Macromolecules Inorganic Molecules Nonliving matter Water Salt Organic





- Slides: 16

Macromolecules

• Inorganic Molecules – Non-living matter • Water • Salt • Organic Molecules – Always contain carbon make-up of carbon allows it to covalently bend with four other atoms

Organization of molecules of Life • Monomer – Smaller molecules • Polymer – Macromolecule ---chain of monomers Monomers Polymers Monosaccharide Polysaccharide Amino acids Protein Nucleotide

Building and Breaking Macromolecules • Synthesis – Combine and produce larger molecules and water • Hydrolysis – Uses water and breaks down into smaller molecules.

Four classes of the molecules of Life • Carbohydrate – Contain C H O C 6 H 12 O 6 – Simple – Monosaccharides Glucose blood Fructose Fruit Galactose milk

• Complex Disaccharide Sucrose table sugar Maltose Polysaccharide Starch storage of glucose in plants Glycogen storage of glucose in animals Cellulose makes up cell walls of plants – indigestible for humans, fiber or roughage

• Lipids – – – C 3 H 28 O 6 Contains C H O Do not dissolve in water Long term energy storage Insulation Protective layer around organs

• Fats – Animal origin solid at room temp • Oils – Plant origin liquid room temp. • Glycerol & 3 fatty-acids – Fat molecule and water

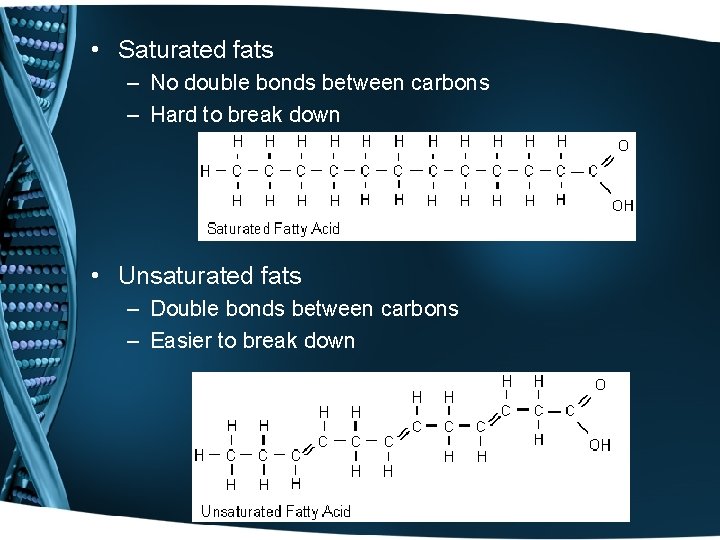
• Saturated fats – No double bonds between carbons – Hard to break down • Unsaturated fats – Double bonds between carbons – Easier to break down

• Emulsification – Adding emulsifiers to fats to allow them to mix with water – Bile produced by the gall bladder aids in the emulsification of fats in the stomach • Phospholipids – Two fatty-acids and a phosphate group – Make up cell membranes • Steroids – Differ in structure from fats – Backbone of four fused carbon rings & different arrangement of ring & attached functional groups determines type • Cholesterol • Estrogen • Testosterone

• Proteins – C H O N C 14 H 16 O 10 N 2 – Amino Acids • The building blocks of life • monomers • 20 different amino acids • Proteins are polymers of amino acids • Peptide bonds join amino acids to form proteins • Build structures in the body – Hair, nails, muscles, bones

