Richardson PG. et al ASH 2015, MMRF 2016

Recently approved new agents New Immunomodulator Pomalidomide (Pomalyst/Imnovid) Used in relapse (including relapse post Lenalidomide) Double or Triple combinations Toxicity similar to Lenalidomide n 33

Recently approved new agents New Immunomodulator n New Proteasome Inhibitors ► Carfilzomib ( Kyprolis) - IV infusion on 2 consecutive days n - less neurotoxicity but more cardio toxicity than Bortezomib - at higher doses carfilzomibn pkus dex superior to bortezomib plus dex - in relapse (double or triple combinations) - studies in frontline treatment 34

Recently approved new agents New Immunomodulator n New Proteasome Inhibitors ► Carfilzomib ( Kyprolis) ► Ixazomib (Ninlaro) n - oral (once weekly) - well tolerated - convenient for elderly patients and maintenance? - optimal dose ? 35

Recently approved new agents New Immunomodulator n New Proteasome Inhibitors n Antibodies ► Elotuzumab ► anti CD 38 (Daratumumab) - IV infusion (SC currently evaluated) n - well tolerated except infusion-related events with the first infusion) - the most effective new agent 36

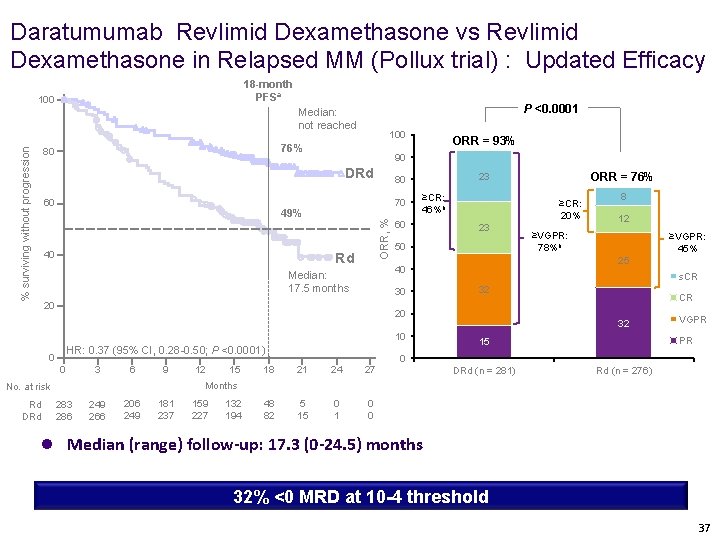
Daratumumab Revlimid Dexamethasone vs Revlimid Dexamethasone in Relapsed MM (Pollux trial) : Updated Efficacy 18 -month PFSa 100 P <0. 0001 Median: not reached 76% 80 % surviving without progression 100 90 DRd 60 ORR, % Rd ≥CR: 46%b 60 23 50 30 20 HR: 0. 37 (95% CI, 0. 28 -0. 50; P <0. 0001) 0 0 3 6 9 12 15 18 21 24 27 48 82 5 15 0 1 0 0 8 12 ≥VGPR: 78%b ≥VGPR: 45% 25 s. CR 32 20 10 CR 32 VGPR PR 15 0 DRd (n = 281) Rd (n = 276) Months No. at risk Rd DRd ≥CR: 20% 40 Median: 17. 5 months ORR = 76% 23 80 70 49% 40 ORR = 93% 283 286 249 266 206 249 181 237 159 227 132 194 l Median (range) follow-up: 17. 3 (0 -24. 5) months 32% <0 MRD at 10 -4 threshold 37

WHAT IS THE NEXT STEP? IFM 2018 Objectives Increase the rate of MRD negativity – by using second-generation new agents n 38

