Macromolecules of Life Carbohydrates Proteins Lipids Nucleic Acids

Macromolecules of Life Carbohydrates, Proteins, Lipids, Nucleic Acids (Ch 3)

�H 2 O 1 big Oxygen, 2 little Hydrogens �Polar Why is water AND that it is POLAR, SOOO important? �Keeps temperature �Helps plants move water �Dissolves substances Water is a polar molecule
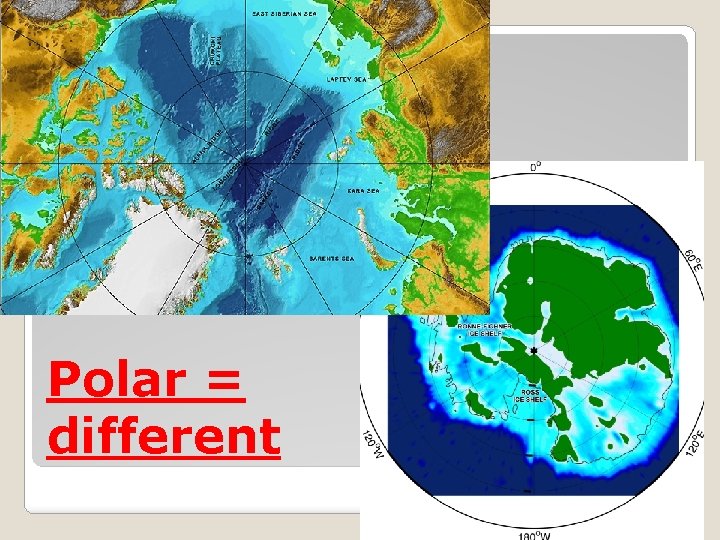
Polar = different
� 4 types ◦ 1. Carbohydrates ◦ 2. Proteins ◦ 3. Lipids ◦ 4. Nucleic Acids �Made of Monomers – little parts ◦ Monomer + Monomer = Polymer Large Molecules
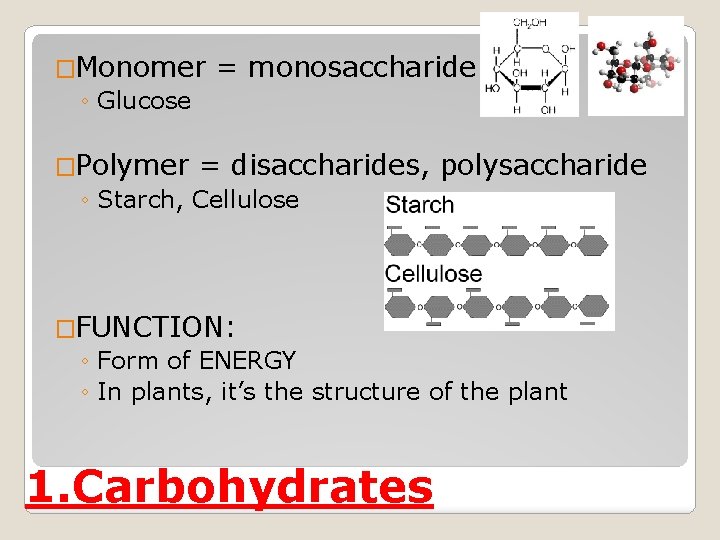
