Macromolecules Large Carbon Compounds Introduction and Carbohydrates Objectives

Macromolecules: Large Carbon Compounds Introduction and Carbohydrates

Objectives • Distinguish between organic and inorganic compounds • Summarize how large carbon molecules are synthesized and broken down

Organic vs inorganic • All living organisms are composed primarily of carbon atoms • Organic compounds = contain Carbon • Inorganic compounds = do not contain Carbon
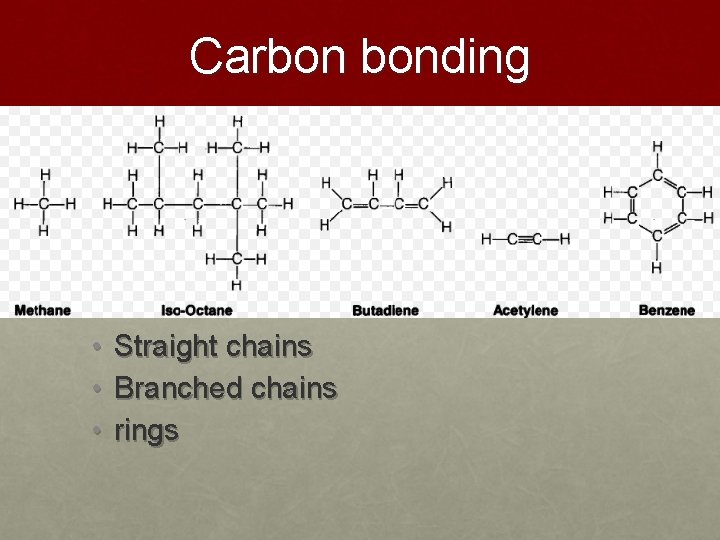
Carbon bonding • Carbon can form large complex molecules • Readily bonds with other carbon atoms to form • Straight chains • Branched chains • rings
Macromolecules • Large compounds of multiple smaller molecules of carbon are called Macromolecules • Monomers: small, simple molecules of carbon • Polymers: a large molecule that consists of repeated, linked units • Monomers link together to form polymers • There are many types of macromolecules: carbohydrates, proteins, lipids and nucleic acids

Chemical reactions • Condensation or Dehydration Synthesis: • Monomers link to form polymers • Hydrolysis: • Polymers are broken down into their monomer parts
CHAPTER 3 The Structure and Function of Macromolecules “You are what you eat!”
Objectives • Distinguish among proteins, carbohydrates, lipids, and nucleic acids. • Identify the major structural components and functions of the four major macromolecules

