Macromolecules Molecules that make up living things are

















- Slides: 53
Macromolecules

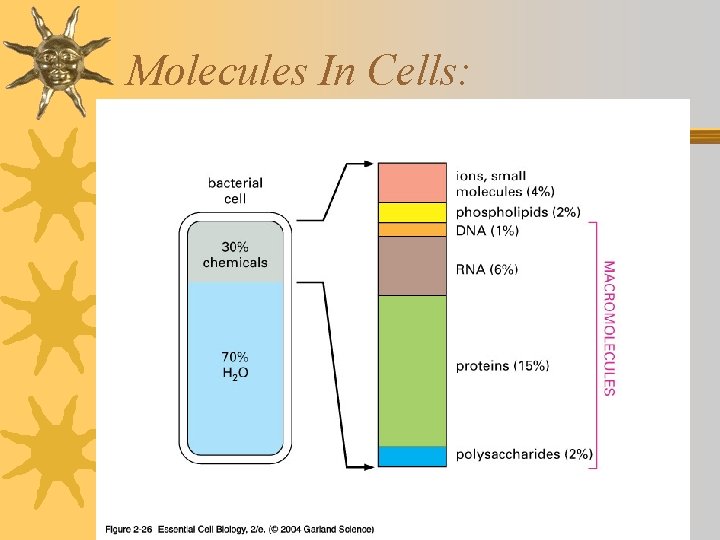
Molecules that make up living things are made of the following elements: CHNOPS

Two Types of Molecules ¬Organic Molecules ¬Inorganic Molecules

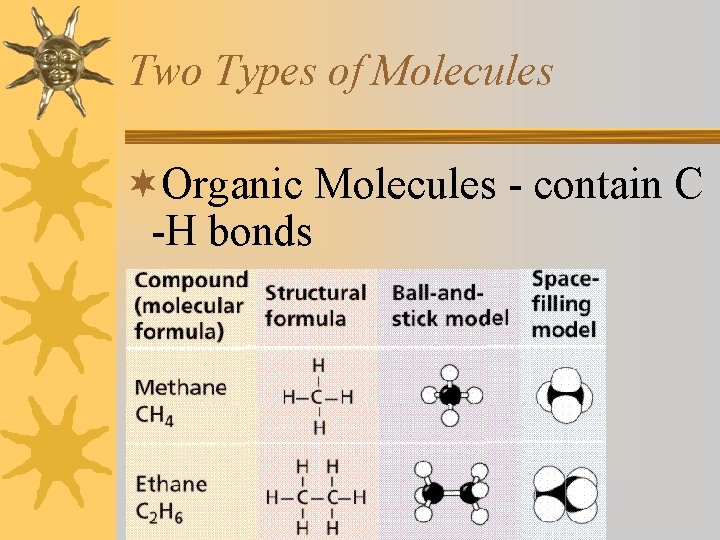
Two Types of Molecules ¬Organic Molecules - contain C -H bonds ¬Inorganic Molecules -

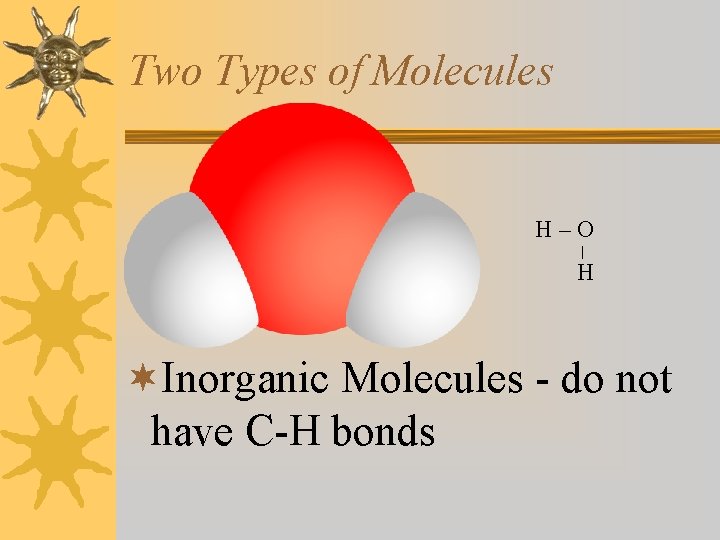
Two Types of Molecules H–O H ¬Inorganic Molecules - do not have C-H bonds

Molecules In Cells:

Metabolism ¬all of the chemical reactions that happen in an organism’s body and allow it to survive ¬Anabolic reactions – build molecules ¬Catabolic reactions – take apart molecules

