Macromolecules Notes Powerpoint Templates Page 1 Organic Compounds













- Slides: 29

Macromolecules Notes Powerpoint Templates Page 1

Organic Compounds • Compounds that contain CARBON are called organic • Macromolecules are large organic molecules Powerpoint Templates Page 2

Uses of Organic Molecules • Americans consume an average of 140 pounds of sugar person per year Cellulose, found in plant cell walls, is the most abundant organic compound on Earth Powerpoint Templates Page 3

Uses of Organic Molecules • A typical cell in your body has about 2 meters of DNA A typical cow produces over 200 pounds of methane gas each year Powerpoint Templates Page 4

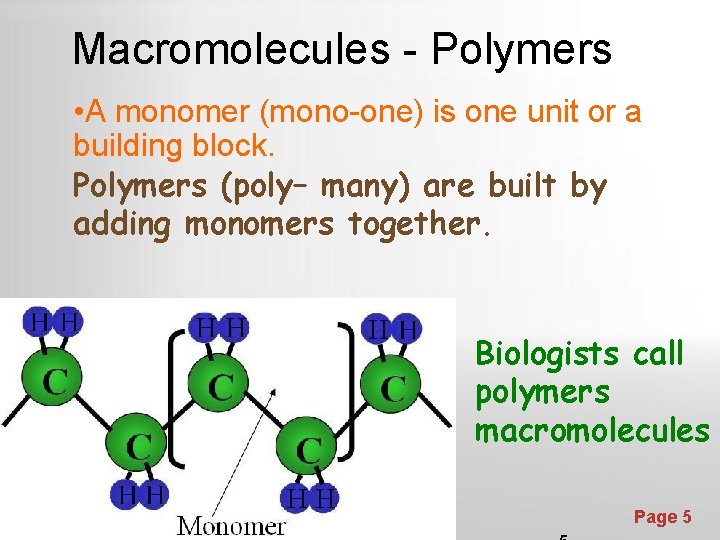
Macromolecules - Polymers • A monomer (mono-one) is one unit or a building block. Polymers (poly– many) are built by adding monomers together. Biologists call polymers macromolecules Powerpoint Templates Page 5

Lipids Powerpoint Templates 6 Page 6

Lipids • Lipids are hydrophobic – • Hydro- water • Phobic – fear of Do NOT mix with water Powerpoint Templates Includes fats, waxes, steroids, & oils Page 7

Lipids Four functions of lipids: 1. Long term energy storage 2. Protection against water loss 3. Chemical messengers (hormones) 4. Major component of cell membranes (phospholipids) Powerpoint Templates 8 Page 8

Fatty Acids -Lipids are made up of monomer called Fatty Acids -Fatty Acids are made of C, H, and O. 1. Saturated fatty acids: no double bonds (bad) 2. Unsaturated fatty acids: double bonds (good) Powerpoint Templates Page 9

Examples of Steroids Cholesterol is the “base steroid” from which your body produces other steroids. You get cholesterol from your diet. Estrogen & testosterone are also steroids Powerpoint Templates Page 10

Synthetic Anabolic Steroids • They are variants of testosterone Some athletes use them to build up their muscles quickly They can pose Powerpoint Templates serious health risks Page 11

Nucleic Acids Powerpoint Templates Page 12

Nucleic Acids • Store hereditary information Contain information for making all of the body’s proteins Two types exist --- DNA & RNA • ATP is another nucleotide. It’s used by cells for energy Powerpoint Templates Page 13

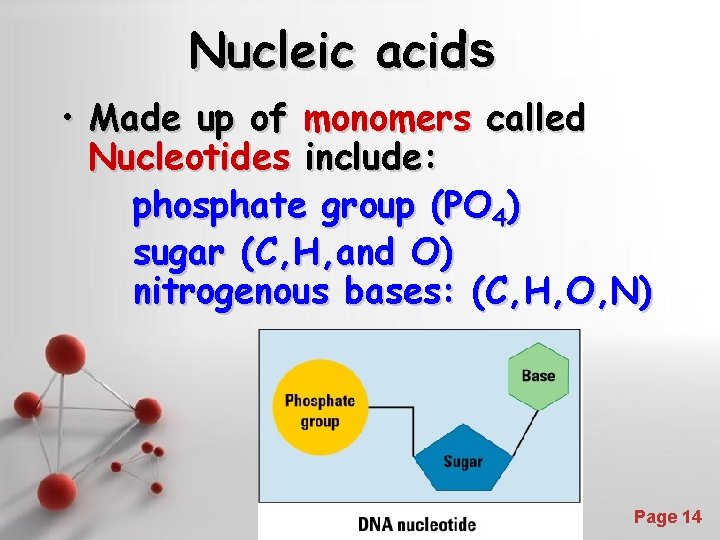
Nucleic acids • Made up of monomers called Nucleotides include: phosphate group (PO 4) sugar (C, H, and O) nitrogenous bases: (C, H, O, N) Powerpoint Templates Page 14