Shoulder Partners
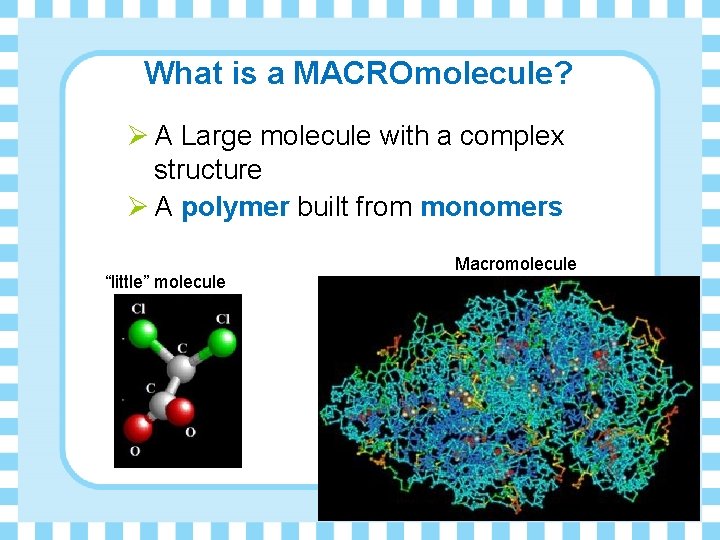
What is a MACROmolecule? Ø A Large molecule with a complex structure Ø A polymer built from monomers “little” molecule Macromolecule
Mono - mer One Part Ø The “building blocks” of polymers Ø A monomer is a sub-unit of a polymer.
Poly - mer Many Parts • A long molecule made of monomers bonded together
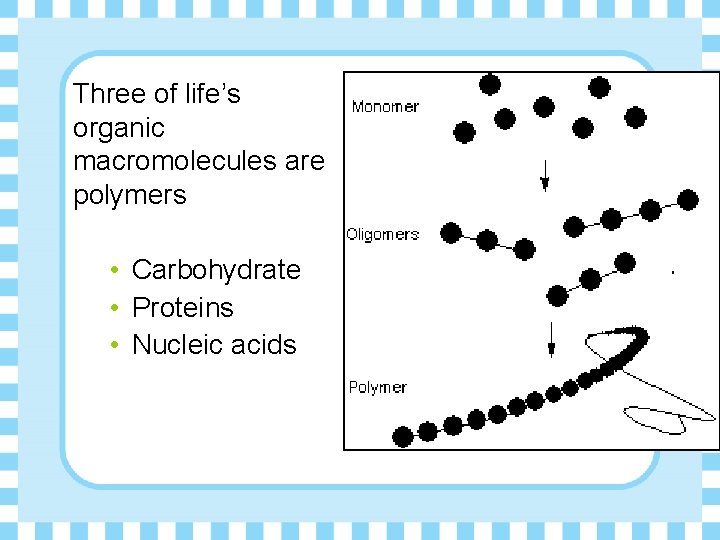
Three of life’s organic macromolecules are polymers • Carbohydrate • Proteins • Nucleic acids

Polar Bears Ø Explain to your penguins the connection between a monomer and a polymer