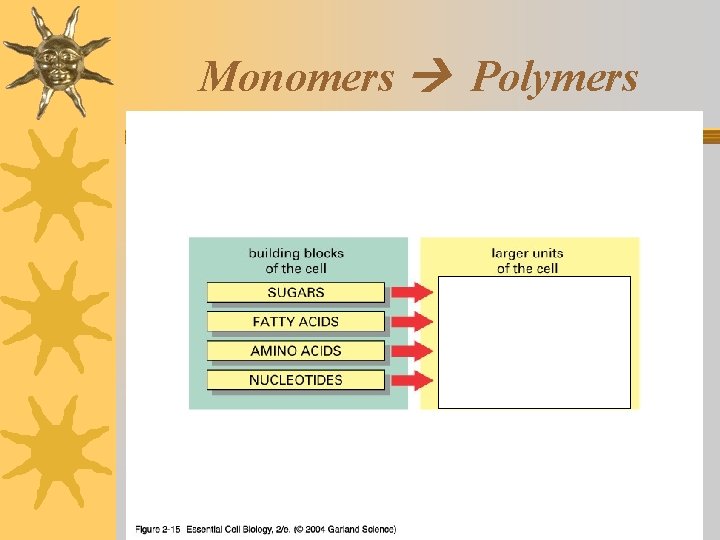
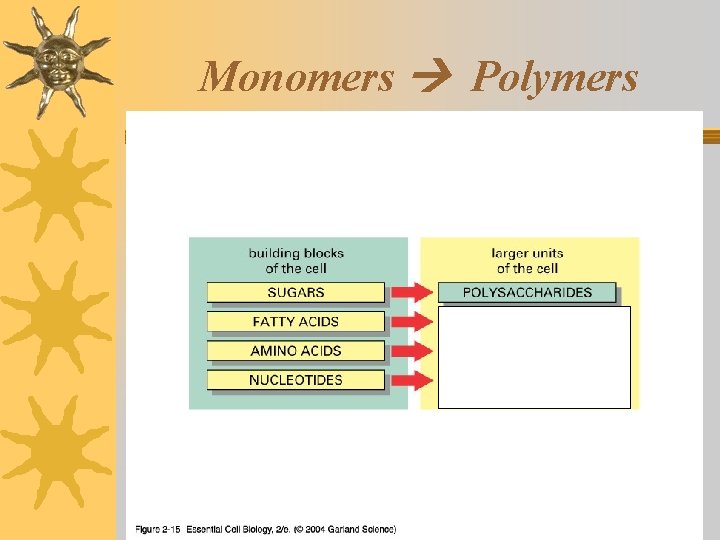
3 Levels of molecules Macromolecules Polymers Monomers


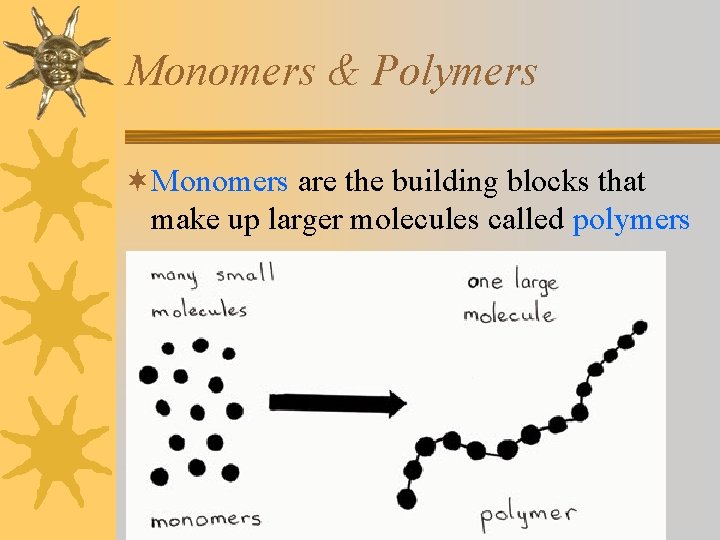
Monomers & Polymers ¬Monomers are the building blocks that make up larger molecules called polymers

¬There are only 4 different monomers found in living things, but, like letters of the alphabet, their arrangement/sequence can produce different polymers. This creates diversity among living things.

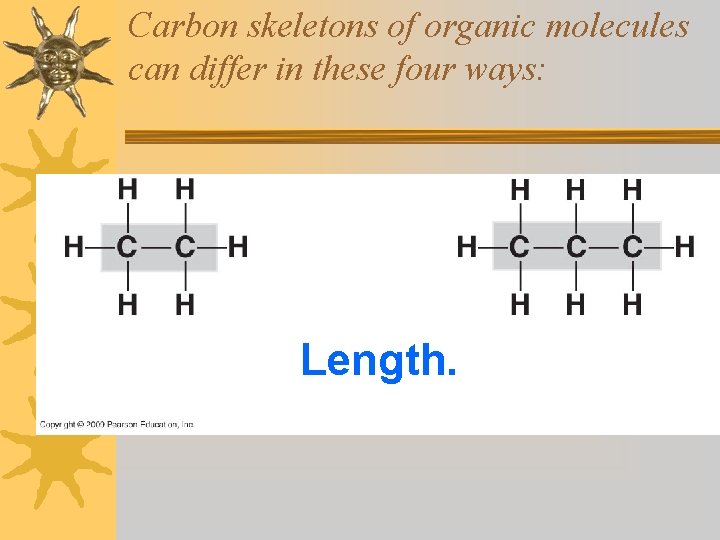
Carbon skeletons of organic molecules can differ in these four ways: Length.

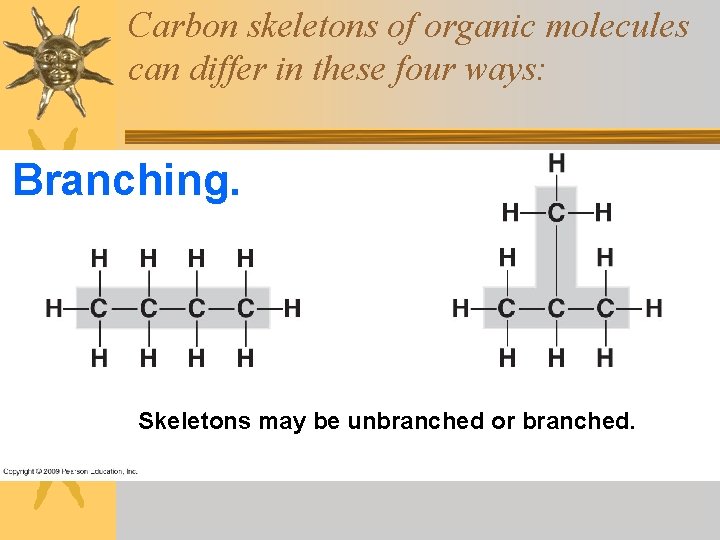
Carbon skeletons of organic molecules can differ in these four ways: Branching. Skeletons may be unbranched or branched.

