Macromolecules Carbohydrates Proteins Lipids Nucleic acids All macromolecules
Macromolecules • Carbohydrates • Proteins • Lipids • Nucleic acids
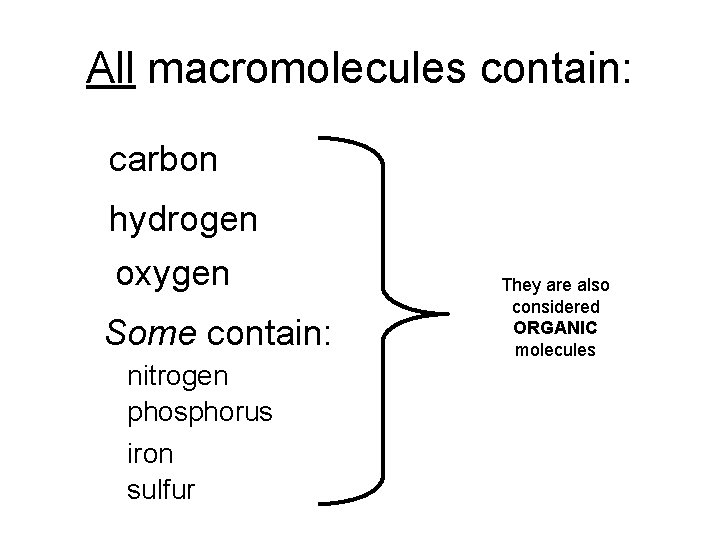
All macromolecules contain: carbon hydrogen oxygen Some contain: nitrogen phosphorus iron sulfur They are also considered ORGANIC molecules
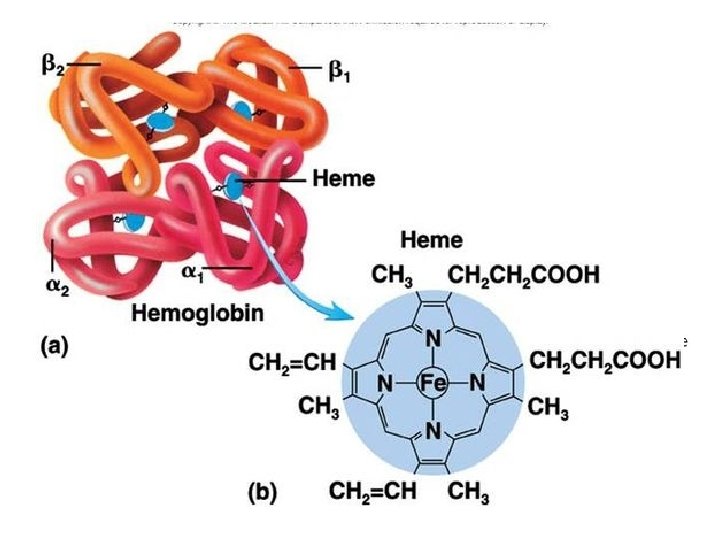
Where would you find iron in a macromolecule?
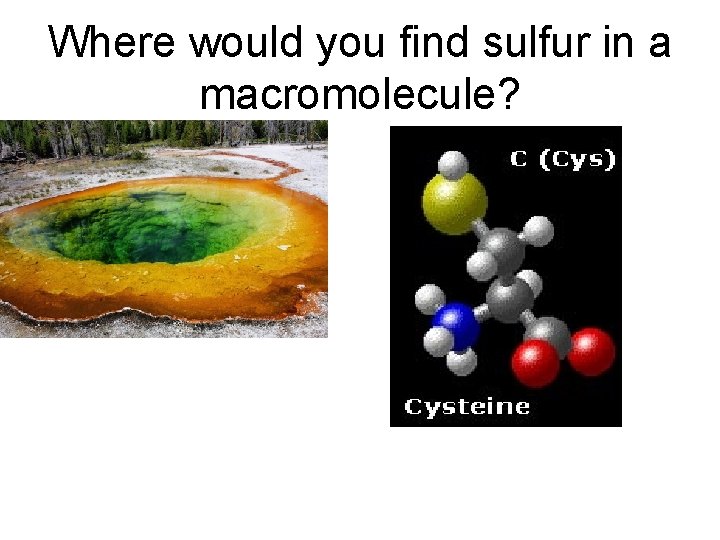
Where would you find sulfur in a macromolecule?
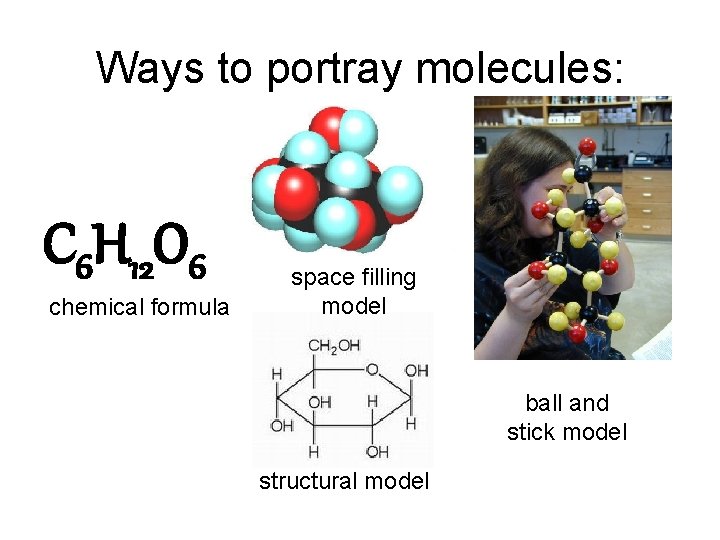
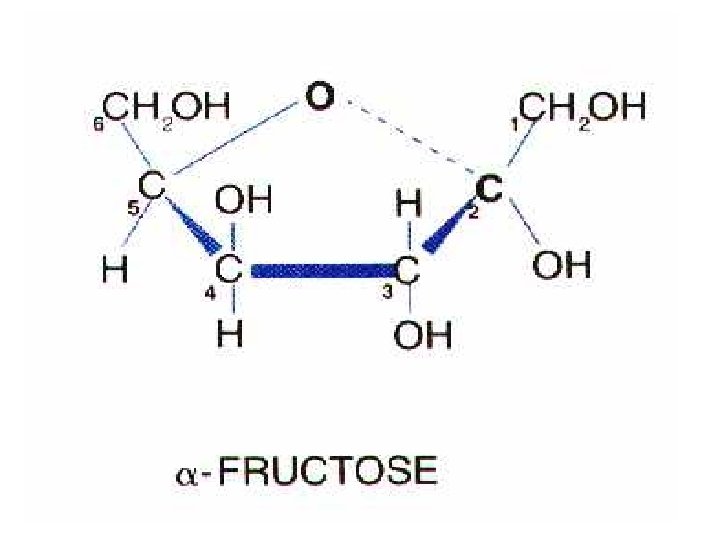
Ways to portray molecules: C 6 H 12 O 6 chemical formula space filling model ball and stick model structural model

