Macromolecules in Biology also known as biomolecules Chapter

Macromolecules in Biology (also known as biomolecules) Chapter 3
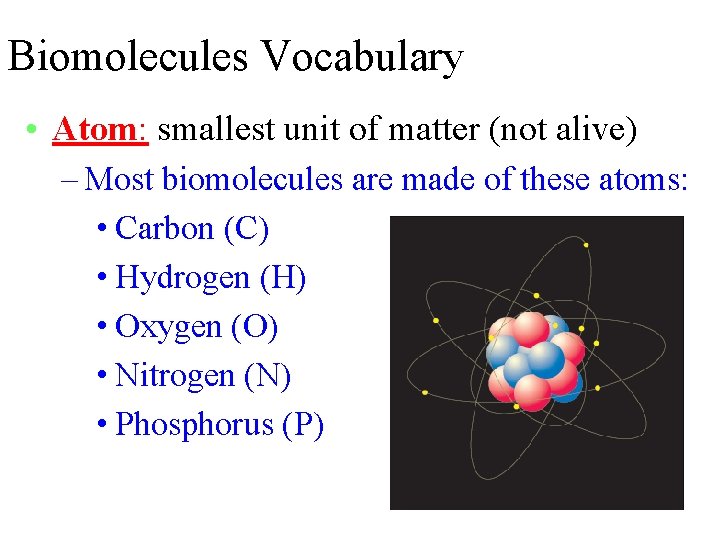
Biomolecules Vocabulary • Atom: smallest unit of matter (not alive) – Most biomolecules are made of these atoms: • Carbon (C) • Hydrogen (H) • Oxygen (O) • Nitrogen (N) • Phosphorus (P)
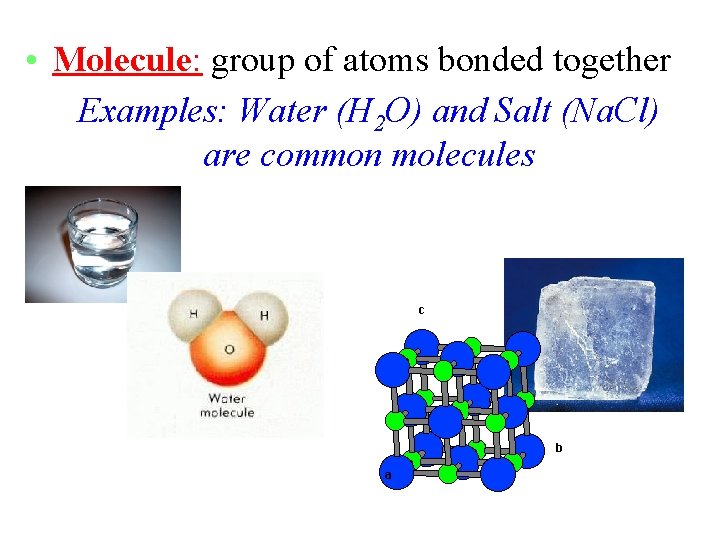
• Molecule: group of atoms bonded together Examples: Water (H 2 O) and Salt (Na. Cl) are common molecules
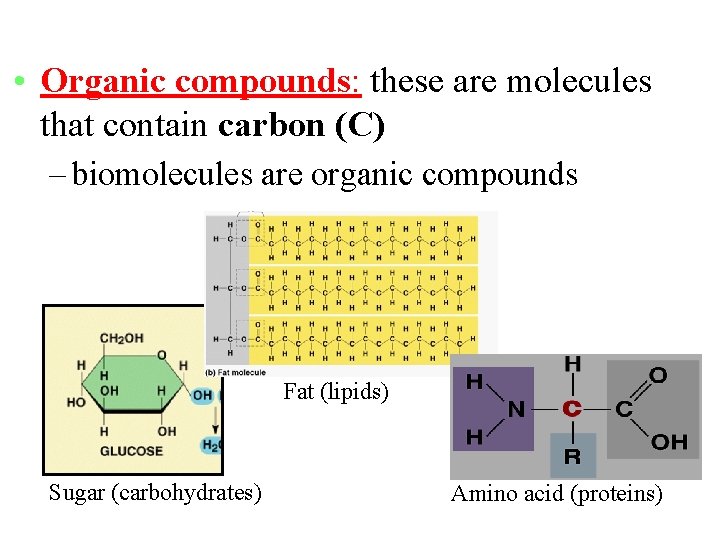
• Organic compounds: these are molecules that contain carbon (C) – biomolecules are organic compounds Fat (lipids) Sugar (carbohydrates) Amino acid (proteins)

• Monomer: simple molecules that link up to form a bigger compound (polymer) (It is kind of like one link in a chain. )
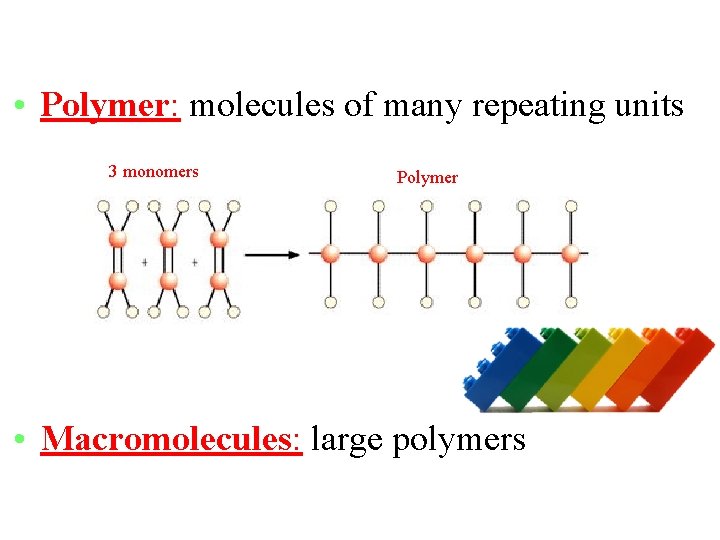
