Macromolecules Notes Macromolecules Contain Carbon Carbon is important

Macromolecules Notes

Macromolecules Contain Carbon! Carbon is important to life because: • It can form 4 strong covalent bonds • It can bind to itself and form LIMITLESS chains • It can form single, double, or triple bonds with another atom • Organic Chemistry studies carbon compounds

Hydrocarbons • Contain CARBON and HYDROGEN –Highly flammable
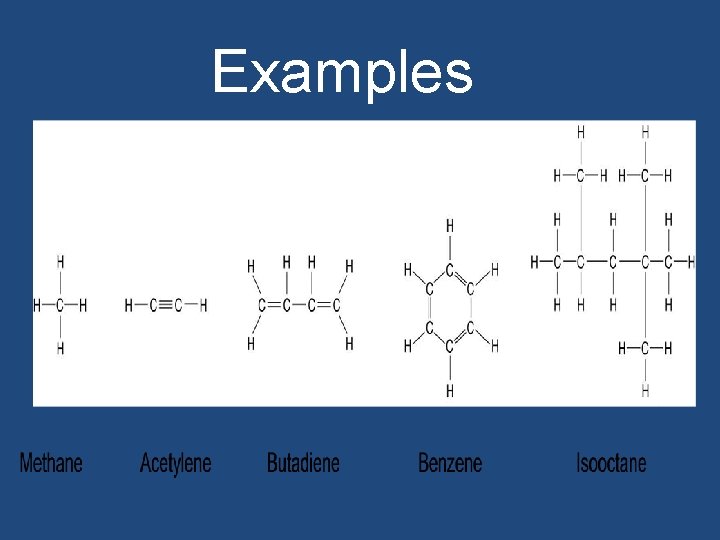
Examples
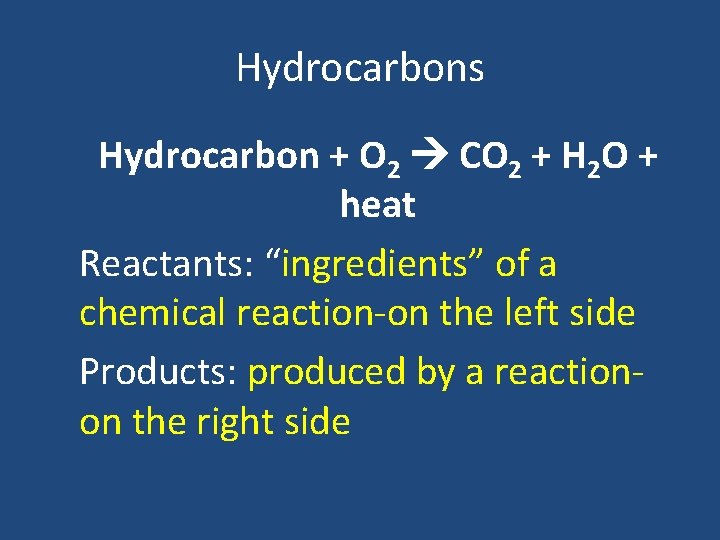
Hydrocarbons Hydrocarbon + O 2 CO 2 + H 2 O + heat Reactants: “ingredients” of a chemical reaction-on the left side Products: produced by a reactionon the right side