carbohydrates
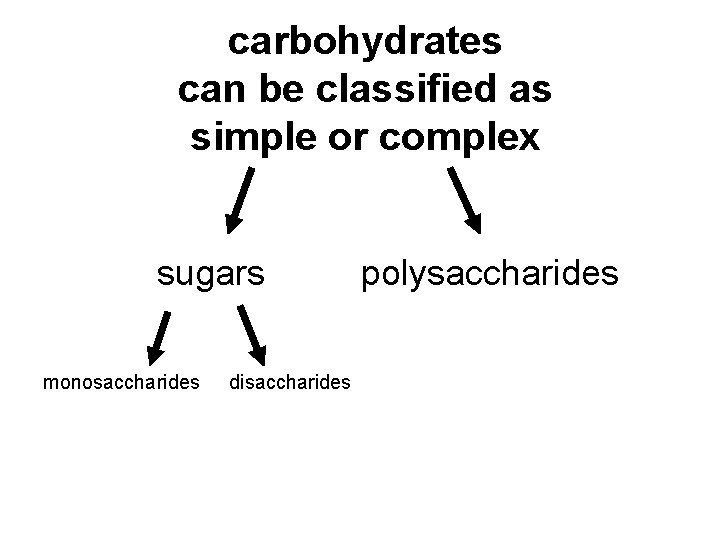
carbohydrates can be classified as simple or complex sugars monosaccharides disaccharides polysaccharides