• Polymer: molecules of many repeating units 3 monomers Polymer • Macromolecules: large polymers

• Macromolecules: organic molecules needed for life – Proteins – Nucleic Acids (DNA and RNA) – Carbohydrates (sugars) – Lipids (Fats)
• Function: Used by all cells for quick energy and used by plants for structure. • Structure: Includes all sugars, from simple sugars to complex polymer sugars • Elements: Carbon, Hydrogen, Oxygen (CHO) • Energy is stored in the bonds between carbon, hydrogen, and oxygen
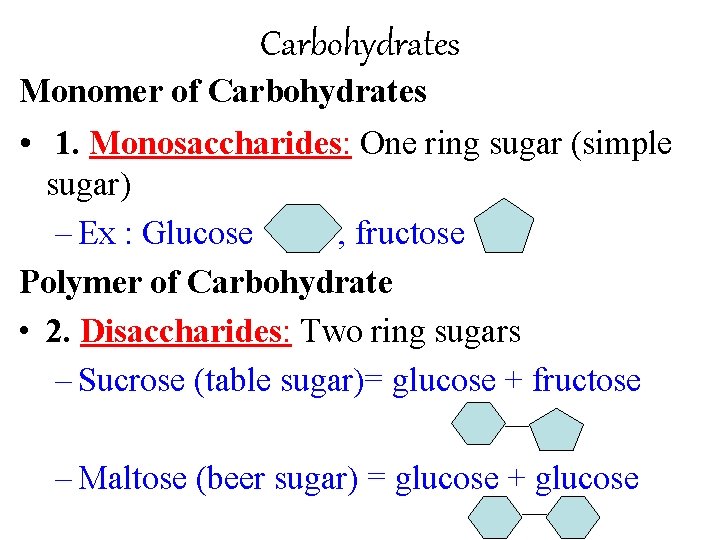
Carbohydrates Monomer of Carbohydrates • 1. Monosaccharides: One ring sugar (simple sugar) – Ex : Glucose , fructose Polymer of Carbohydrate • 2. Disaccharides: Two ring sugars – Sucrose (table sugar)= glucose + fructose – Maltose (beer sugar) = glucose + glucose
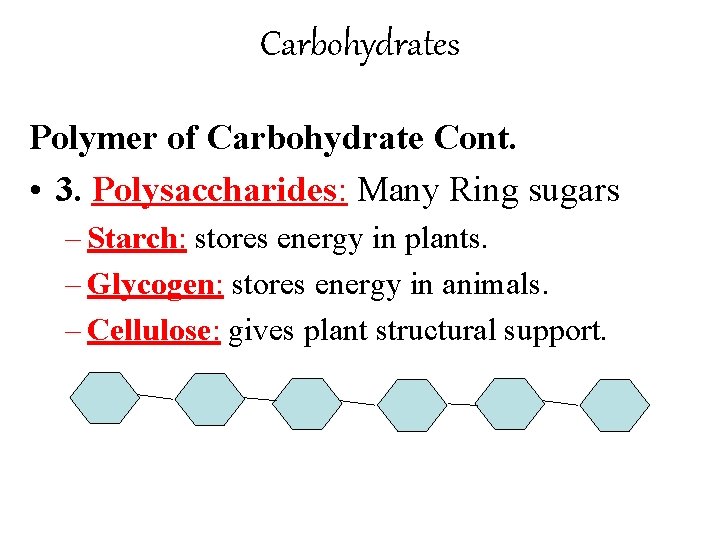