EXAMPLES

Penguins Ø Explain to your partner how these Lego structures are like Polymers
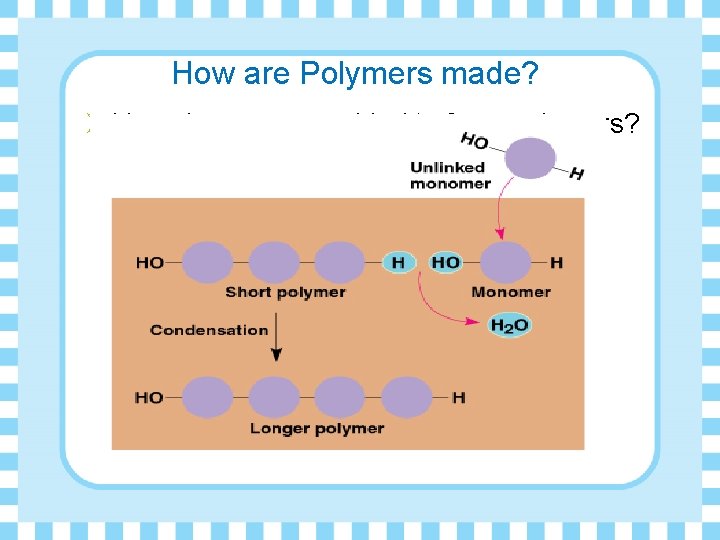
How are Polymers made? Ø How do monomers bind to form polymers? • condensation reactions called dehydration synthesis (removal of water)
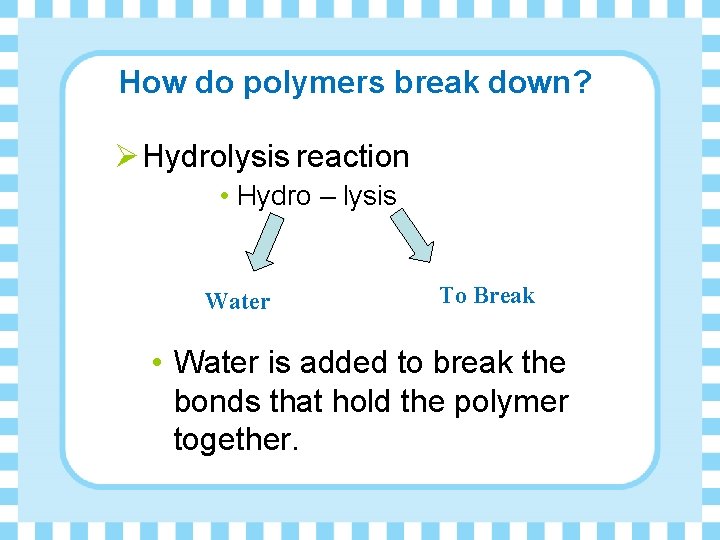
How do polymers break down? Ø Hydrolysis reaction • Hydro – lysis Water To Break • Water is added to break the bonds that hold the polymer together.
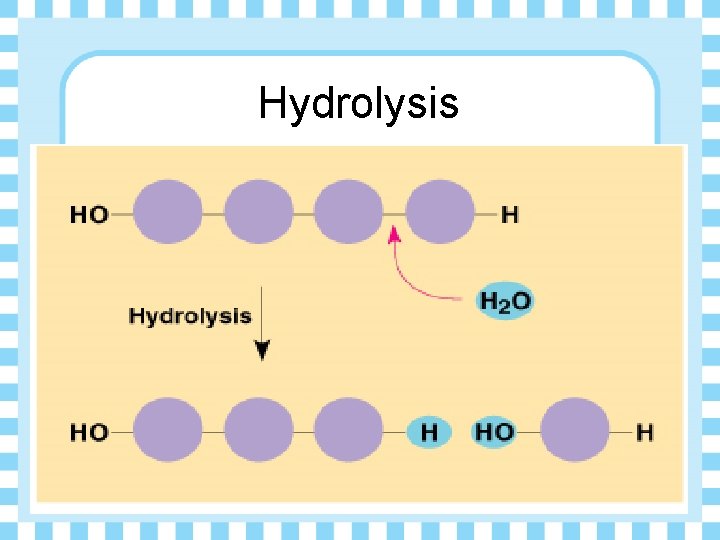
Hydrolysis

Think – Pair – Share Together Why would polymers need to be “broken down”?

Classes of Organic Macromolecules: • • Carbohydrates Proteins Lipids Nucleic Acids

CARBOHYDRATES
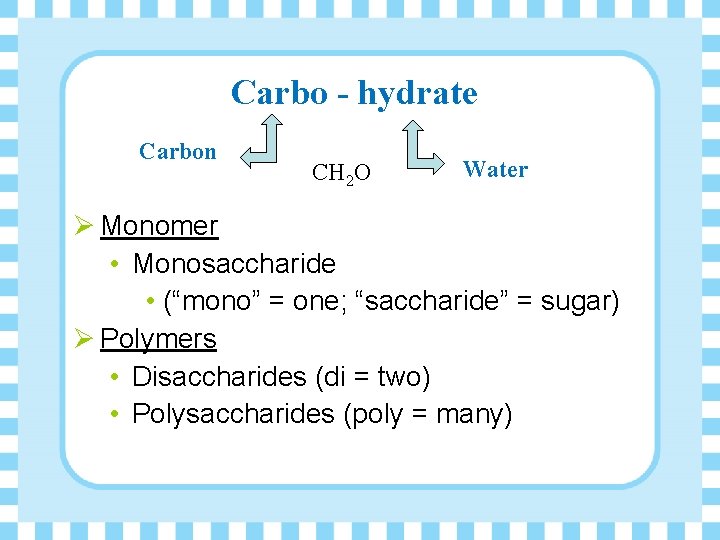
Carbo - hydrate Carbon CH 2 O Water Ø Monomer • Monosaccharide • (“mono” = one; “saccharide” = sugar) Ø Polymers • Disaccharides (di = two) • Polysaccharides (poly = many)