Carbohydrates are only composed of: carbon, hydrogen, and oxygen…right? You are right! There is one exception
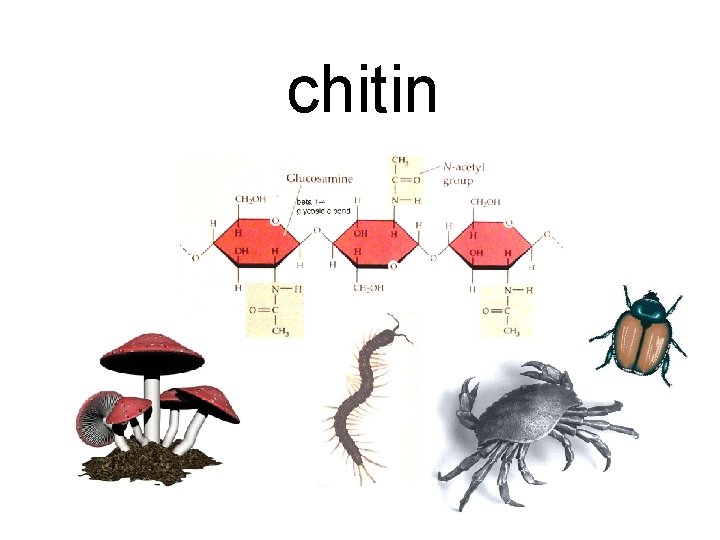
chitin
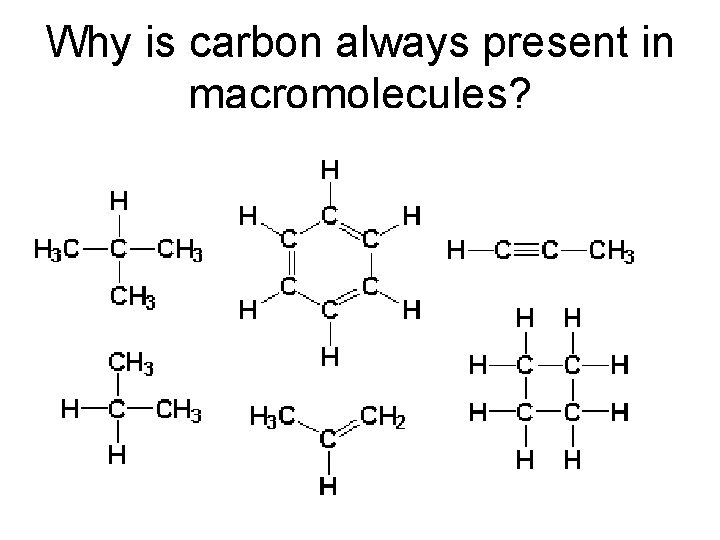
Why is carbon always present in macromolecules?
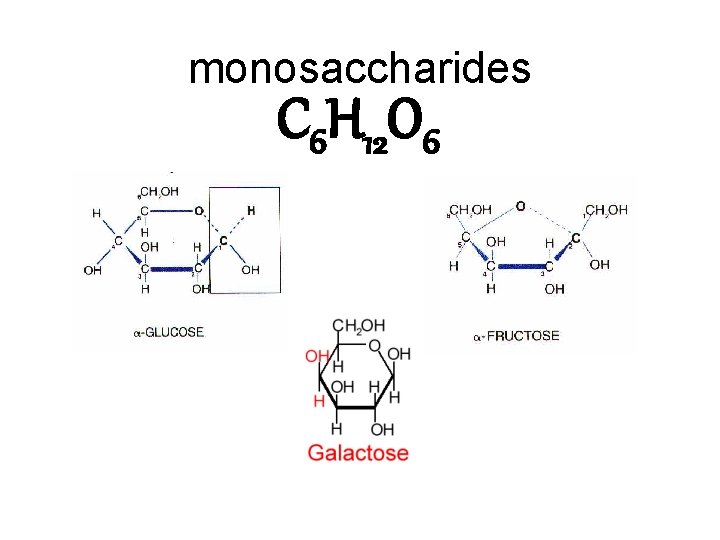
monosaccharides C 6 H 12 O 6
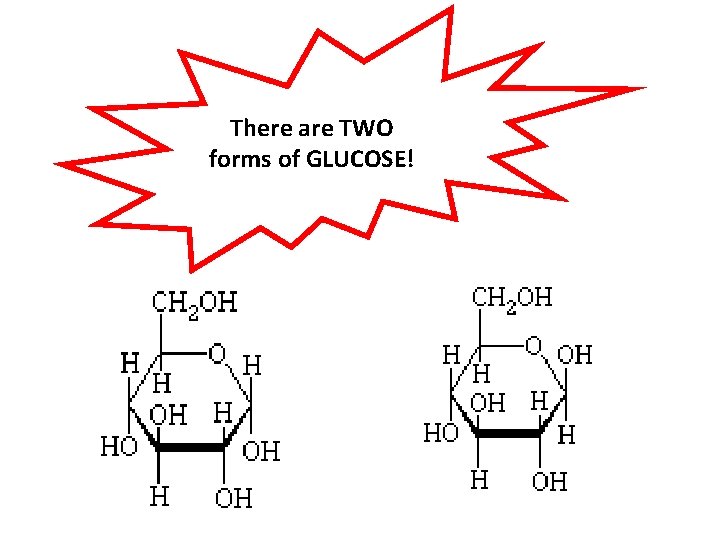
There are TWO forms of GLUCOSE!

Disaccharides include: lactose GALACTOSE + GLUCOSE maltose GLUCOSE + GLUCOSE sucrose GLUCOSE + FRUCTOSE
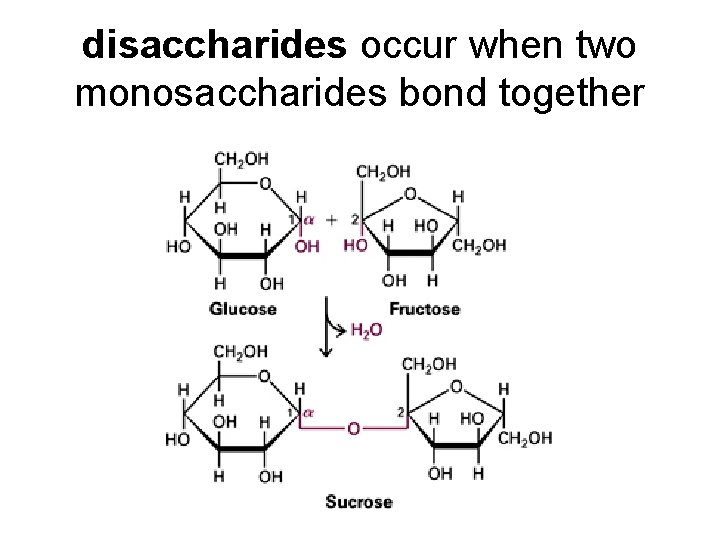
disaccharides occur when two monosaccharides bond together
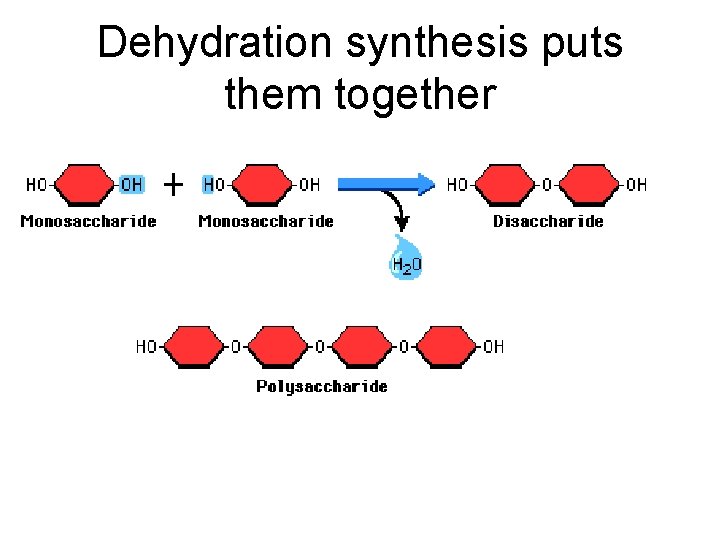
Dehydration synthesis puts them together