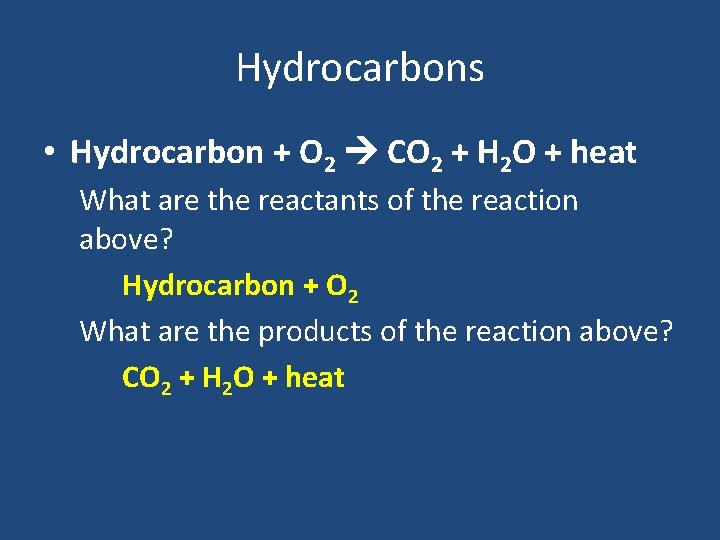
Hydrocarbons • Hydrocarbon + O 2 CO 2 + H 2 O + heat What are the reactants of the reaction above? Hydrocarbon + O 2 What are the products of the reaction above? CO 2 + H 2 O + heat
Polymers • Long, relatively complex chains called macromolecules • Poly “many" and meros "part” • The parts are called monomers
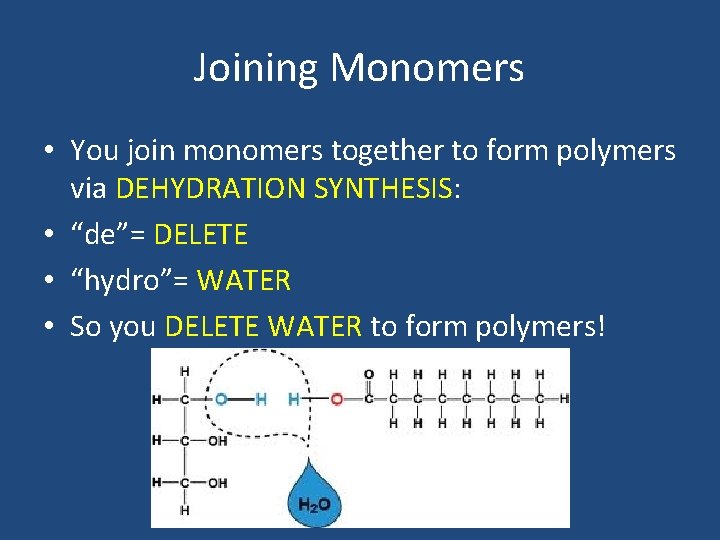
Joining Monomers • You join monomers together to form polymers via DEHYDRATION SYNTHESIS: • “de”= DELETE • “hydro”= WATER • So you DELETE WATER to form polymers!