�Monomer ◦ Glucose = monosaccharide �Polymer = disaccharides, ◦ Starch, Cellulose polysaccharide �FUNCTION: ◦ Form of ENERGY ◦ In plants, it’s the structure of the plant 1. Carbohydrates

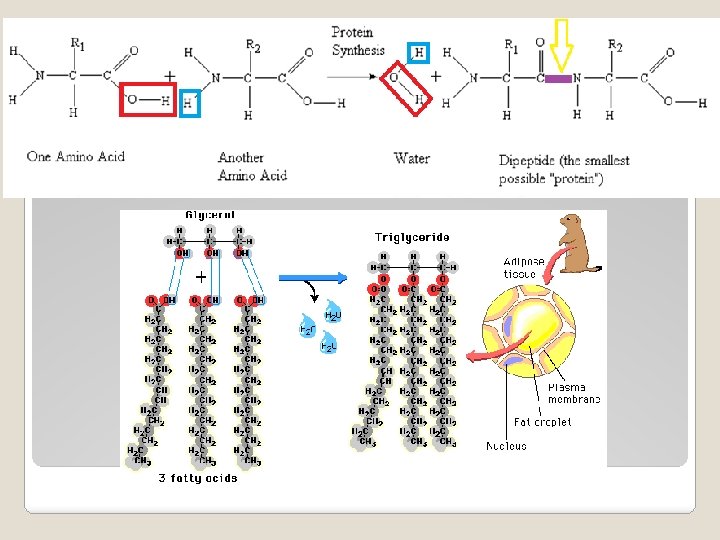
�Monomer = Amino Acid ◦ Examples: Glycine, Alanine �Polymer = Polypeptide �FUNCTION: ◦ Builds your body ◦ Enzymes �Lock and Key = helps chemical reactions take place 2. Protein
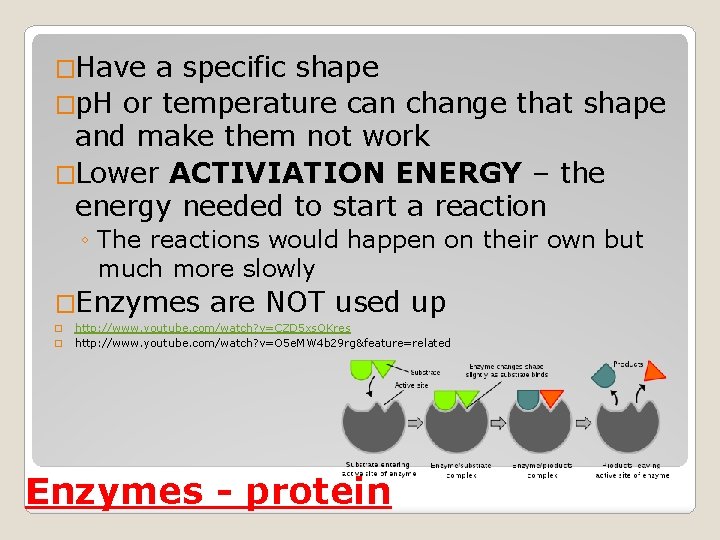
�Have a specific shape �p. H or temperature can change that shape and make them not work �Lower ACTIVIATION ENERGY – the energy needed to start a reaction ◦ The reactions would happen on their own but much more slowly �Enzymes � � are NOT used up http: //www. youtube. com/watch? v=CZD 5 xs. OKres http: //www. youtube. com/watch? v=O 5 e. MW 4 b 29 rg&feature=related Enzymes - protein

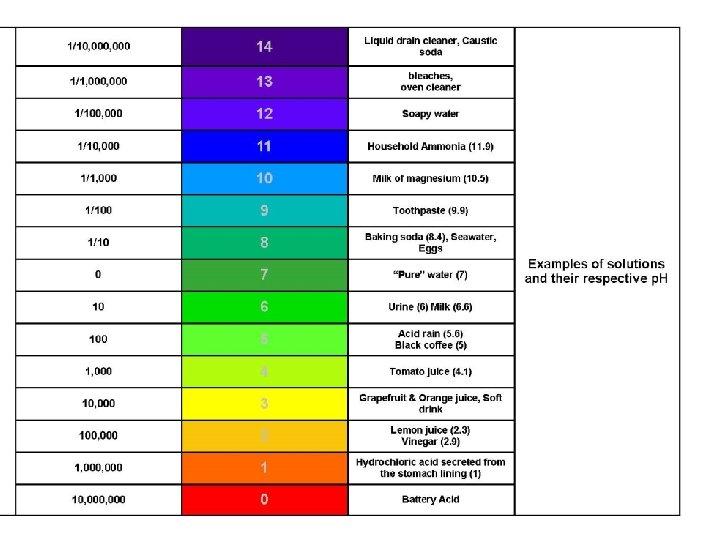
�Scale from 0 -14 ◦ 0 -6 acid (sour) ◦ 8 -14 base (slippery, bitter) ◦ 7 neutral p. H
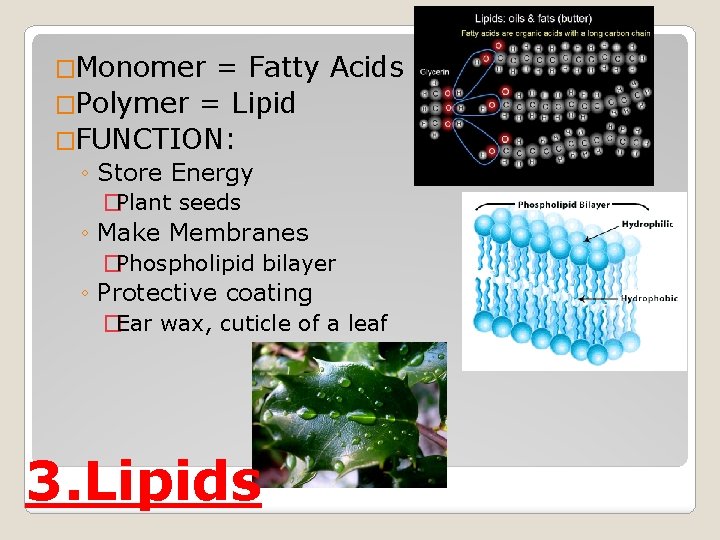
�Monomer = Fatty Acids �Polymer = Lipid �FUNCTION: ◦ Store Energy �Plant seeds ◦ Make Membranes �Phospholipid bilayer ◦ Protective coating �Ear wax, cuticle of a leaf 3. Lipids