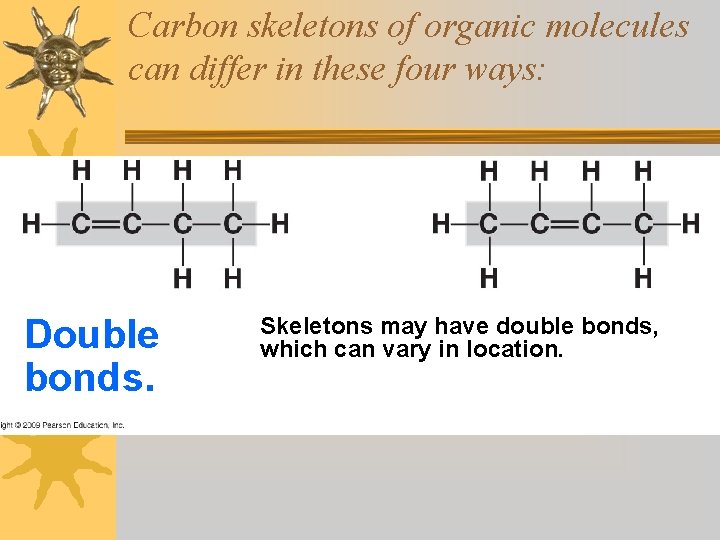
Carbon skeletons of organic molecules can differ in these four ways: Double bonds. Skeletons may have double bonds, which can vary in location.

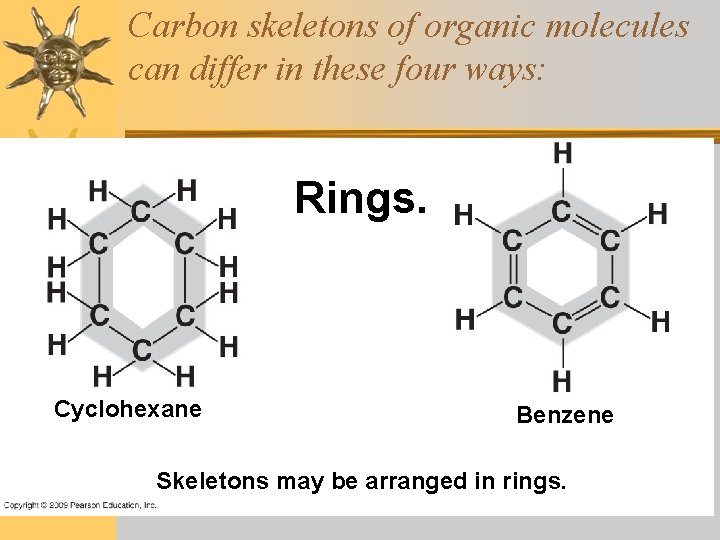
Carbon skeletons of organic molecules can differ in these four ways: Rings. Cyclohexane Benzene Skeletons may be arranged in rings.

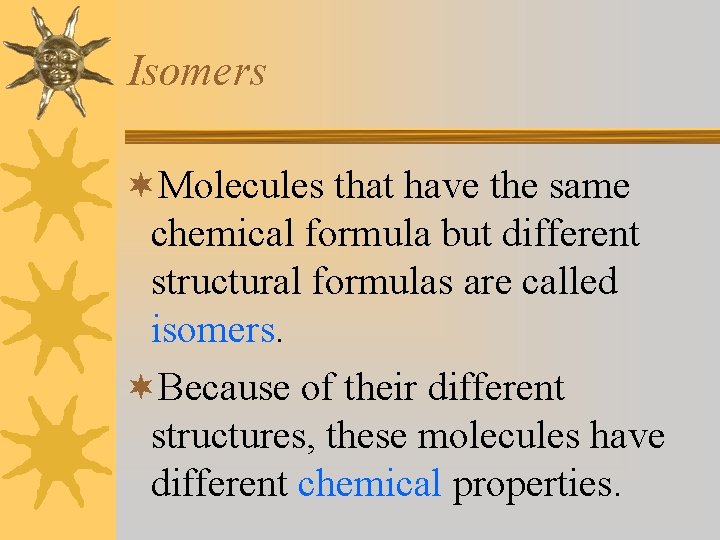
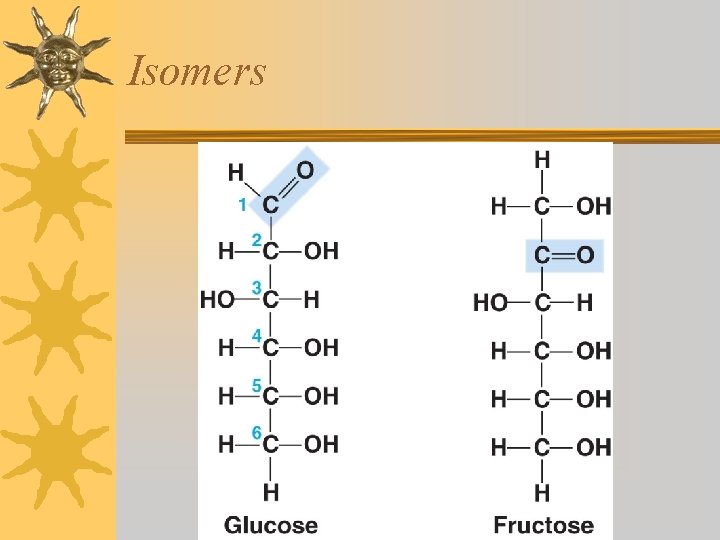
Isomers ¬Molecules that have the same chemical formula but different structural formulas are called isomers. ¬Because of their different structures, these molecules have different chemical properties.