Hydrolysis takes them apart
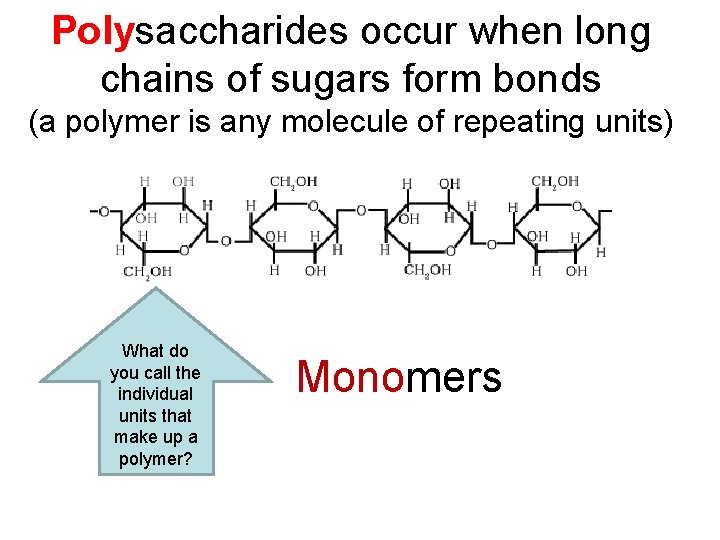
Polysaccharides occur when long chains of sugars form bonds (a polymer is any molecule of repeating units) What do you call the individual units that make up a polymer? Monomers

Polysaccharides are not just single lines, but numerous lines bonded together. What kind of bond holds these lines together? ?
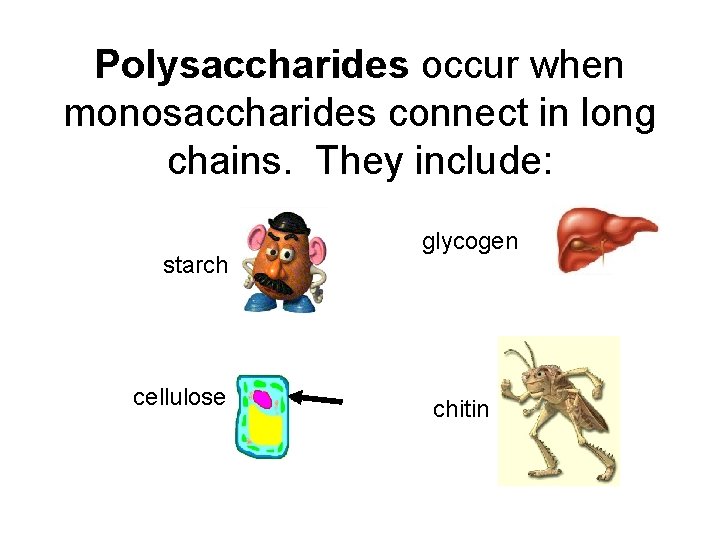
Polysaccharides occur when monosaccharides connect in long chains. They include: starch cellulose glycogen chitin

Their functions vary: starch Energy storage in plants cellulose Structural support in plants glycogen Energy storage in animals chitin Structural support in animals

Even though we eat a lot of it, we can’t digest cellulose Dietary fiber
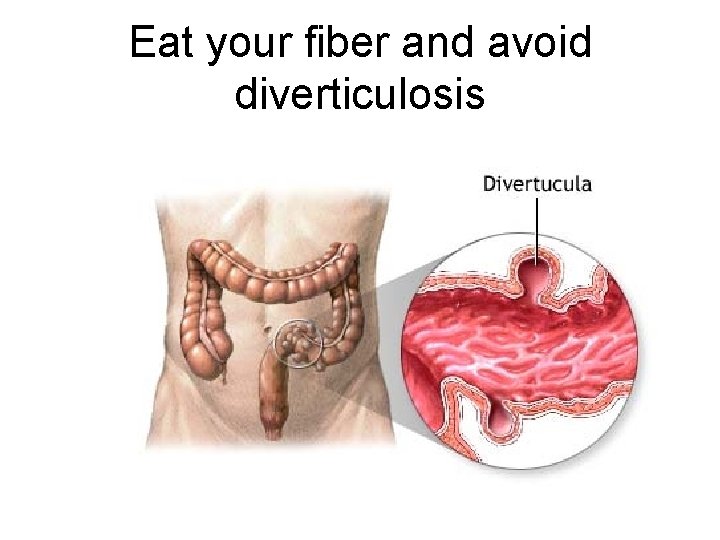
Eat your fiber and avoid diverticulosis