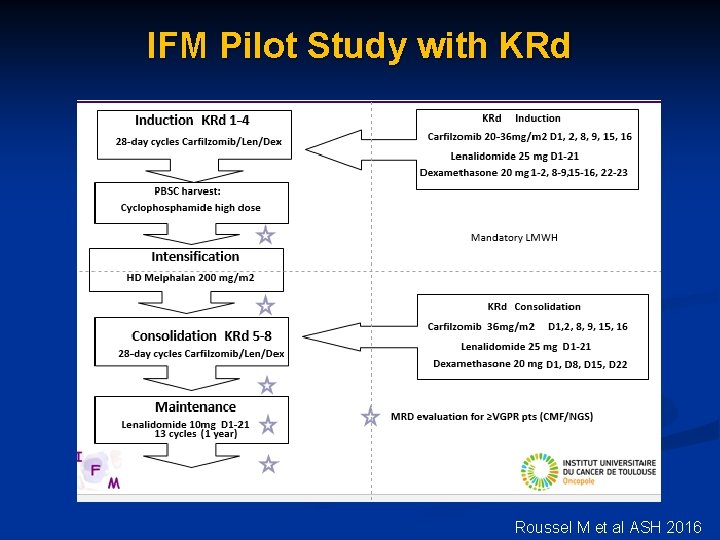
IFM Pilot Study with KRd Roussel M et al ASH 2016

RESPONSE RATES at the completion of Consolidation N=46 n % s. CR 26 57 MRD - CMF 32 70 MRD - NGS 23/34 68 68 At least CR 28 61 At least VGPR 39 85 ORR 41 89 PD 1 2 4 patients were not evaluable due to toxicities MRD CMF 10 -4/10 -5 MRD NGS clono. SEQ Adaptive 10 -6 IFM CRD

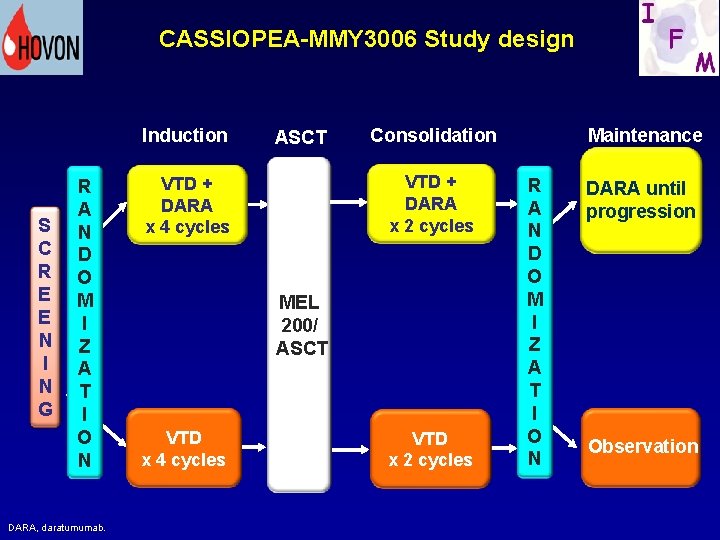
41 CASSIOPEA-MMY 3006 Study design Induction S C R E E N I N G R A N D O M I Z A T I O N DARA, daratumumab. ASCT Consolidation VTD + DARA x 2 cycles VTD + DARA x 4 cycles MEL 200/ ASCT VTD x 4 cycles VTD x 2 cycles Maintenance R A N D O M I Z A T I O N DARA until dara progression Observation