Isomers

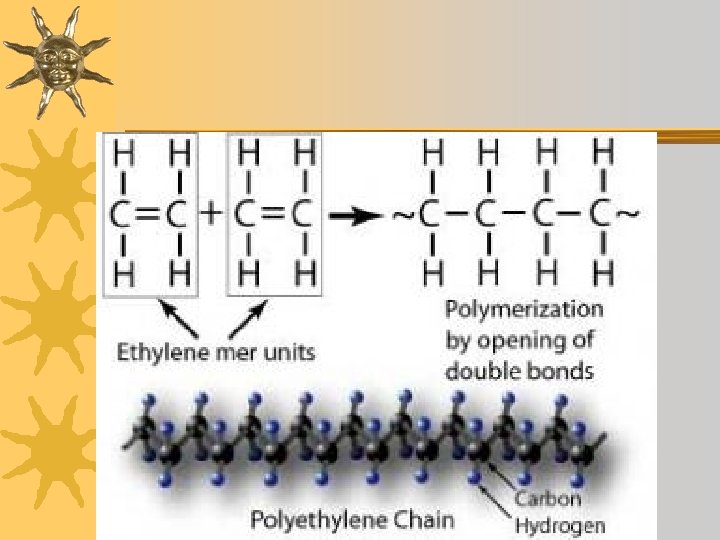
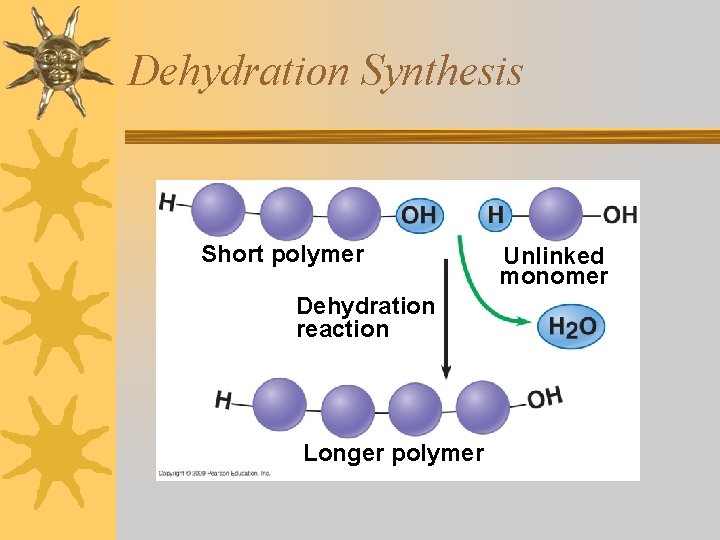
Dehydration Synthesis ¬The process by which monomers are bonded together to form polymers. ¬One monomer gives up OH and the other gives up H. (forms water) ¬A covalent bond holds monomers together

Dehydration Synthesis Short polymer Dehydration reaction Longer polymer Unlinked monomer

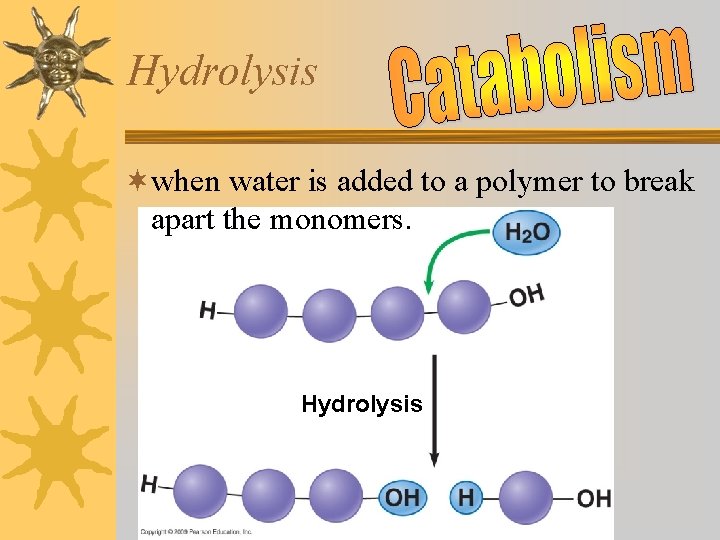
Hydrolysis ¬when water is added to a polymer to break apart the monomers. Hydrolysis

Uses ¬Dehydration Synthesis? ¬Hydrolysis?

¬Both of these reactions would not happen at a fast enough rate without the help of enzymes, enzymes special proteins that help speed up reactions