fats
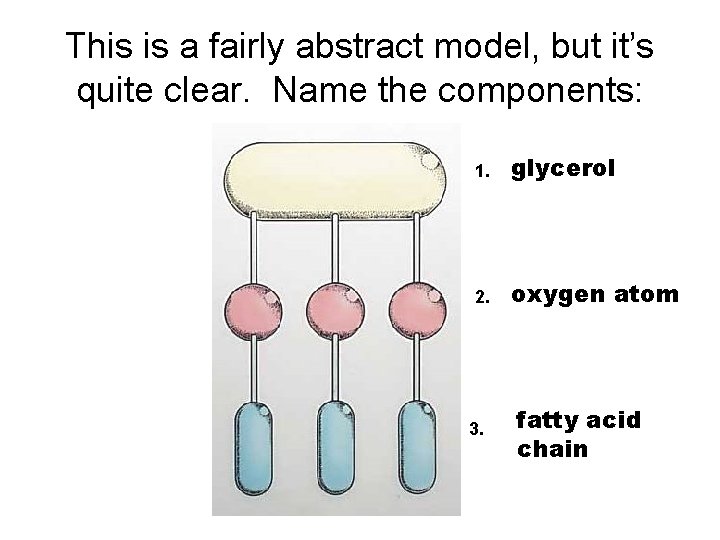
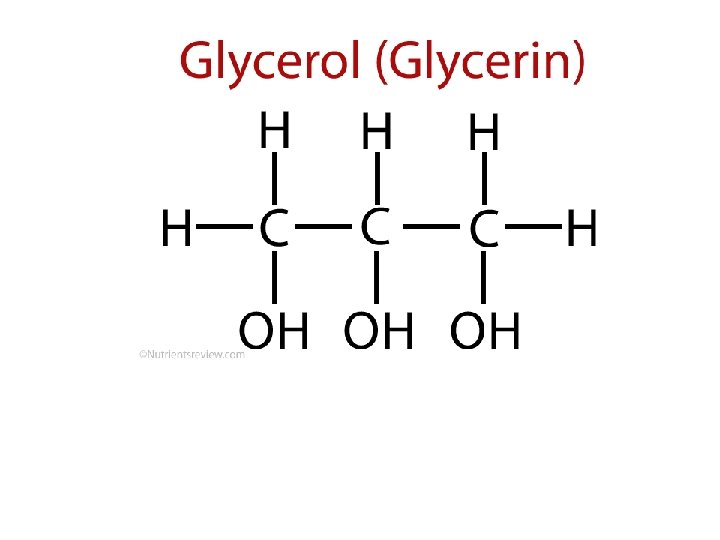
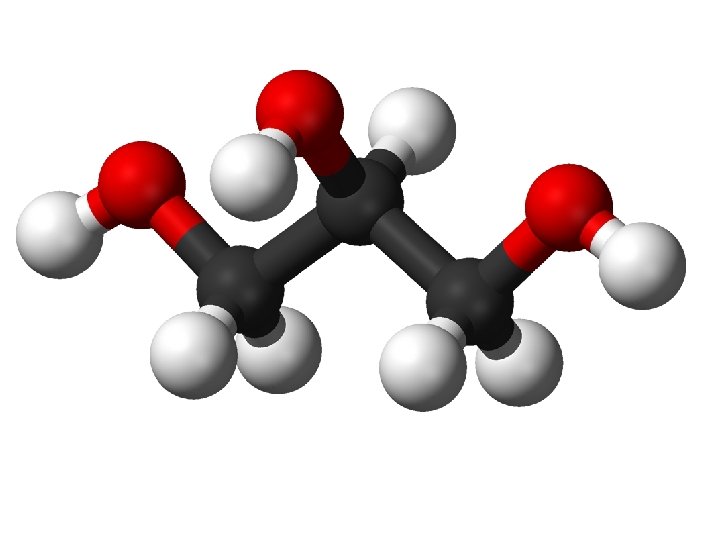
This is a fairly abstract model, but it’s quite clear. Name the components: 1. glycerol 2. oxygen atom 3. fatty acid chain
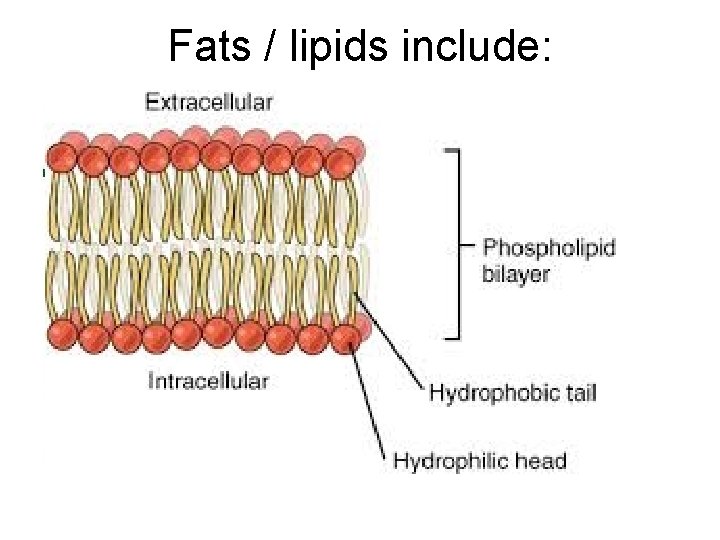
Fats / lipids include: Phospholipids
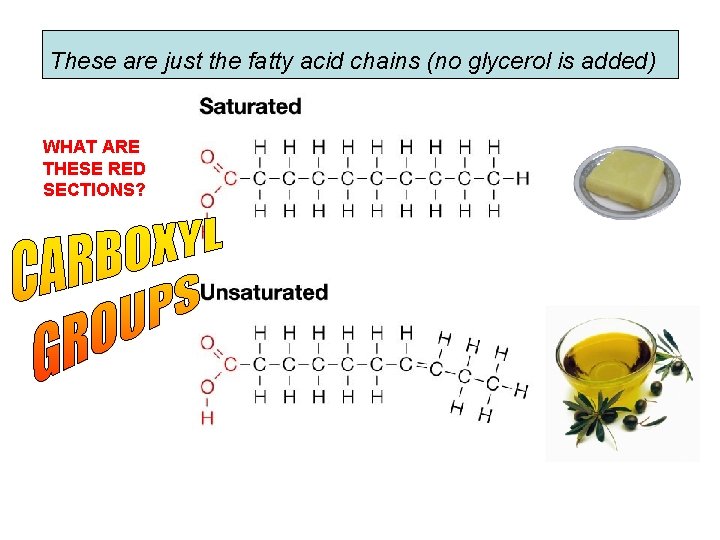
These are just the fatty acid chains (no glycerol is added) WHAT ARE THESE RED SECTIONS?
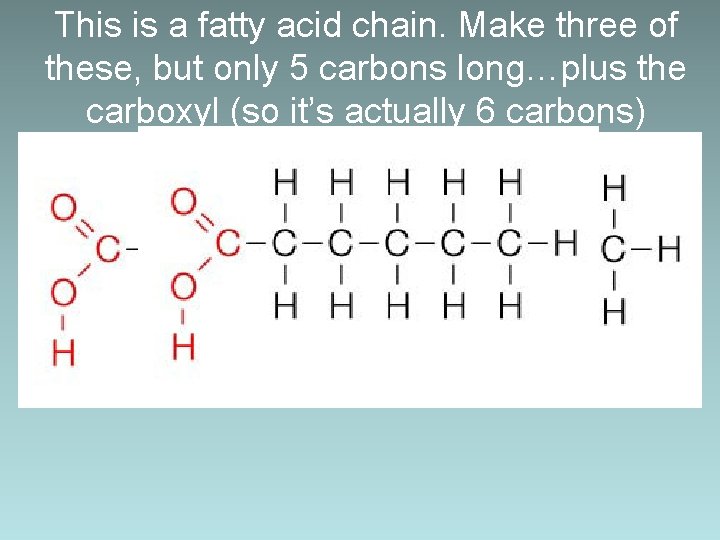
This is a fatty acid chain. Make three of these, but only 5 carbons long…plus the carboxyl (so it’s actually 6 carbons)