Polar Bears Tell your penguin some functions of carbohydrates
Ø Functions of Carbohydrates in living things: • Major fuel/energy source • Energy storage • Can be used as raw materials for other Macromolecules • Structural/building material in plants

Structure of Monosaccharides Ø Contain only C, H, O Ø All have the molecular formula (CH 2 O)n
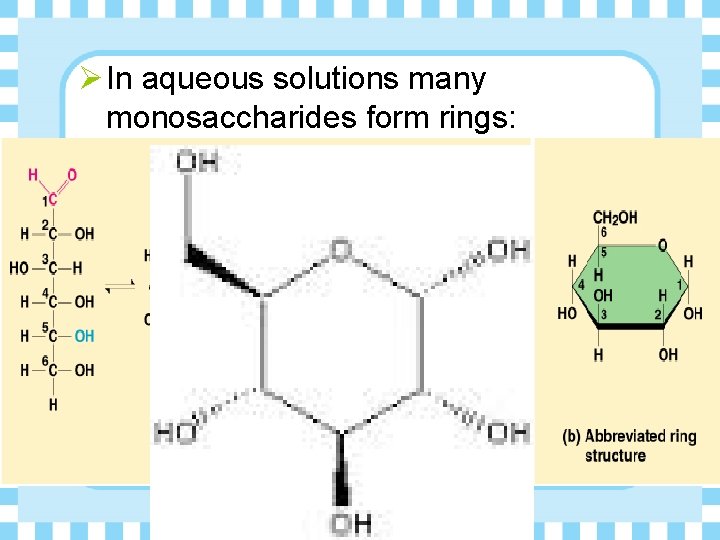
Ø In aqueous solutions many monosaccharides form rings:
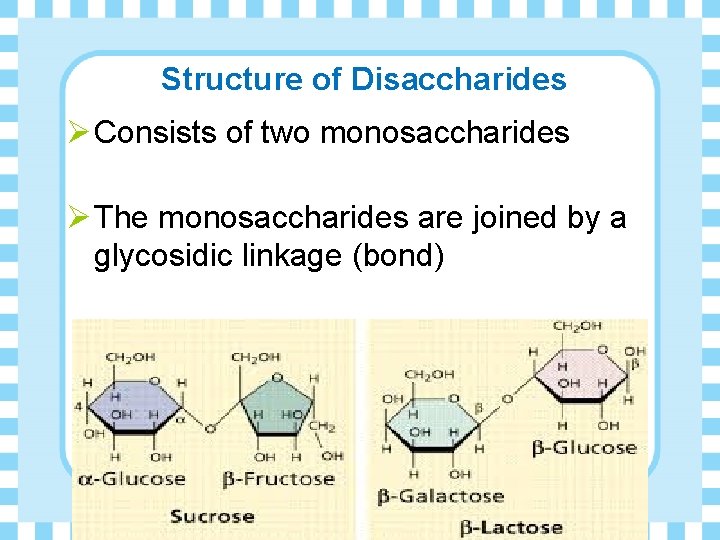
Structure of Disaccharides Ø Consists of two monosaccharides Ø The monosaccharides are joined by a glycosidic linkage (bond)