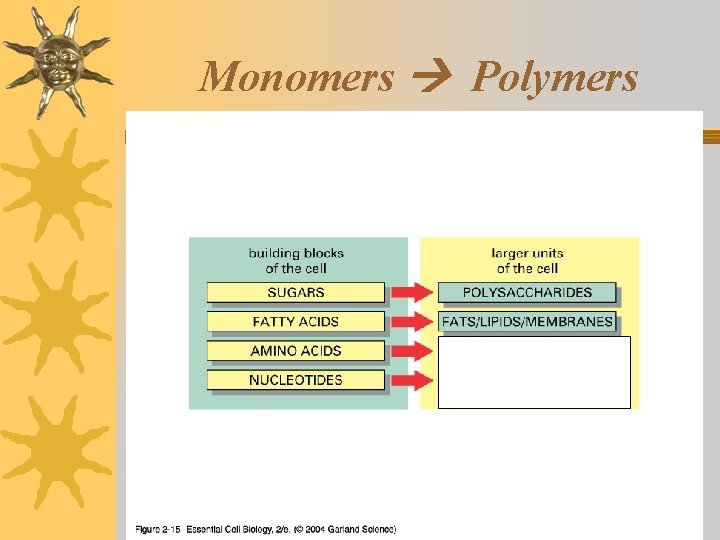
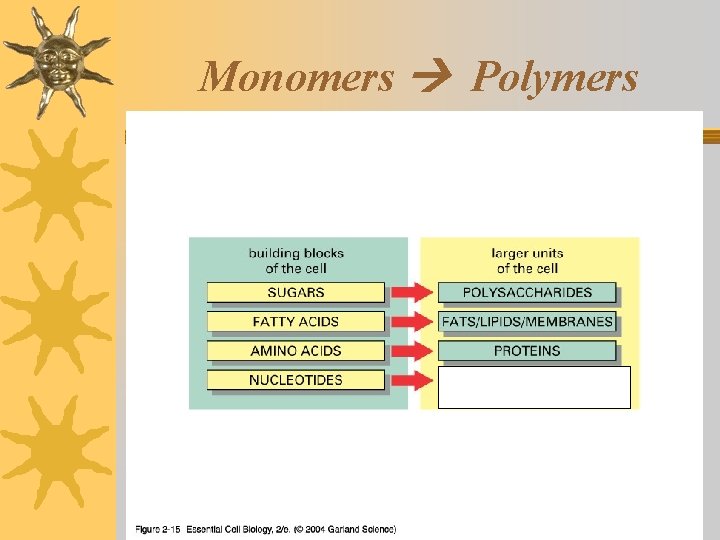
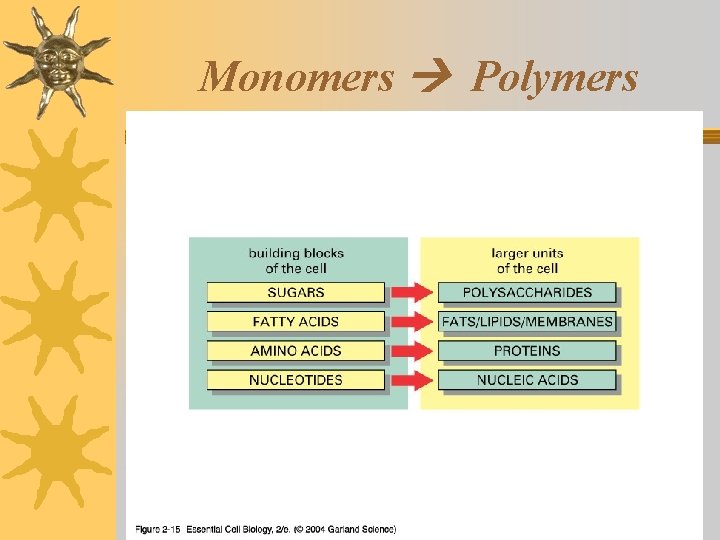
Living things are made of 4 classes of organic polymers ¬Carbohydrates / Polysaccharides ¬Lipids / Fats ¬Proteins / Polypeptides ¬Nucleic Acids

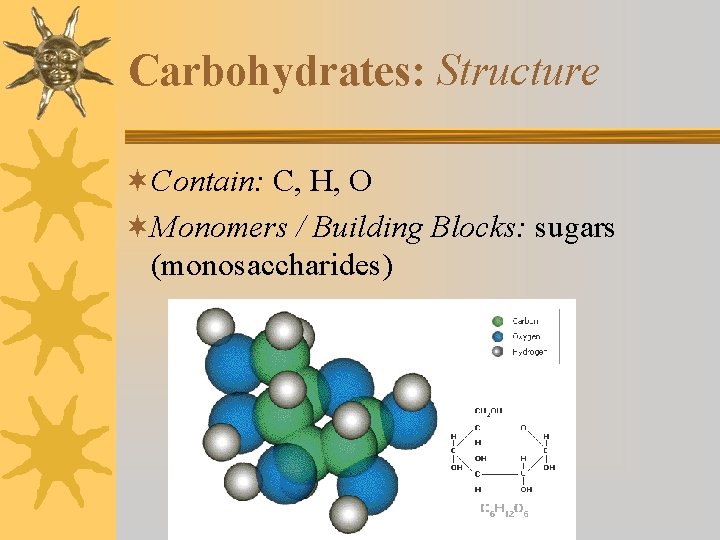
Carbohydrates: Structure ¬Contain: C, H, O ¬Monomers / Building Blocks: sugars (monosaccharides)

Carbohydrates: Role/Functions ¬The main energy source for living things ¬Structural components of cells (cell wall, etc) Found in: – Grains (bread, cereal, flour) – Fruits – Veggies – Sugars

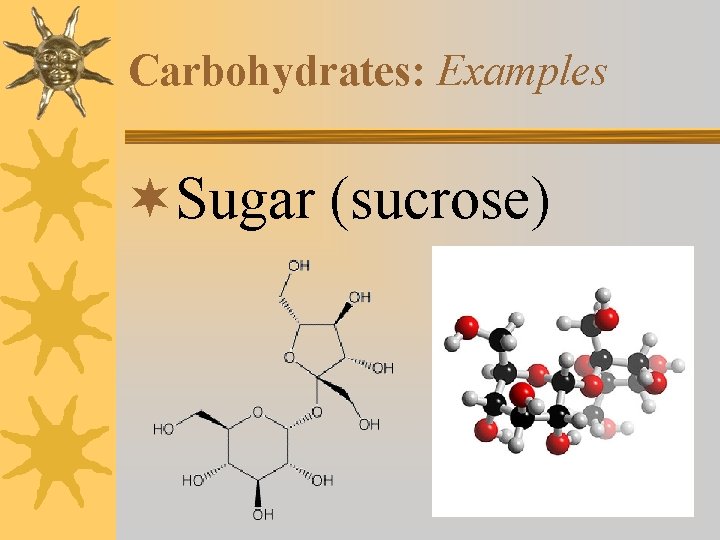
Carbohydrates: Examples ¬Sugar (sucrose)