Carbohydrates Polymer of Carbohydrate Cont. • 3. Polysaccharides: Many Ring sugars – Starch: stores energy in plants. – Glycogen: stores energy in animals. – Cellulose: gives plant structural support.
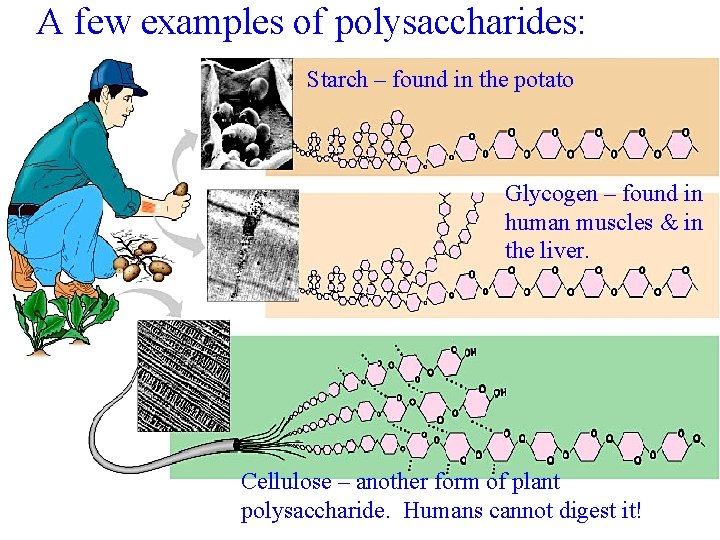
A few examples of polysaccharides: Starch – found in the potato Glycogen – found in human muscles & in the liver. Cellulose – another form of plant polysaccharide. Humans cannot digest it!
Cellulose = dietary fiber. Helps “scrub” out your digestive tract.

Pair Share • What type of carbohydrate is the single-ring sugar called glucose: – Monosaccharide? – Disaccharide? – Polysaccharide?
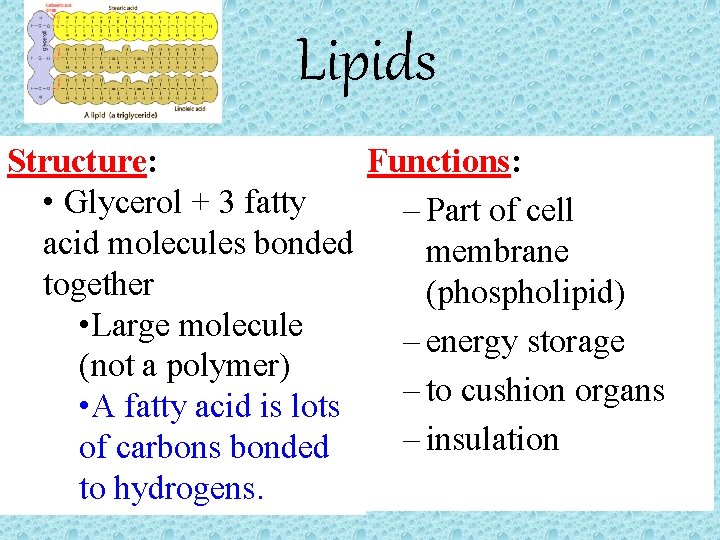
Lipids Structure: Functions: • Glycerol + 3 fatty – Part of cell acid molecules bonded membrane together (phospholipid) • Large molecule – energy storage (not a polymer) – to cushion organs • A fatty acid is lots – insulation of carbons bonded to hydrogens.