The advantage of lipids? ?
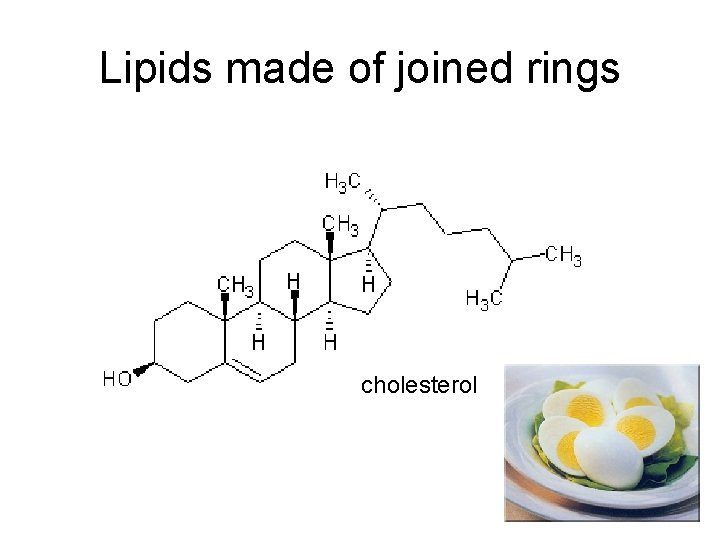
Lipids made of joined rings cholesterol
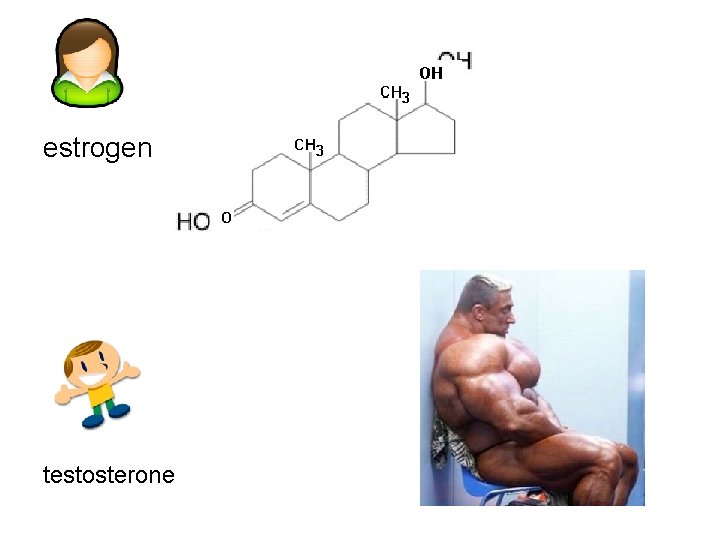
estrogen testosterone