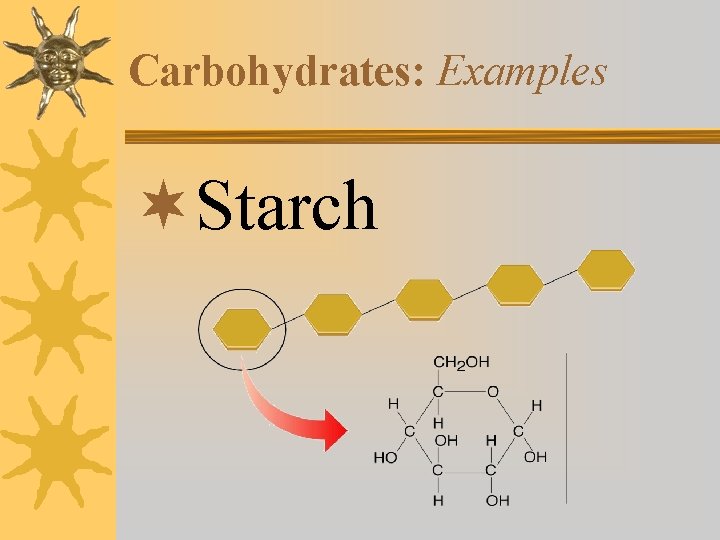
Carbohydrates: Examples ¬Starch

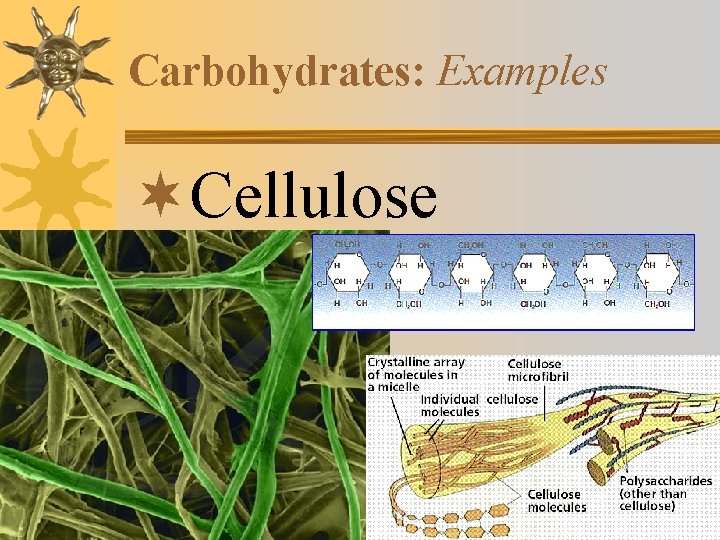
Carbohydrates: Examples ¬Cellulose

Carbs often end in _____

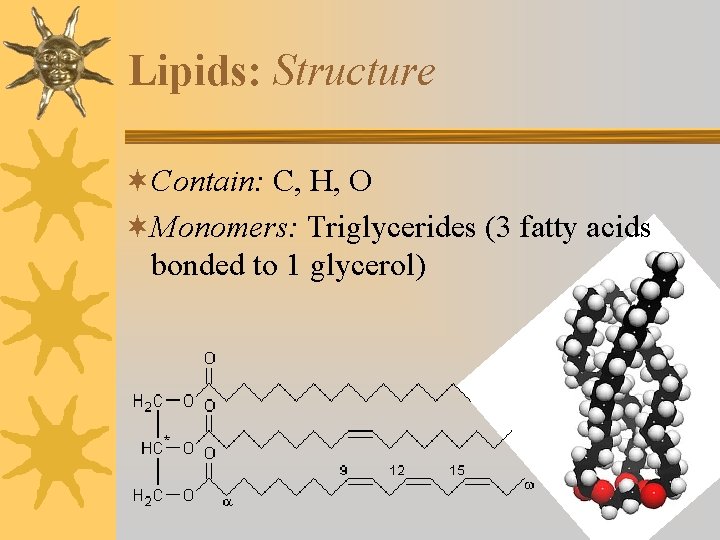
Lipids: Structure ¬Contain: C, H, O ¬Monomers: Triglycerides (3 fatty acids bonded to 1 glycerol)

Lipids: Role ¬Energy Storage ¬Insulation / Waterproofing ¬Biochemical Signals ¬Make up most of the cell membrane Found in: – Oils, Butter, Shortening – Dairy Products – Meat – Some veggies (like avocado) – Junk Food