Polar Bears Ø What reaction forms the glycosidic linkage (bond) between the monosaccharides to become a disaccharide? • Dehydration synthesis
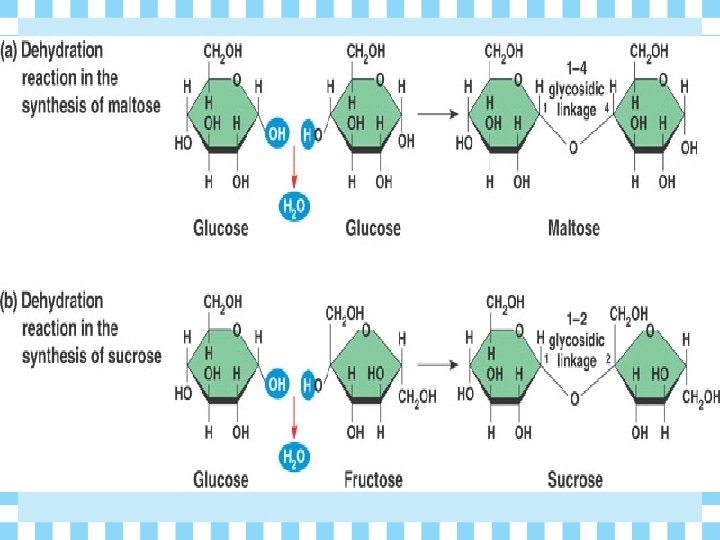
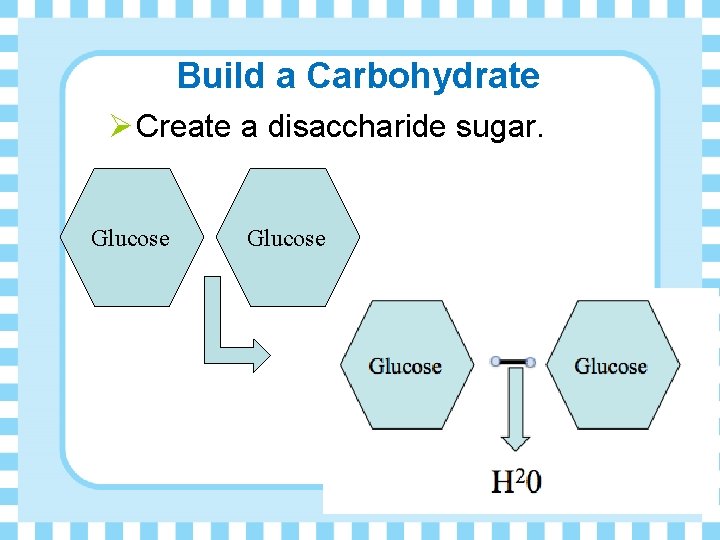
Build a Carbohydrate Ø Create a disaccharide sugar. Glucose
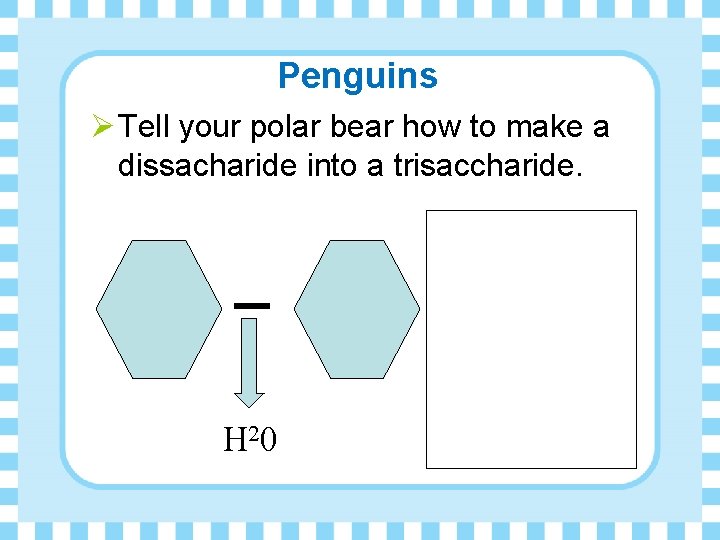
Penguins Ø Tell your polar bear how to make a dissacharide into a trisaccharide. H 20
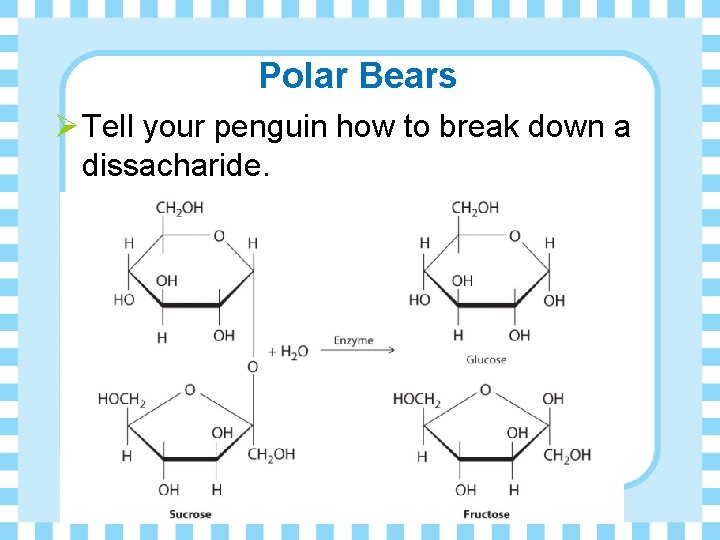