Breaking Polymers • You break polymers apart to form monomers via HYDROLYSIS: • “Hydro”= Water • “lysis”= to break • So you ADD WATER to form monomers
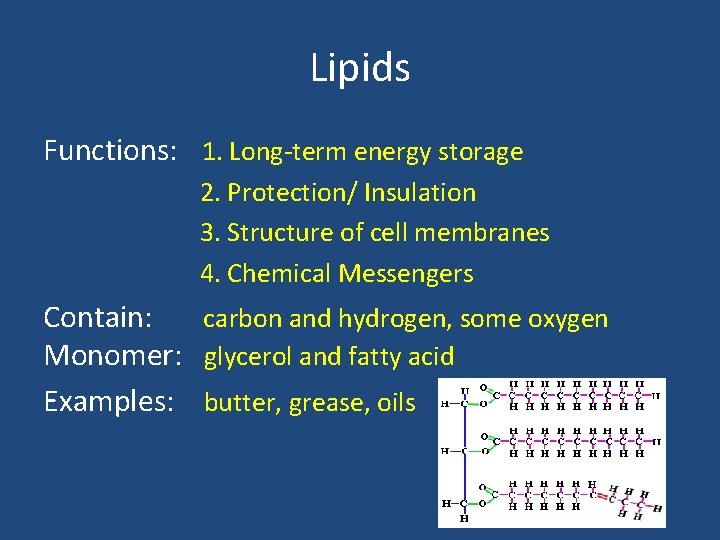
Lipids Functions: 1. Long-term energy storage 2. Protection/ Insulation 3. Structure of cell membranes 4. Chemical Messengers Contain: carbon and hydrogen, some oxygen Monomer: glycerol and fatty acid Examples: butter, grease, oils
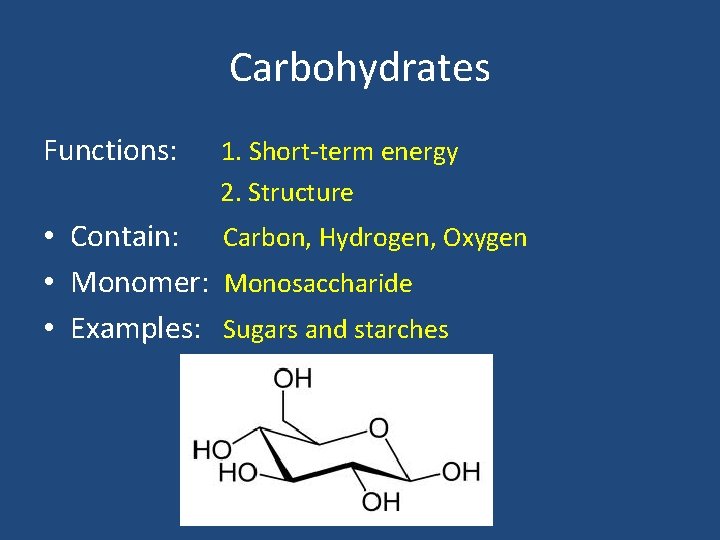
Carbohydrates Functions: 1. Short-term energy 2. Structure • Contain: Carbon, Hydrogen, Oxygen • Monomer: Monosaccharide • Examples: Sugars and starches
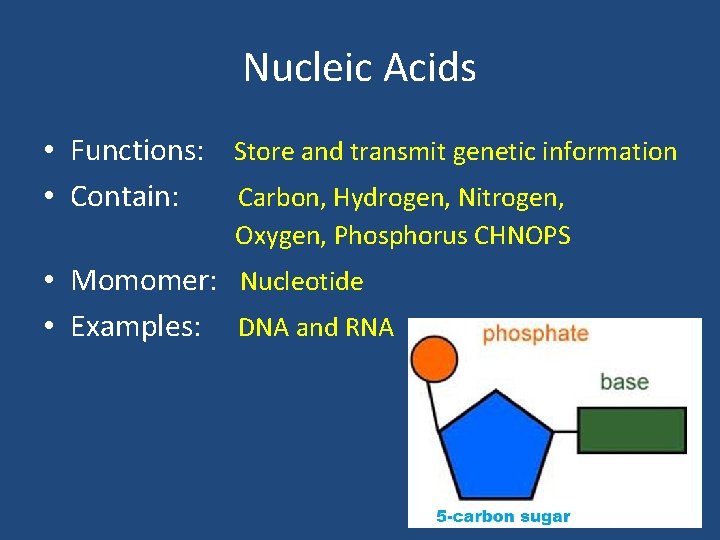