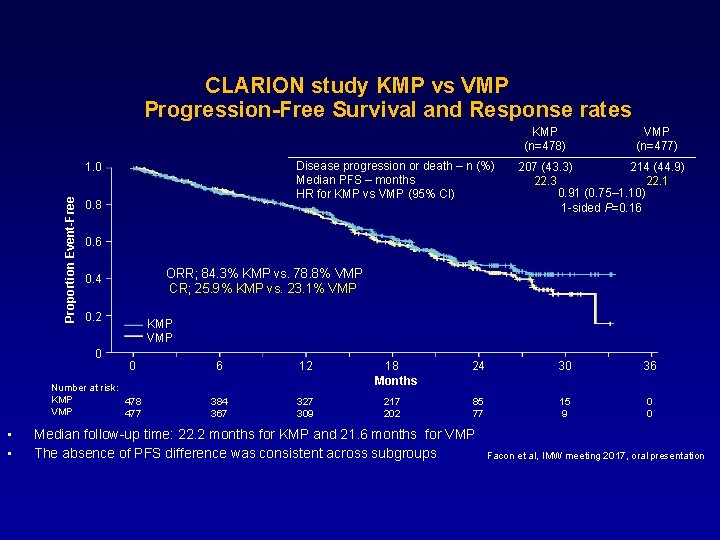
CLARION study KMP vs VMP Progression-Free Survival and Response rates KMP (n=478) Disease progression or death – n (%) Median PFS – months HR for KMP vs VMP (95% CI) Proportion Event-Free 1. 0 0. 8 207 (43. 3) 214 (44. 9) 22. 3 22. 1 0. 91 (0. 75– 1. 10) 1 -sided P=0. 16 0. 6 ORR; 84. 3% KMP vs. 78. 8% VMP CR; 25. 9% KMP vs. 23. 1% VMP 0. 4 0. 2 0 KMP VMP 0 Number at risk: KMP 478 VMP 477 • • VMP (n=477) 6 12 384 367 327 309 18 Months 217 202 24 30 36 85 77 15 9 0 0 Median follow-up time: 22. 2 months for KMP and 21. 6 months for VMP The absence of PFS difference was consistent across subgroups Facon et al, IMW meeting 2017, oral presentation

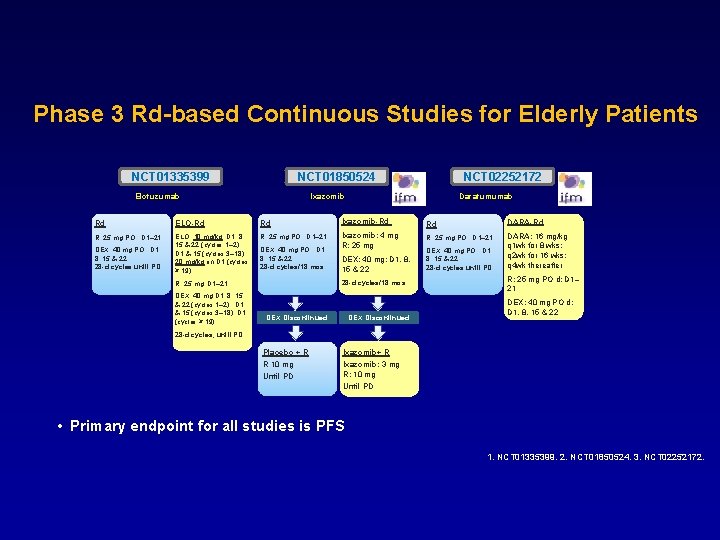
Phase 3 Rd-based Continuous Studies for Elderly Patients NCT 01335399 NCT 01850524 Elotuzumab NCT 02252172 Ixazomib Daratumumab Ixazomib-Rd DARA-Rd Rd ELO-Rd Rd R: 25 mg PO ; D 1– 21 ELO: 10 mg/kg; D 1, 8, 15 & 22 (cycles 1– 2); D 1 & 15 (cycles 3– 18); 20 mg/kg on D 1 (cycles ≥ 19) Ixazomib: 4 mg R: 25 mg PO ; D 1– 21 DEX: 40 mg PO ; D 1, 8, 15 & 22 28 -d cycles until PD DEX: 40 mg PO ; D 1, 8, 15 & 22 28 -d cycles/18 mos DEX: 40 mg; D 1, 8, 15 & 22 DEX: 40 mg PO ; D 1, 8, 15 & 22 28 -d cycles until PD 28 -d cycles/18 mos R: 25 mg, D 1– 21 DEX: 40 mg D 1, 8, 15 & 22 (cycles 1– 2); D 1 & 15 (cycles 3– 18); D 1 (cycles ≥ 19) R: 25 mg Rd DEX Discontinued DARA: 16 mg/kg q 1 wk for 8 wks; q 2 wk for 16 wks; q 4 wk thereafter R: 25 mg PO d; D 1– 21 DEX: 40 mg PO d; D 1, 8, 15 & 22 28 -d cycles, until PD Placebo + R R 10 mg Until PD Ixazomib+ R Ixazomib: 3 mg R: 10 mg Until PD • Primary endpoint for all studies is PFS 1. NCT 01335399. 2. NCT 01850524. 3. NCT 02252172.