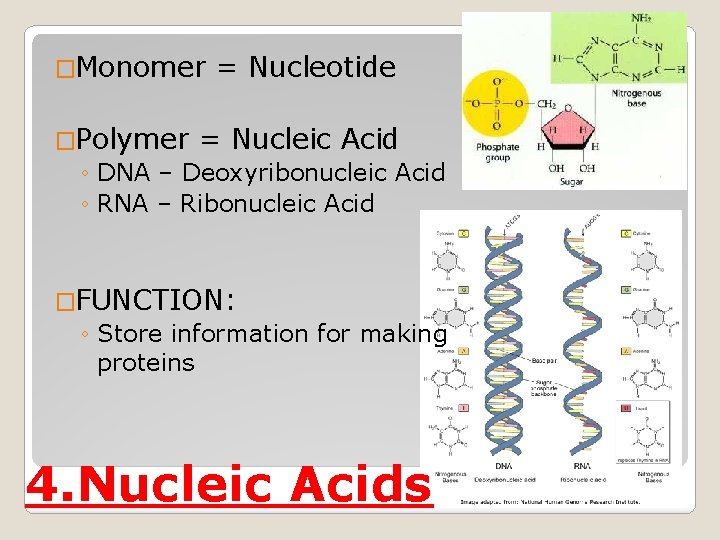
�Monomer = Nucleotide �Polymer = Nucleic Acid ◦ DNA – Deoxyribonucleic Acid ◦ RNA – Ribonucleic Acid �FUNCTION: ◦ Store information for making proteins 4. Nucleic Acids
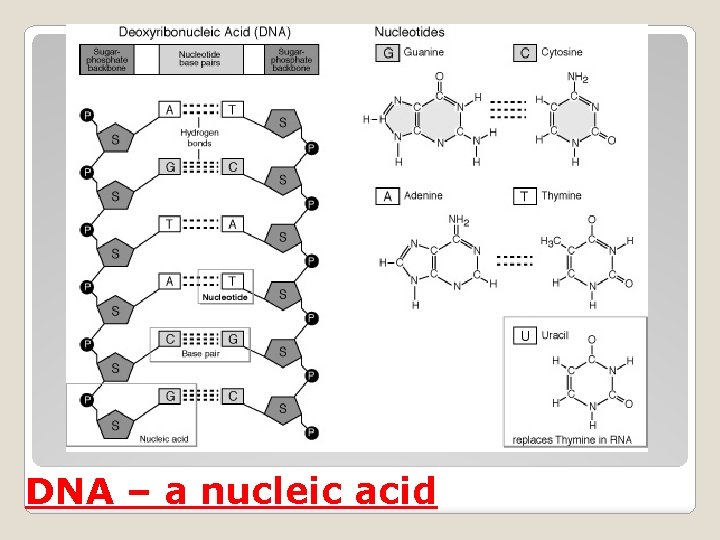
DNA – a nucleic acid
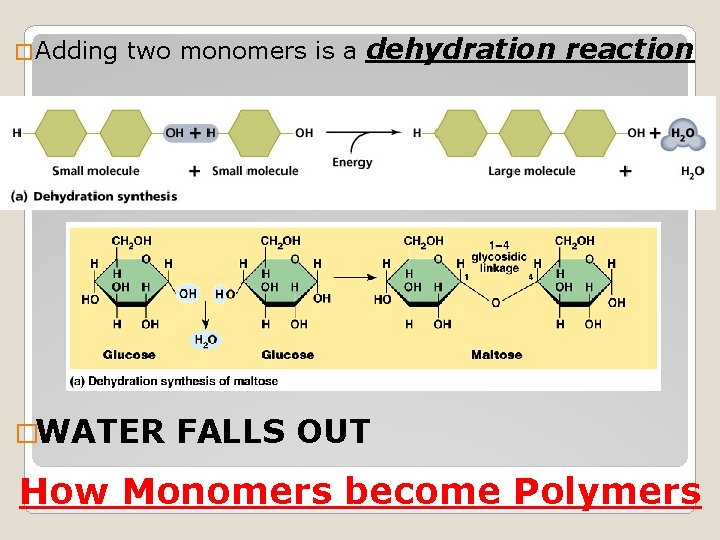
� Adding two monomers is a dehydration reaction �WATER FALLS OUT How Monomers become Polymers
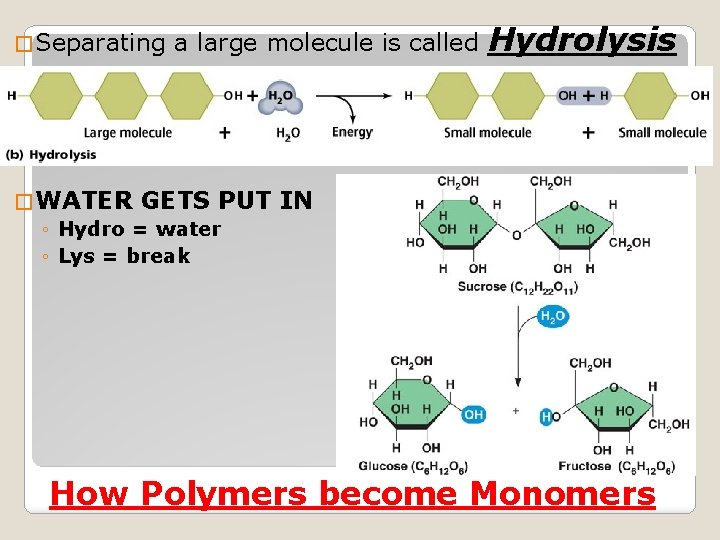
� Separating a large molecule is called � WATER GETS PUT ◦ Hydro = water ◦ Lys = break Hydrolysis IN How Polymers become Monomers

Vocab Words from the Book
- Slides: 16