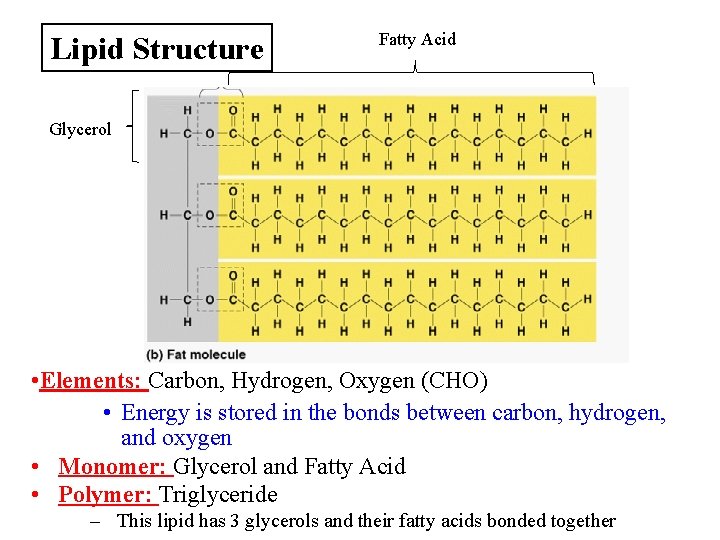
Lipid Structure Fatty Acid Glycerol • Elements: Carbon, Hydrogen, Oxygen (CHO) • Energy is stored in the bonds between carbon, hydrogen, and oxygen • Monomer: Glycerol and Fatty Acid • Polymer: Triglyceride – This lipid has 3 glycerols and their fatty acids bonded together
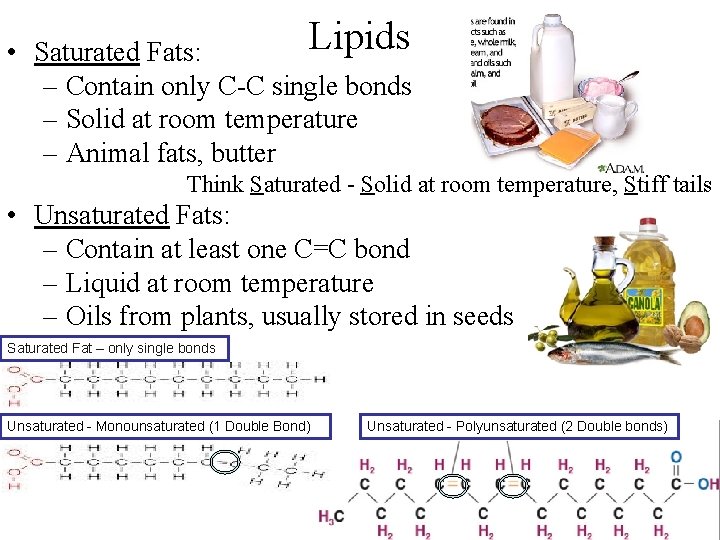
Lipids • Saturated Fats: – Contain only C-C single bonds – Solid at room temperature – Animal fats, butter Think Saturated - Solid at room temperature, Stiff tails • Unsaturated Fats: – Contain at least one C=C bond – Liquid at room temperature – Oils from plants, usually stored in seeds Saturated Fat – only single bonds Unsaturated - Monounsaturated (1 Double Bond) Unsaturated - Polyunsaturated (2 Double bonds)

Pair Share • What is the most important function of a lipid?