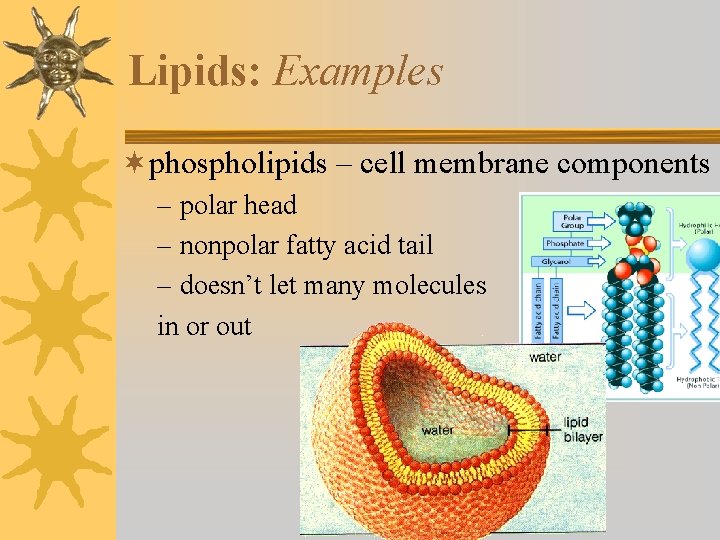
Lipids: Examples ¬phospholipids – cell membrane components – polar head – nonpolar fatty acid tail – doesn’t let many molecules in or out

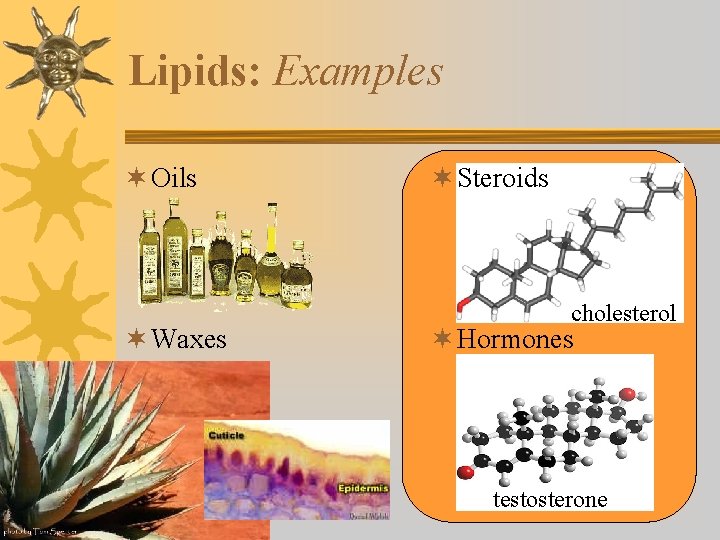
Lipids: Examples ¬ Oils ¬ Waxes ¬ Steroids cholesterol ¬ Hormones testosterone

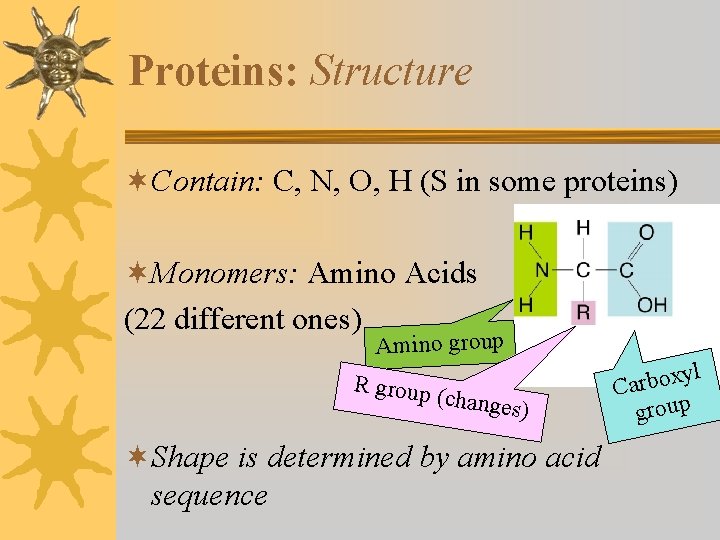
Proteins: Structure ¬Contain: C, N, O, H (S in some proteins) ¬Monomers: Amino Acids (22 different ones) Amino group R group (changes ) ¬Shape is determined by amino acid sequence l y x o b r Ca group

Proteins: Role ¬Enzymes (help reactions take place) ¬Carry out body functions ¬Structural Components (make up muscle, internal cell structure) Found in: – Meat – Dairy – Eggs – Nuts and Legumes

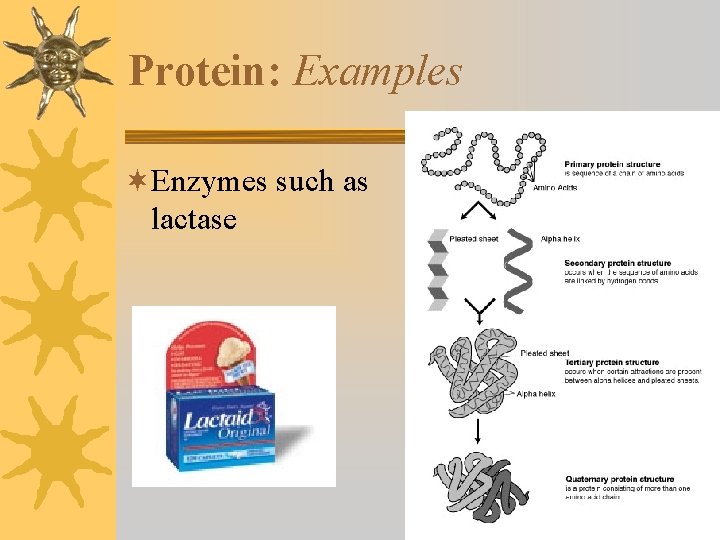
Protein: Examples ¬Enzymes such as lactase