Nucleic acids The monomers link together to form nucleic acids like DNA and RNA. Powerpoint Templates Page 15

Carbohydrates Powerpoint Templates Page 16

Carbohydrates - Carbohydrates AKA sugars - made up of carbon, hydrogen, and oxygen - Found in food such as grains, fruits and vegetables Powerpoint Templates Page 17

Carbs Functions: • Short term energy • Cell recognition on the surface of cells. • Cellulose and Chitin provide structure to plant cells or Powerpoint Templates Page 18 exoskeletons.

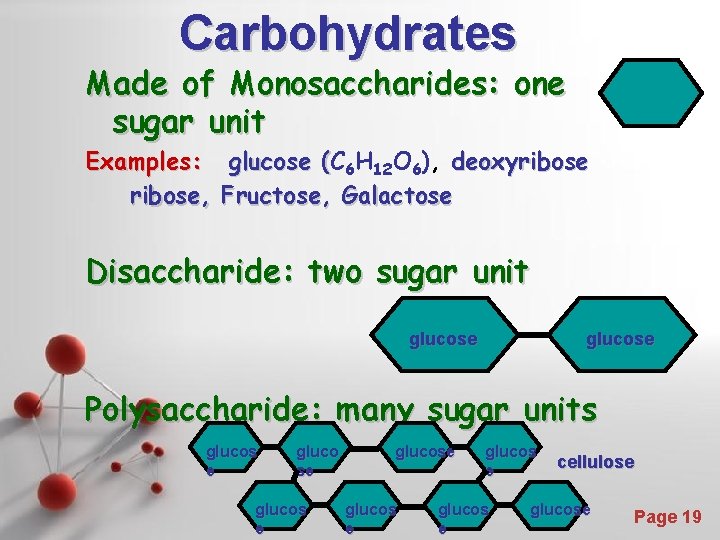
Carbohydrates Made of Monosaccharides: one sugar unit Examples: glucose (C ( 6 H 12 O 6), deoxyribose, Fructose, Galactose Disaccharide: two sugar unit glucose Polysaccharide: many sugar units glucos e gluco se glucos e Powerpoint Templates glucos e e e cellulose glucose Page 19

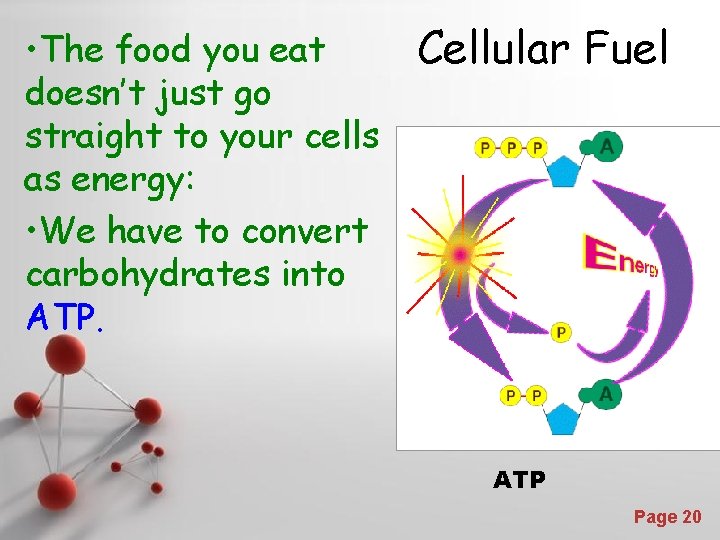
• The food you eat doesn’t just go straight to your cells as energy: • We have to convert carbohydrates into ATP. Cellular Fuel ATP Powerpoint Templates Page 20