Proteins Structure: A chain of amino acids – also called polypeptides. – different proteins have different sequences and amounts of amino acids • Functions: – Creates skin, muscle, hair, teeth, bone & other body structures – Help transport materials from cell to cell – Allows cells to signal each other – Used in immune system defense – Enzymes – Last form of energy storage •
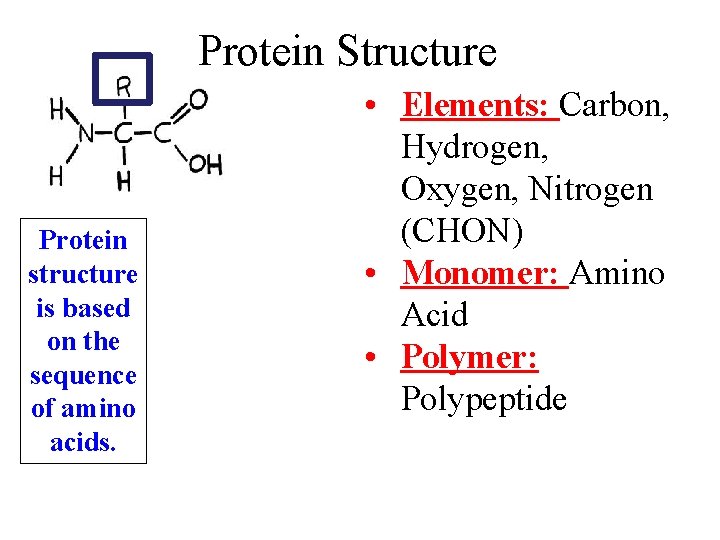
Protein Structure Protein structure is based on the sequence of amino acids. • Elements: Carbon, Hydrogen, Oxygen, Nitrogen (CHON) • Monomer: Amino Acid • Polymer: Polypeptide
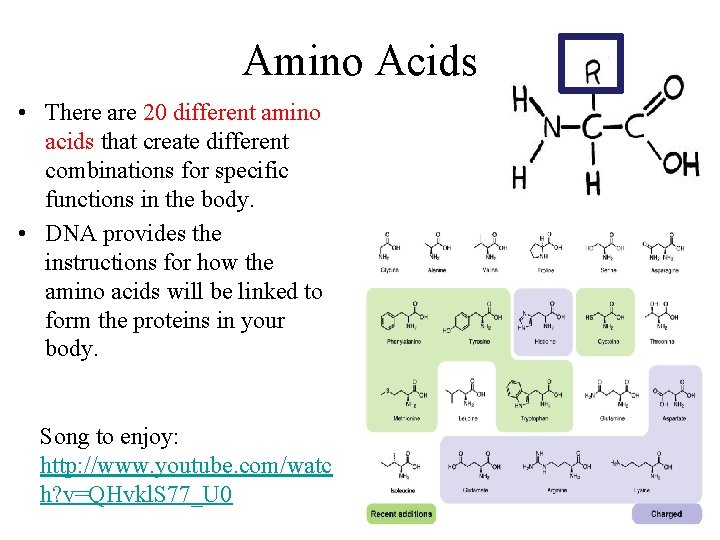
Amino Acids • There are 20 different amino acids that create different combinations for specific functions in the body. • DNA provides the instructions for how the amino acids will be linked to form the proteins in your body. Song to enjoy: http: //www. youtube. com/watc h? v=QHvkl. S 77_U 0