Nucleic Acids • Functions: Store and transmit genetic information • Contain: Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus CHNOPS • Momomer: Nucleotide • Examples: DNA and RNA
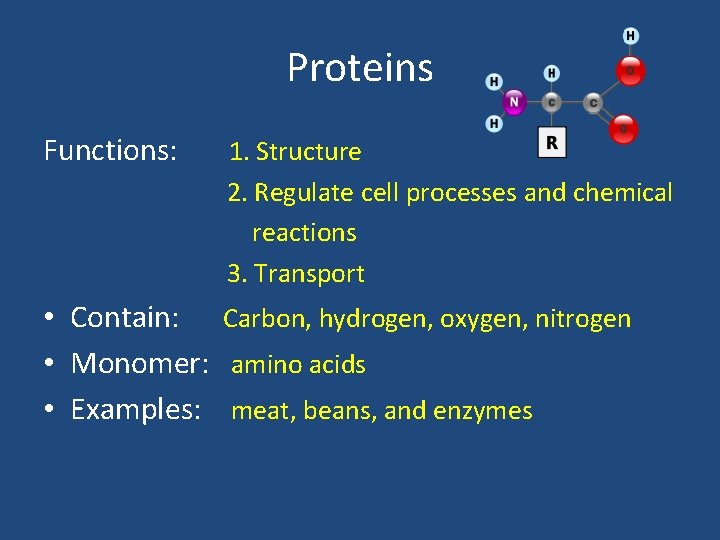
Proteins Functions: 1. Structure 2. Regulate cell processes and chemical reactions 3. Transport • Contain: Carbon, hydrogen, oxygen, nitrogen • Monomer: amino acids • Examples: meat, beans, and enzymes
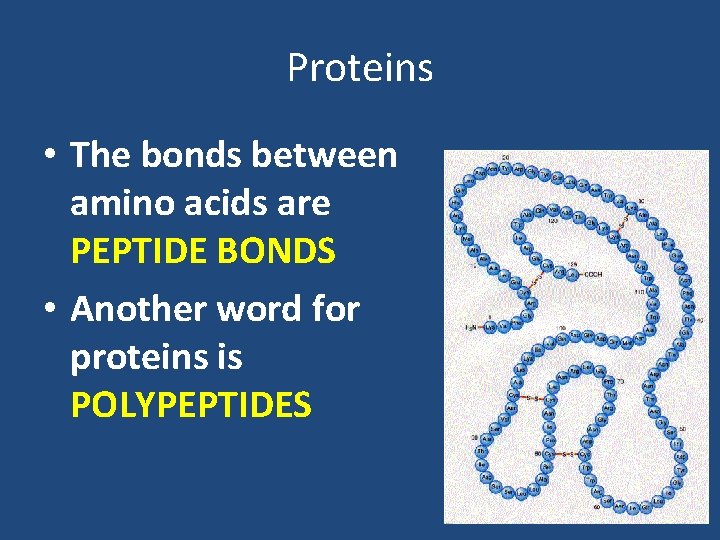
Proteins • The bonds between amino acids are PEPTIDE BONDS • Another word for proteins is POLYPEPTIDES
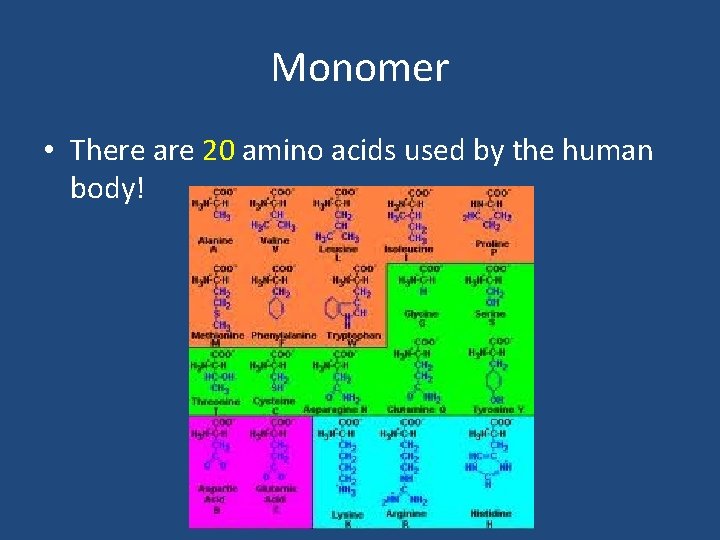
Monomer • There are 20 amino acids used by the human body!