proteins

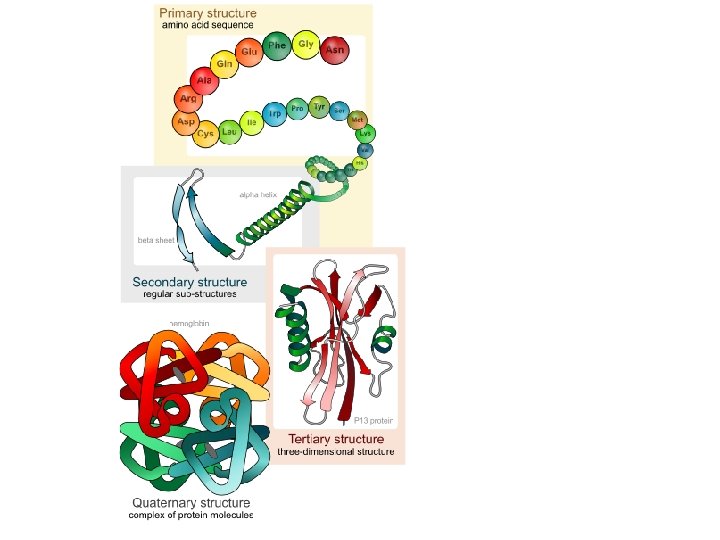
Protein Structure

Proteins are composed of a chain (polymer) of AMINO ? ACIDS. The chain can be a simple string of pearls This is the primary structure


Amino acids include:
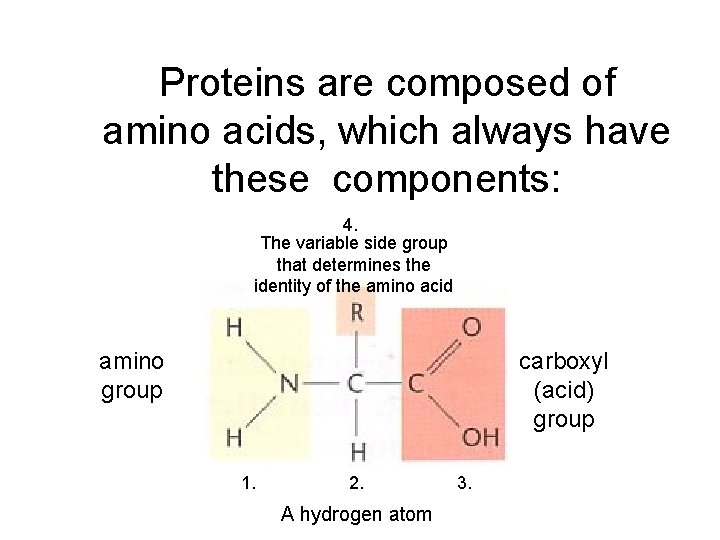
Proteins are composed of amino acids, which always have these components: 4. The variable side group that determines the identity of the amino acid amino group carboxyl (acid) group 1. 2. A hydrogen atom 3.
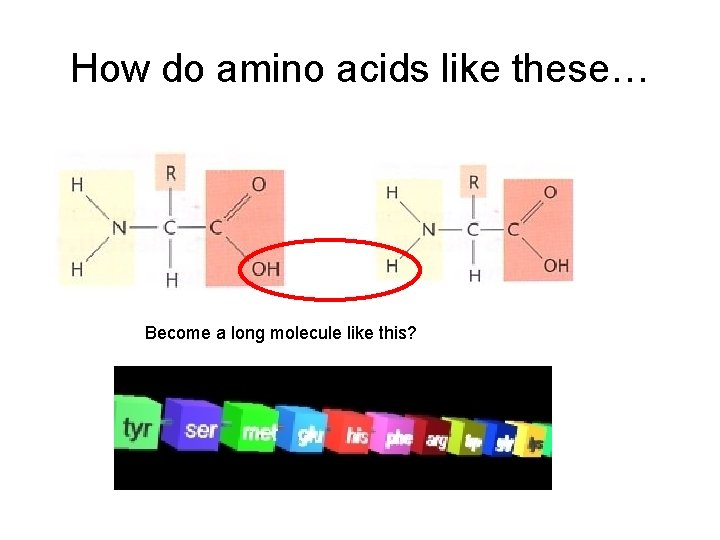
How do amino acids like these… Become a long molecule like this?
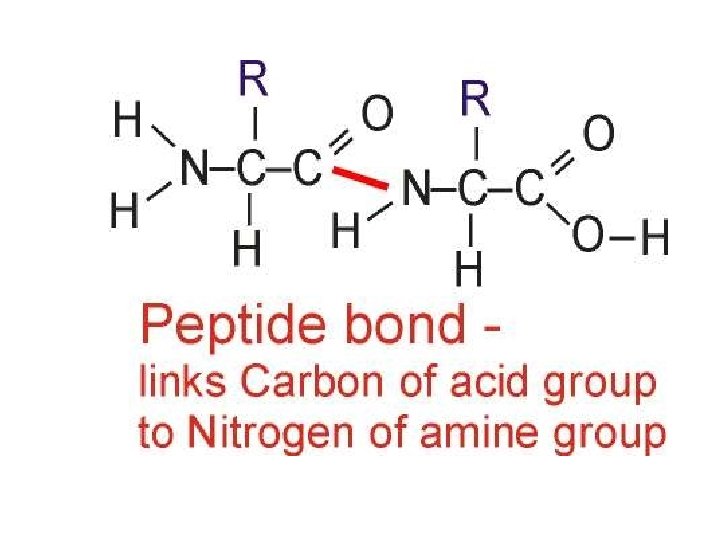
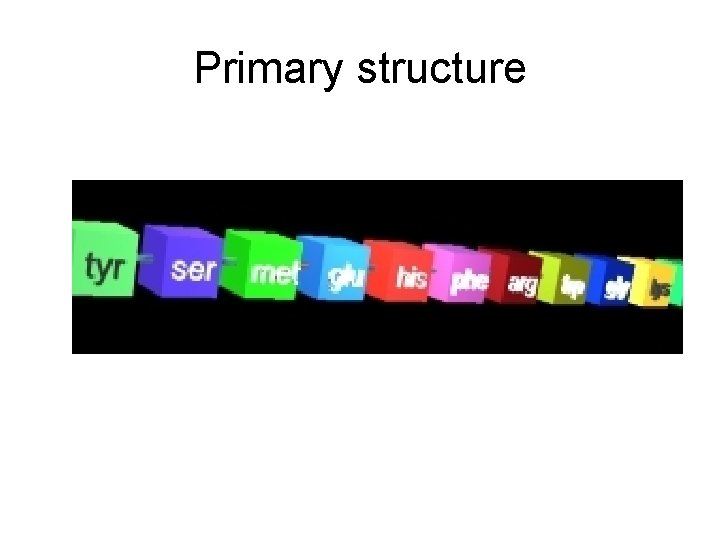
Primary structure