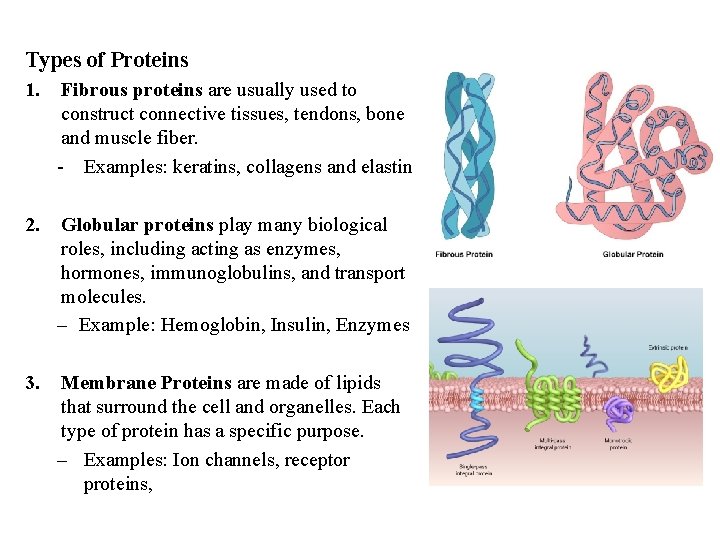
Types of Proteins 1. Fibrous proteins are usually used to construct connective tissues, tendons, bone and muscle fiber. - Examples: keratins, collagens and elastin 2. Globular proteins play many biological roles, including acting as enzymes, hormones, immunoglobulins, and transport molecules. – Example: Hemoglobin, Insulin, Enzymes 3. Membrane Proteins are made of lipids that surround the cell and organelles. Each type of protein has a specific purpose. – Examples: Ion channels, receptor proteins,
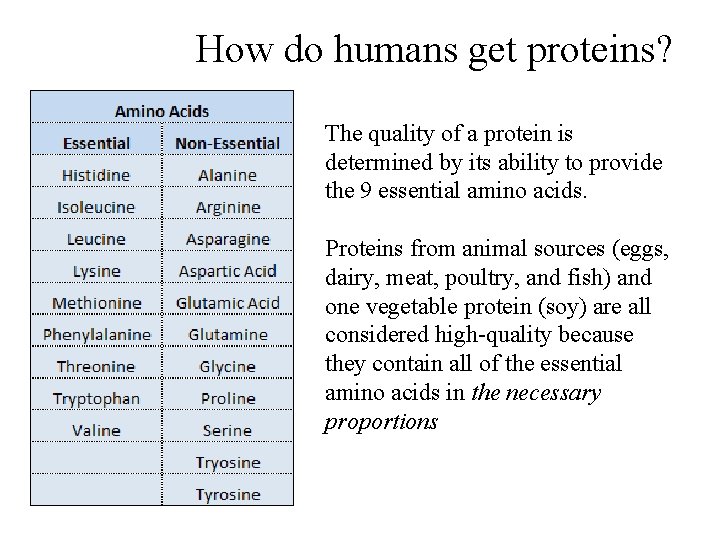
How do humans get proteins? The quality of a protein is determined by its ability to provide the 9 essential amino acids. Proteins from animal sources (eggs, dairy, meat, poultry, and fish) and one vegetable protein (soy) are all considered high-quality because they contain all of the essential amino acids in the necessary proportions

Pair Share • Why is a protein an example of a macromolecule?