Polar Bears Ø Tell your penguin how to break down a dissacharide.
Polysaccharides Ø Structure: Polymers of a few hundred or a few thousand monosaccharides. Ø Functions: • energy storage molecules • structural support
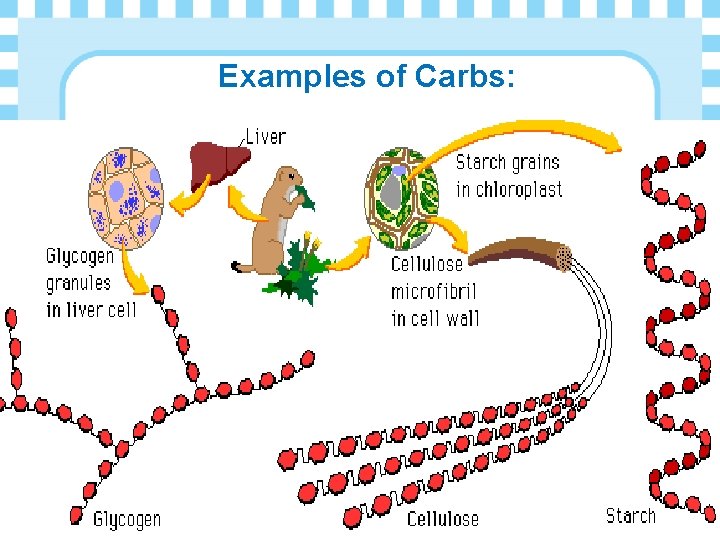
Examples of Carbs:
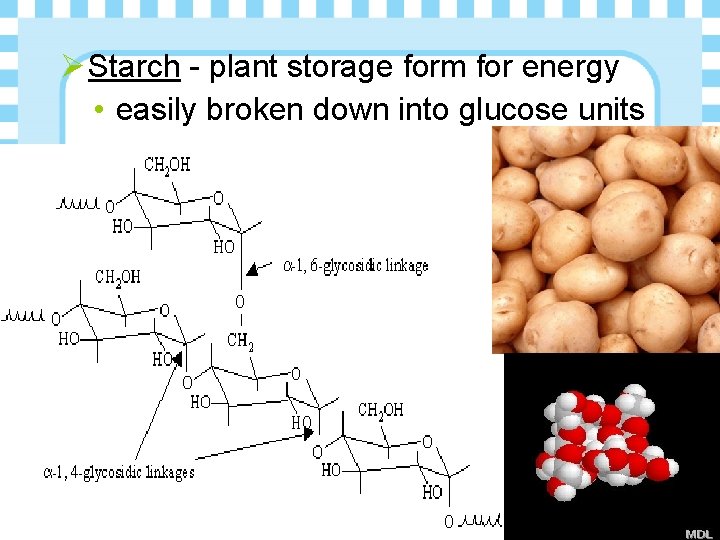
Ø Starch - plant storage form for energy • easily broken down into glucose units
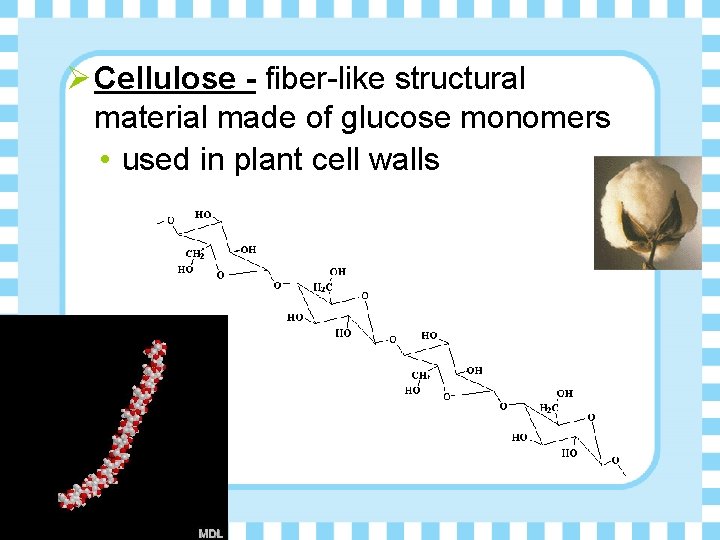
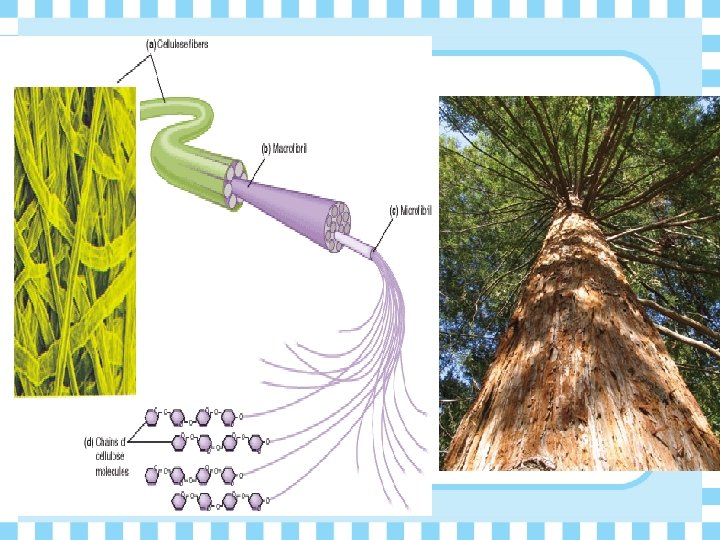