Starch Plant cells store energy in the form of starch. Potatoes and grains are major sources of starch in the human diet Powerpoint Templates Page 21

Cellulose • Cellulose is the most abundant organic compound on Earth. It forms cable-like fibrils in the cell walls in plants. It is also known as dietary fiber Powerpoint Templates Page 22

Proteins Powerpoint Templates copyright cmassengale 23 Page 23

Powerpoint Templates Page 24

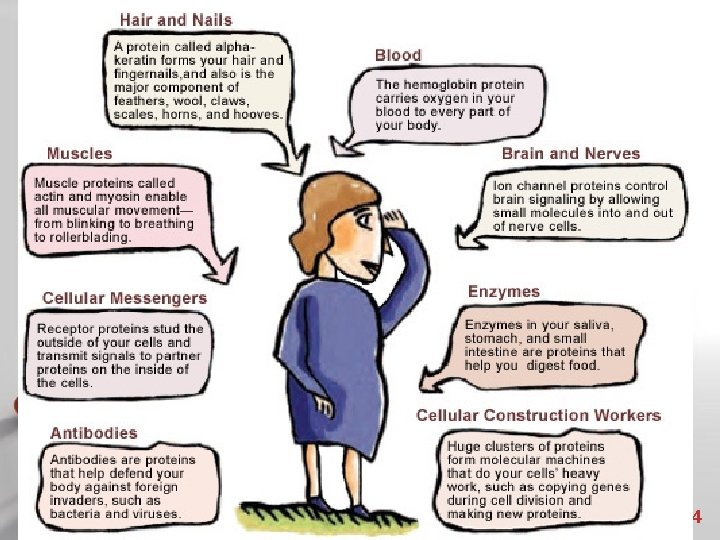
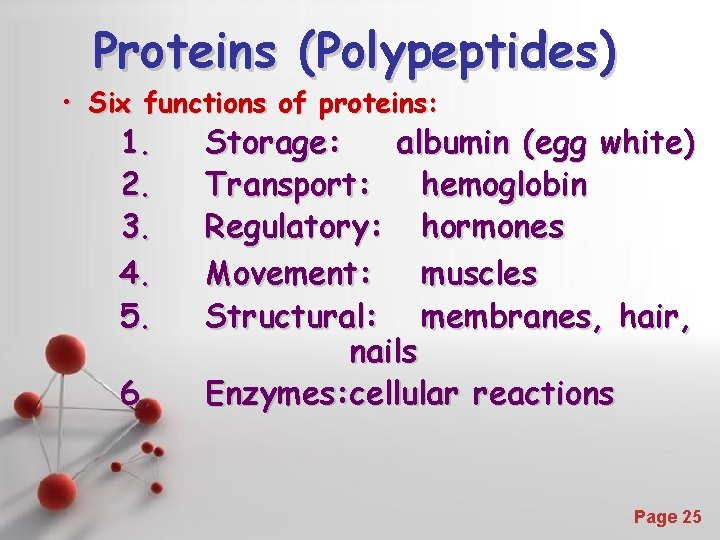
Proteins (Polypeptides) • Six functions of proteins: 1. 2. 3. 4. 5. 6. Storage: albumin (egg white) Transport: hemoglobin Regulatory: hormones Movement: muscles Structural: membranes, hair, nails Enzymes: cellular reactions Powerpoint Templates Page 25