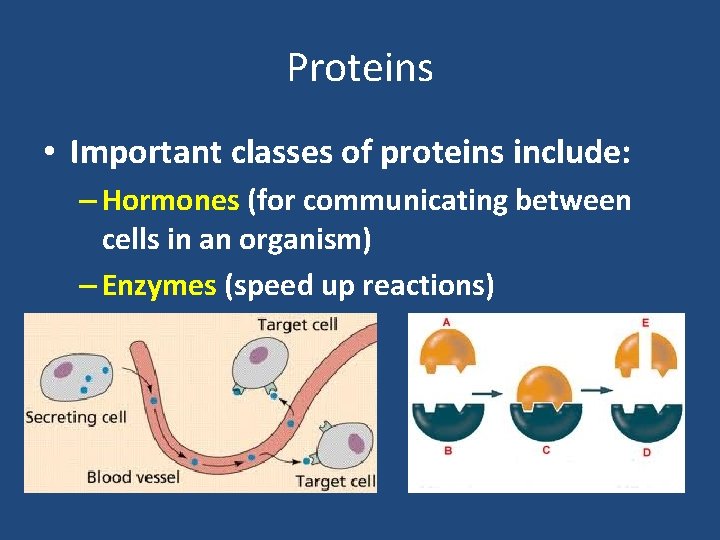
Proteins • Important classes of proteins include: – Hormones (for communicating between cells in an organism) – Enzymes (speed up reactions)
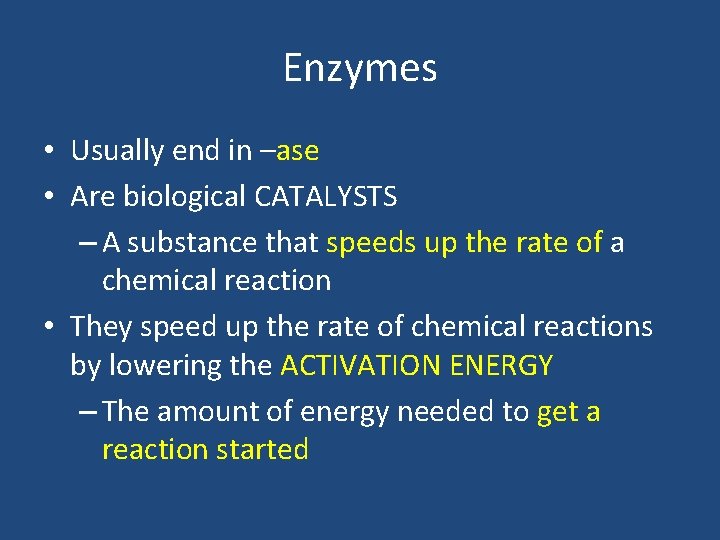
Enzymes • Usually end in –ase • Are biological CATALYSTS – A substance that speeds up the rate of a chemical reaction • They speed up the rate of chemical reactions by lowering the ACTIVATION ENERGY – The amount of energy needed to get a reaction started
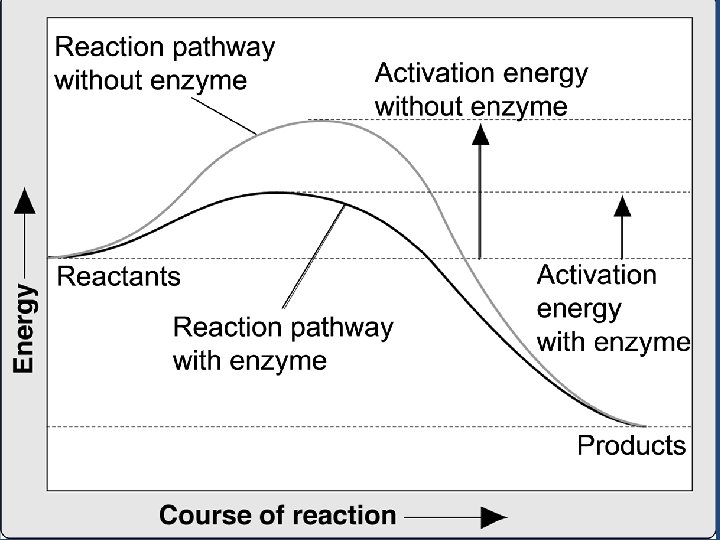

Enzymes • Enzymes bind to a substance called a substrate (reactants). –Enzymes have an active site. –The active site is the groove (space) where the substrate will fit.