Ø Cellulose - fiber-like structural material made of glucose monomers • used in plant cell walls
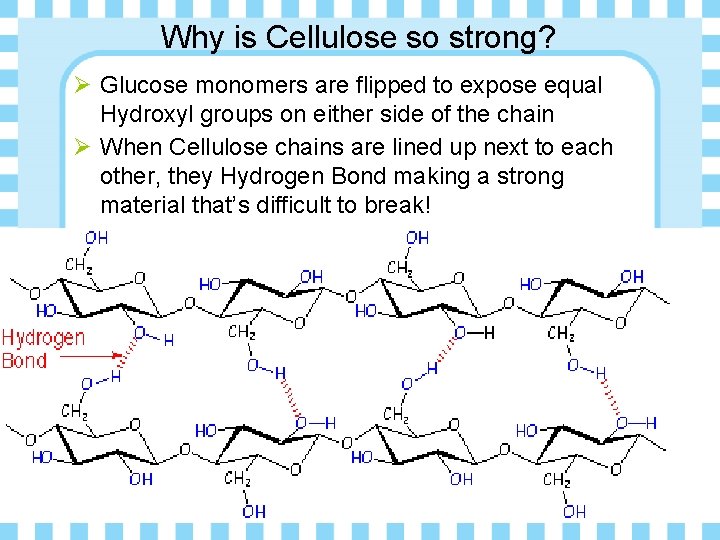
Why is Cellulose so strong? Ø Glucose monomers are flipped to expose equal Hydroxyl groups on either side of the chain Ø When Cellulose chains are lined up next to each other, they Hydrogen Bond making a strong material that’s difficult to break!
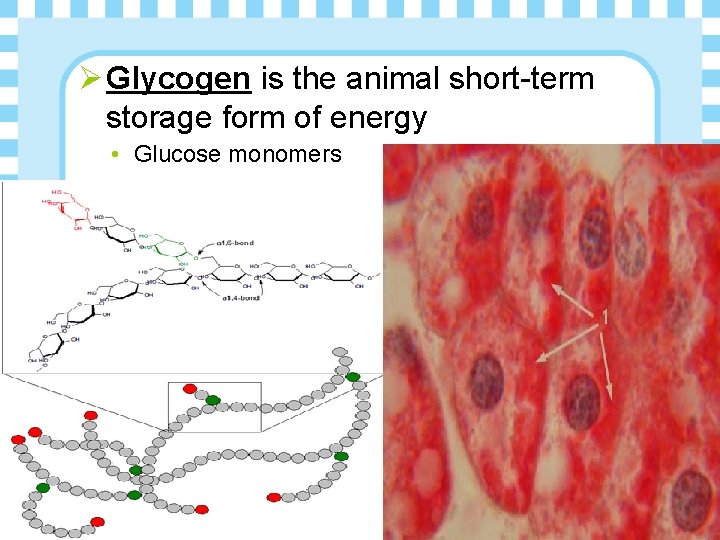
Ø Glycogen is the animal short-term storage form of energy • Glucose monomers