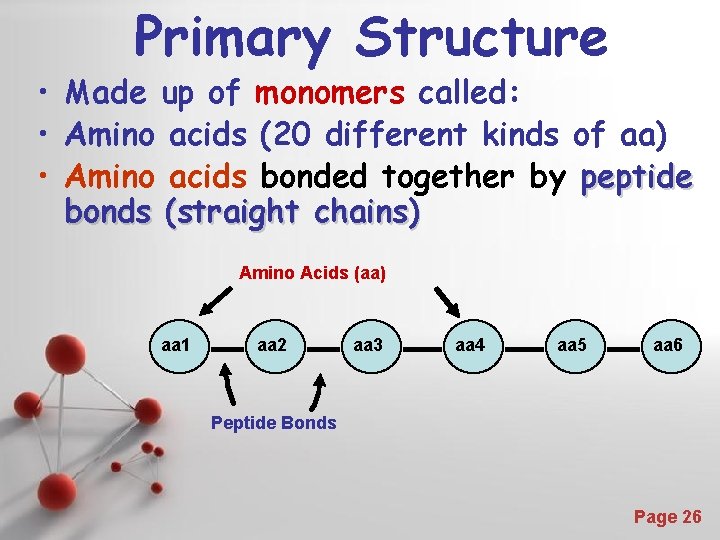
Primary Structure • Made up of monomers called: • Amino acids (20 different kinds of aa) • Amino acids bonded together by peptide bonds (straight chains) Amino Acids (aa) aa 1 aa 2 aa 3 aa 4 aa 5 aa 6 Peptide Bonds Powerpoint Templates Page 26

Proteins as Enzymes Many proteins act as biological catalysts or enzymes Thousands of different enzymes exist in the body Enzymes control the rate of chemical reactions by weakening bonds, and lowering the amount of activation energy needed for the reaction Powerpoint Templates Page 27

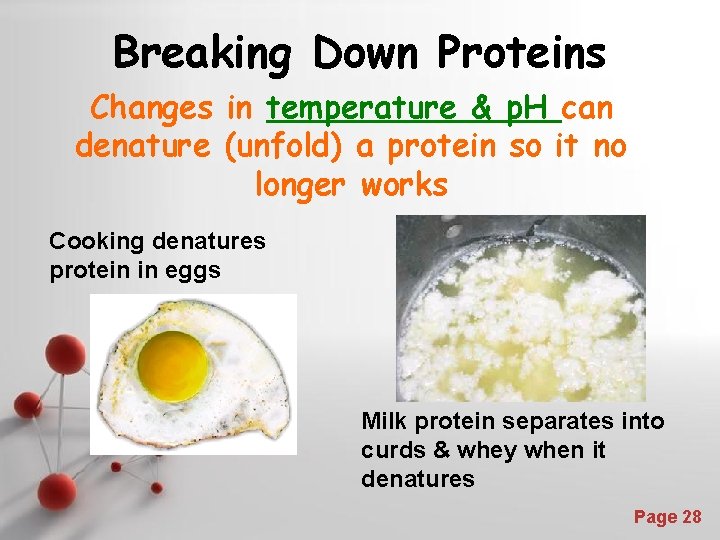
Breaking Down Proteins Changes in temperature & p. H can denature (unfold) a protein so it no longer works Cooking denatures protein in eggs Milk protein separates into curds & whey when it denatures Powerpoint Templates Page 28

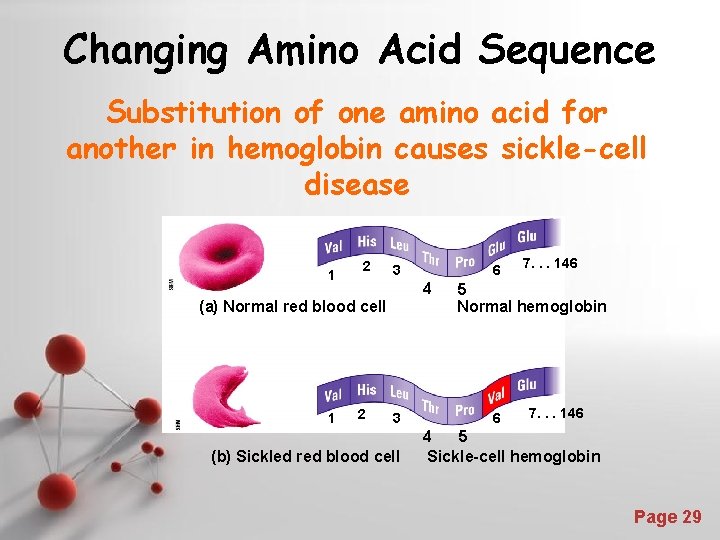
Changing Amino Acid Sequence Substitution of one amino acid for another in hemoglobin causes sickle-cell disease 1 2 3 4 (a) Normal red blood cell 1 2 6 3 (b) Sickled red blood cell 7. . . 146 5 Normal hemoglobin 6 7. . . 146 4 5 Sickle-cell hemoglobin Powerpoint Templates Page 29