WHAT IS THE NEXT STEP? IFM 2018 Objectives Increase the rate of MRD negativity – by using new agents – by prolonging the duration of induction and consolidation n Adapt treatment to results of MRD assessment n Address again the question of the role of ASCT in standard risk patients with <0 MRD after induction n Tailor treatment to initial prognostic factors (double ASCT ? ) n 44

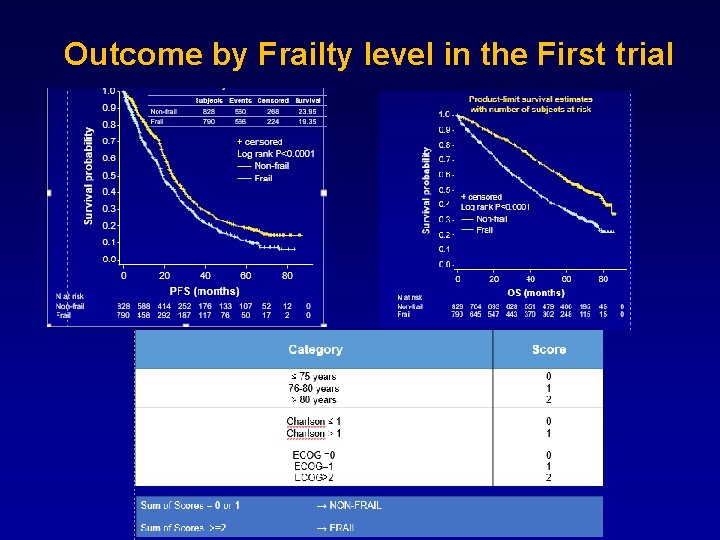
Outcome by Frailty level in the First trial PFS OS

A french study for frail elderly NDMM patients; IFM 2018 -01 A dexamethasone sparing study LT Follow-Up Active Treatment + PFS Follow-up Phase LEN + Lo-DEX continously: 1 Arm B Rd LENALIDOMIDE 25 mg D 1 -21/28 Lo-DEXAMETHASONE 20 mg D 1, 8, 15 & 22/28 Subsequent anti-MM Tx Arm A RDara. SC PD, OS and 2 LENALIDOMIDE 25 mg D 1 -21/28 DARATUMUMAB SC 1800 mg. SC Q 1 Wk for 8 weeks 1800 mg SC Q 2 Wk for 16 weeks 1800 mg SC Q 4 Wk thereafter PD or Unacceptable Toxicity RANDOMIZATION 1: 2 LEN + Dara SC continuously: Randomization will be stratified by International Staging System (I vs III) and age (<80 vs ≥ 80 s In Arm A Cycle 1 and 2 then Methylprednisolone (with SC Dara)