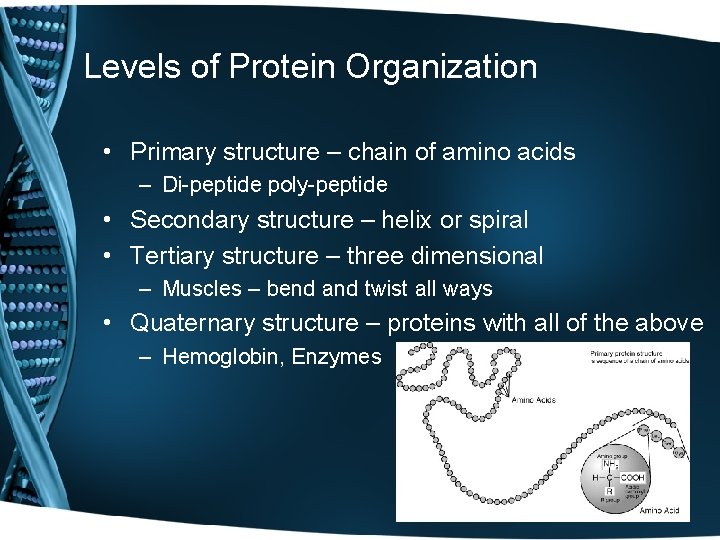
Levels of Protein Organization • Primary structure – chain of amino acids – Di-peptide poly-peptide • Secondary structure – helix or spiral • Tertiary structure – three dimensional – Muscles – bend and twist all ways • Quaternary structure – proteins with all of the above – Hemoglobin, Enzymes

• Final shape of the protein is important in determining it function • Exposing proteins to extremes in heat and p. H can permanently change their shape – Acid + egg white = curdling • Denaturation – Heat + egg white = coagulation • Protein can no longer perform its normal function

• Nucleic Acids – CHOPN – DNA – Deoxyribonucleic Acid • Genes – store info to make proteins • Structure – Double strand • Bases – Adenine, cytosine, thymine, guanine – RNA – Ribonucleic Acid • Takes into from DNA & helps make protein • Structure – Single strand • Bases – Adenine, cytosine, uracil, guanine

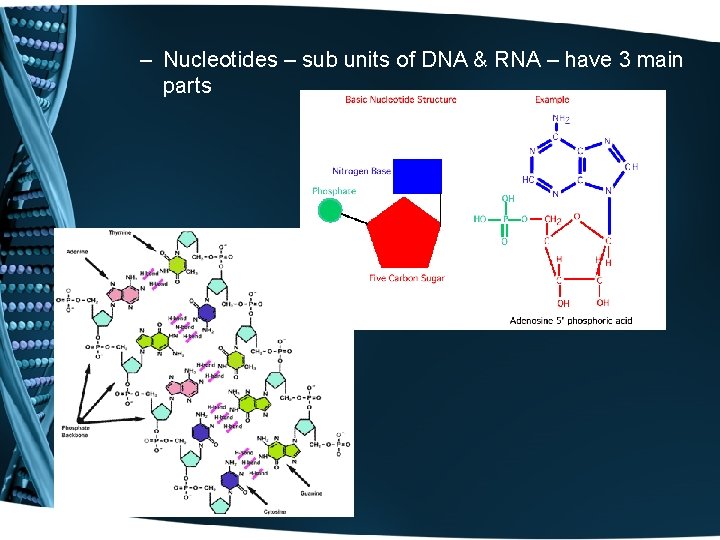
– Nucleotides – sub units of DNA & RNA – have 3 main parts

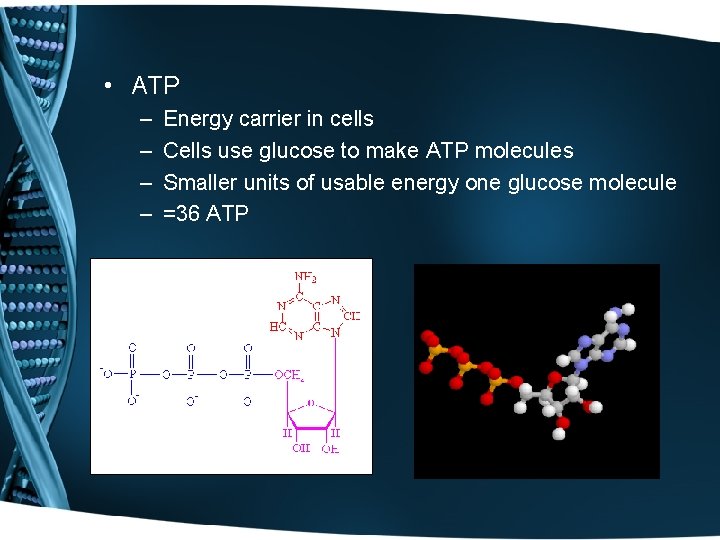
• ATP – – Energy carrier in cells Cells use glucose to make ATP molecules Smaller units of usable energy one glucose molecule =36 ATP