Enzymes • The idea that the enzyme and the substrate fit together perfectly: – Lock-and-key hypothesis
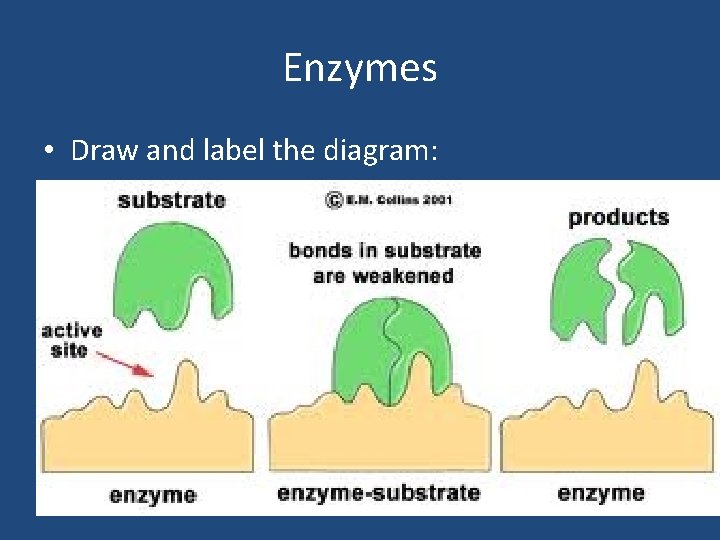
Enzymes • Draw and label the diagram:
Factors that Affect Enzymes • Many enzymes work best at a p. H between 6 and 8 (close to neutral). • The optimum (best) temperature for enzymes is between 35 o. C and 40 o. C (around human body temperature). • Out of this p. H or above this temperature, enzymes denature or lose their shape. • *Some enzymes such as those in the stomach work best at an acidic p. H
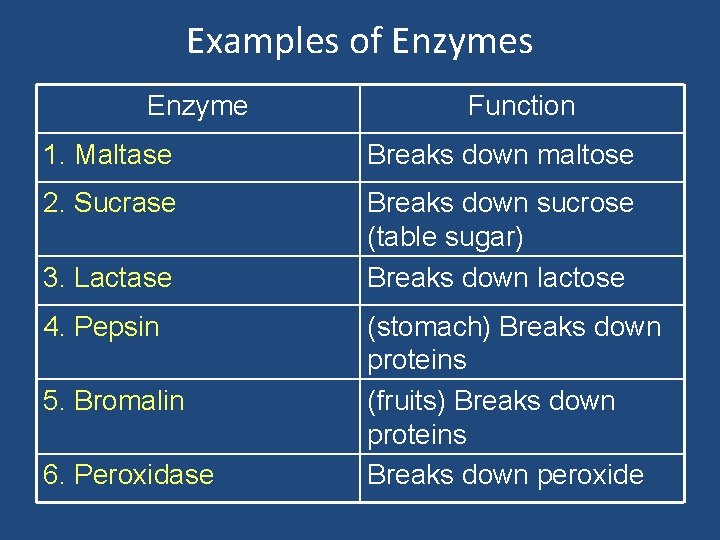
Examples of Enzymes Enzyme Function 1. Maltase Breaks down maltose 2. Sucrase Breaks down sucrose (table sugar) Breaks down lactose 3. Lactase 4. Pepsin 5. Bromalin 6. Peroxidase (stomach) Breaks down proteins (fruits) Breaks down proteins Breaks down peroxide
- Slides: 23