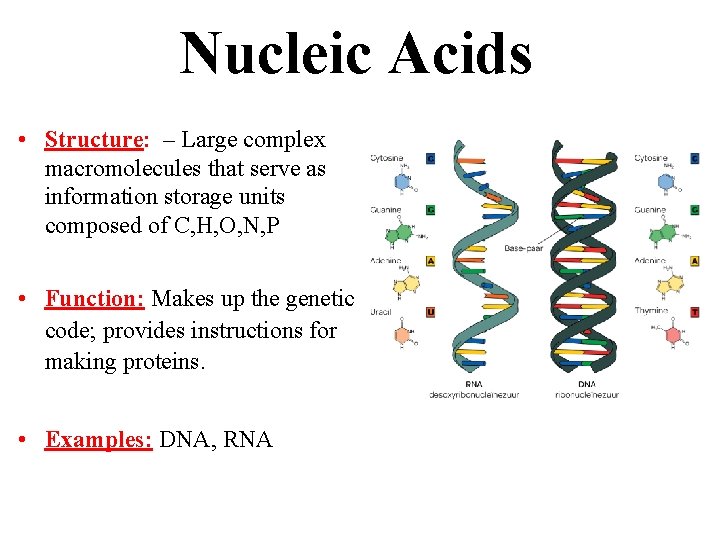
Nucleic Acids • Structure: – Large complex macromolecules that serve as information storage units composed of C, H, O, N, P • Function: Makes up the genetic code; provides instructions for making proteins. • Examples: DNA, RNA
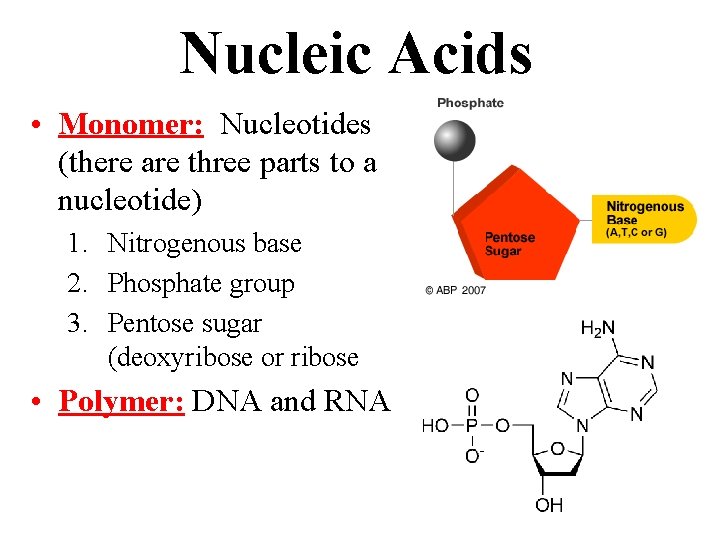
Nucleic Acids • Monomer: Nucleotides (there are three parts to a nucleotide) 1. Nitrogenous base 2. Phosphate group 3. Pentose sugar (deoxyribose or ribose • Polymer: DNA and RNA
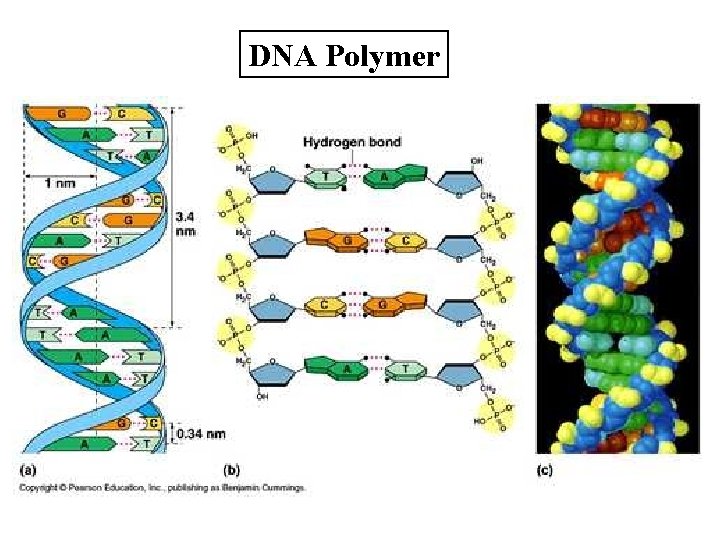
DNA Polymer
Pair Share • Why are DNA and RNA examples of macromolecules?

Pair Share • In your own words, define macromolecule.
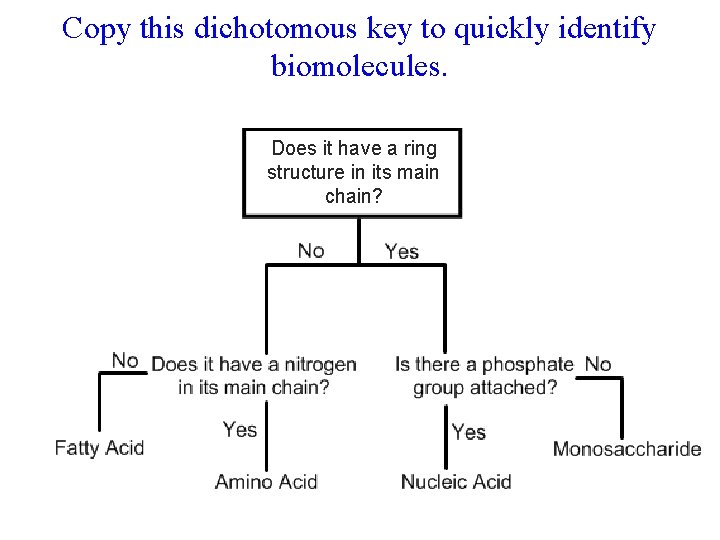
Copy this dichotomous key to quickly identify biomolecules. Does it have a ring structure in its main chain?

Biomolecule Crash Course Video (14 min. ) • http: //www. youtube. com/watch? v=H 8 WJ 2 KENl. K 0
- Slides: 31