Penguins Ø What reaction breaks the glycosidic linkage (bond) between the glucose molecules in glycogen so the monomers can be used for fuel? • Hydrolysis
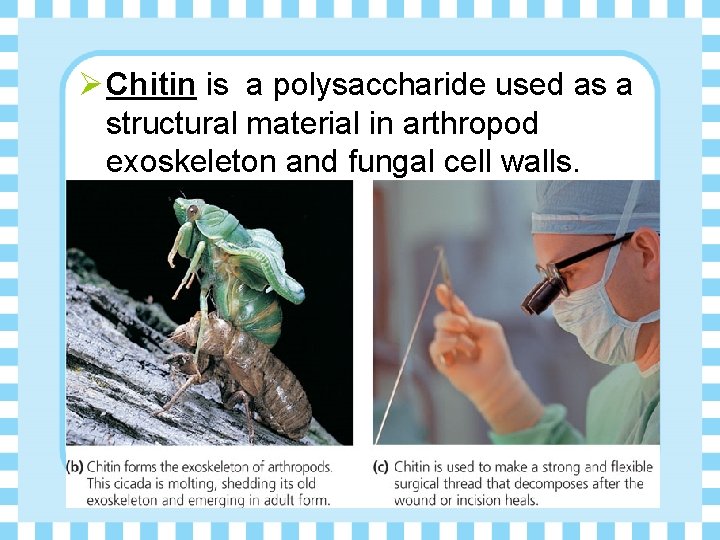
Ø Chitin is a polysaccharide used as a structural material in arthropod exoskeleton and fungal cell walls.

Draw a Carbohydrate Ø Draw a polysaccharide sugar. Ø Be sure to draw water molecules leaving the bond to represent condensation reaction.
Standards ØDistinguish among proteins, carbohydrates, lipids, and nucleic acids. Objectives ØIdentify the major structural components and functions of the four major macromolecules
- Slides: 44