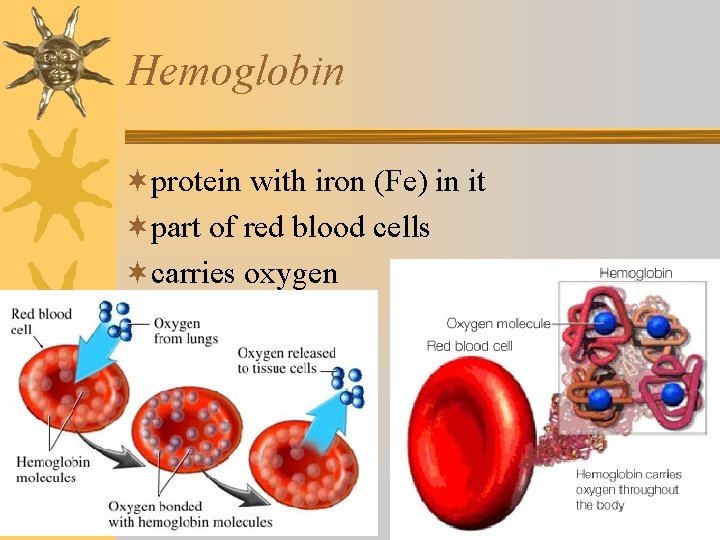
Hemoglobin ¬protein with iron (Fe) in it ¬part of red blood cells ¬carries oxygen

Proteins often end in: ¬-ase (indicates an enzyme) ¬-in or -ein

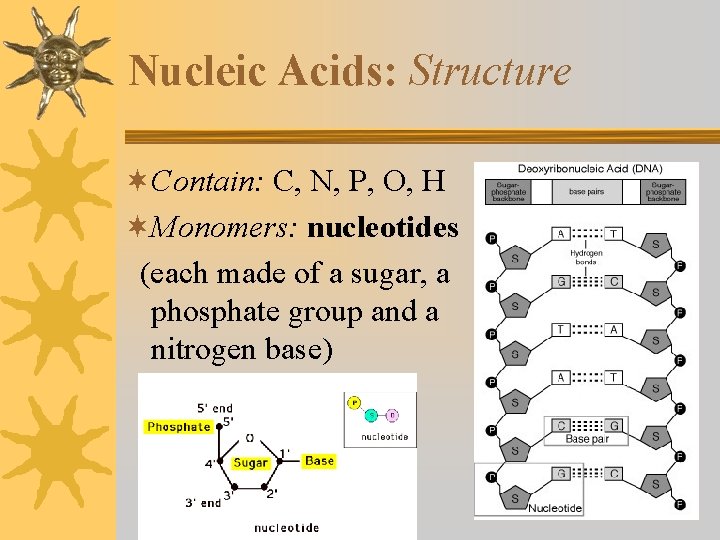
Nucleic Acids: Structure ¬Contain: C, N, P, O, H ¬Monomers: nucleotides (each made of a sugar, a phosphate group and a nitrogen base)

Nucleic Acids: Role ¬Contain genetic information passed on to new cells or during reproduction ¬Instructions / blueprints to make proteins ¬Chemical Energy (ATP) Found in: all living things (not a food source)

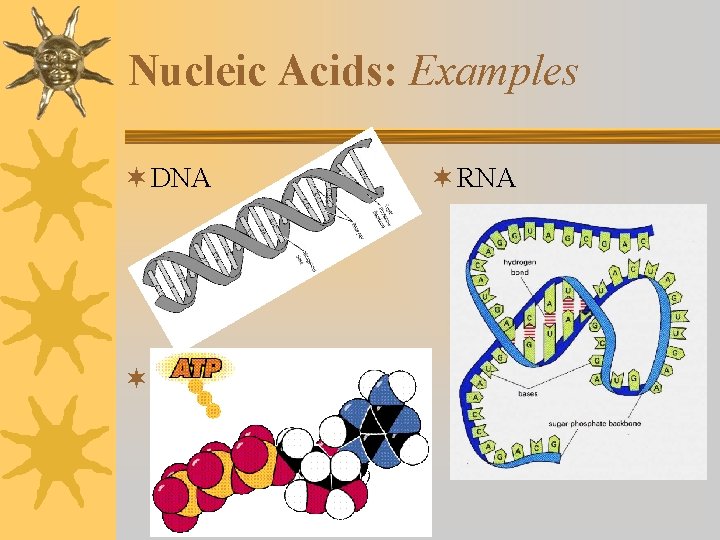
Nucleic Acids: Examples ¬ DNA ¬ ATP ¬ RNA

Monomers Polymers

Monomers Polymers

Monomers Polymers

Monomers Polymers

Monomers Polymers

Vitamins ¬Organic molecules needed by your body in small quantities to regulate body processes

Minerals ¬Inorganic nutrients needed by your body in small amounts ¬Usually one element (iron, zinc, calcium, etc)

¬Vitamins and minerals are MICRONUTRIENTS because they’re needed in small quantities. ¬The RDA or recommended daily allowance tells you how much of these you should consume.

¬Water soluble vitamins – can’t have too much – extra dissolve in urine & are excreted vs. ¬Fat-soluble vitamins – too much can be toxic – build up in fat cells and overwhelm kidneys, liver

EQs / Exit Ticket ¬Make 2 columns on your paper– organic and inorganic. ¬Classify each of the following under one column: – Water – Vitamins – Carbohydrates – Lipids - Nucleic acids - Minerals - Proteins

Homework ¬The skinny on fats article & questions ¬Optional Homework: Make a study wheel for macromolecules Quiz next class!!! (p. H and nutrients)

EQs / Exit Ticket ¬Compare and contrast dehydration synthesis and hydrolysis. In doing so, give an example of why each might occur.