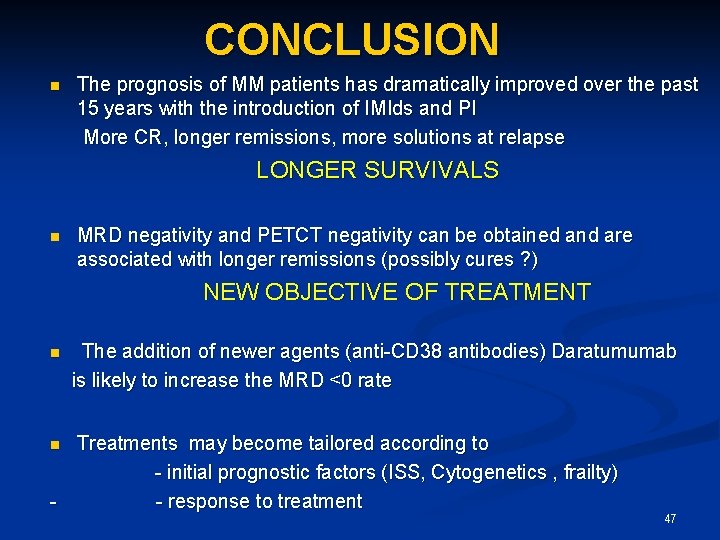
CONCLUSION The prognosis of MM patients has dramatically improved over the past 15 years with the introduction of IMIds and PI More CR, longer remissions, more solutions at relapse n LONGER SURVIVALS n MRD negativity and PETCT negativity can be obtained and are associated with longer remissions (possibly cures ? ) NEW OBJECTIVE OF TREATMENT The addition of newer agents (anti-CD 38 antibodies) Daratumumab is likely to increase the MRD <0 rate n Treatments may become tailored according to - initial prognostic factors (ISS, Cytogenetics , frailty) - - response to treatment n 47


 Recent advances in dental ceramics
Recent advances in dental ceramics Crab criteria multiple myeloma
Crab criteria multiple myeloma Smouldering myeloma
Smouldering myeloma Exercise physiology mascot
Exercise physiology mascot Mielma
Mielma European myeloma network
European myeloma network Plasma cell dyscrasia
Plasma cell dyscrasia Daratumumab macmillan
Daratumumab macmillan Crab kriterleri
Crab kriterleri Waldenstrom macroglobulinemia vs multiple myeloma
Waldenstrom macroglobulinemia vs multiple myeloma Myeloma
Myeloma Kpd myeloma
Kpd myeloma Mayo clinic multiple myeloma
Mayo clinic multiple myeloma Pritesh patel md
Pritesh patel md Myeloma euronet
Myeloma euronet Slidetodoc.com
Slidetodoc.com Loans and advances in tally
Loans and advances in tally What is long term loans and advances
What is long term loans and advances Global oncology trends 2017 advances complexity and cost
Global oncology trends 2017 advances complexity and cost Where is iceland
Where is iceland Iceland economic crisis
Iceland economic crisis Hekla mid atlantic divergent
Hekla mid atlantic divergent Icelandic tale
Icelandic tale Changeling iceland
Changeling iceland Ministry of education iceland
Ministry of education iceland Iceland scavenger hunt
Iceland scavenger hunt Magma type
Magma type Viking
Viking Iceland climate
Iceland climate Northern lights iceland
Northern lights iceland Ngos in iceland
Ngos in iceland Is iceland a stateless nation
Is iceland a stateless nation Mansion sports
Mansion sports Axis powers
Axis powers Advances in technology during wwii
Advances in technology during wwii Chapter 9 intellectual development in the first year
Chapter 9 intellectual development in the first year Advances in real-time rendering in games
Advances in real-time rendering in games Advances in mri
Advances in mri Opto-electronic advances
Opto-electronic advances Asset classification norms
Asset classification norms Advances in memory technology
Advances in memory technology Recent trends in ic engine
Recent trends in ic engine Recent developments in ict
Recent developments in ict Recent developments in object detection
Recent developments in object detection Many recent college graduates have faced
Many recent college graduates have faced Modern trends of foreign trade in india
Modern trends of foreign trade in india Advantages of scanning in reading
Advantages of scanning in reading Current trends in project management
Current trends in project management Recent demographic changes in the uk
Recent demographic changes in the uk Myipsclever
Myipsclever