If the primary structure spirals, it’s now the secondary structure.

There are three types of secondary structure:

What’s the third one? ?
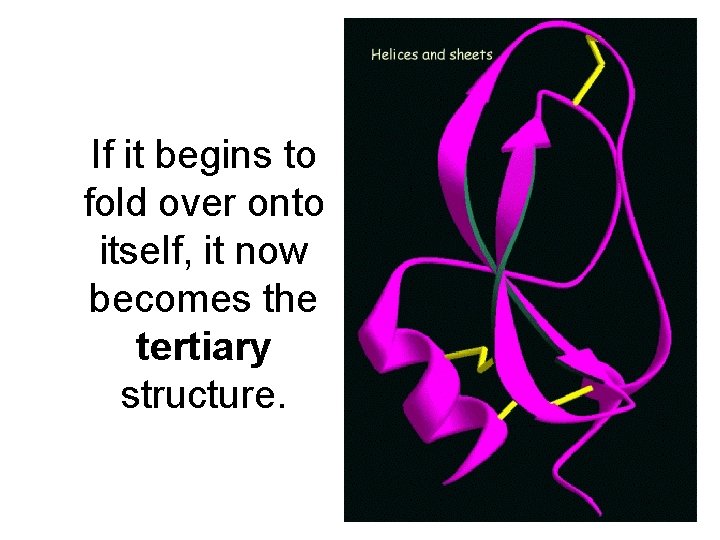
If it begins to fold over onto itself, it now becomes the tertiary structure.

If it incorporates one or more other proteins, it becomes the quaternary structure.

Sometimes it’s very confusing to look at I DON’T GET IT!

Types of Proteins
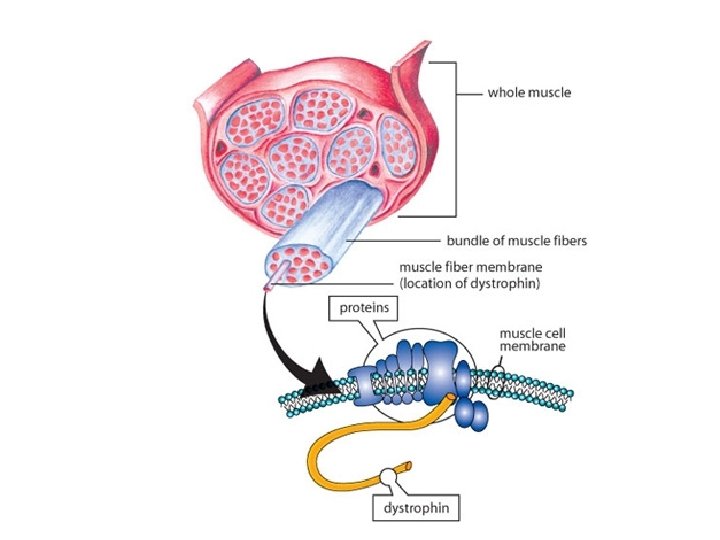
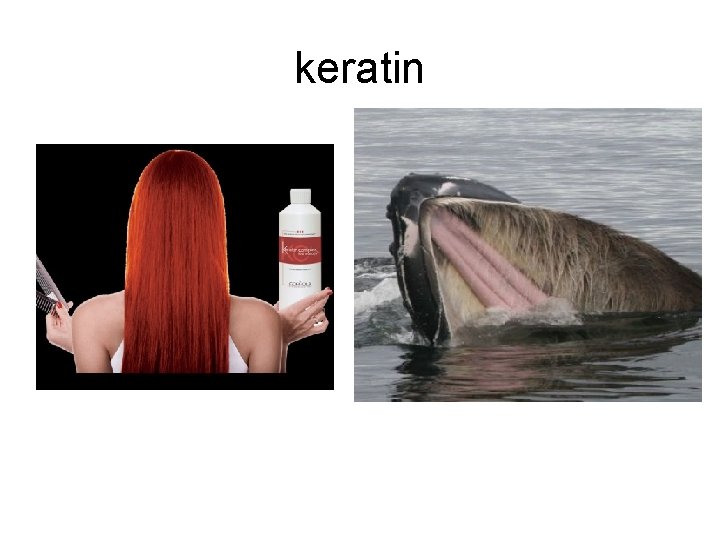
keratin
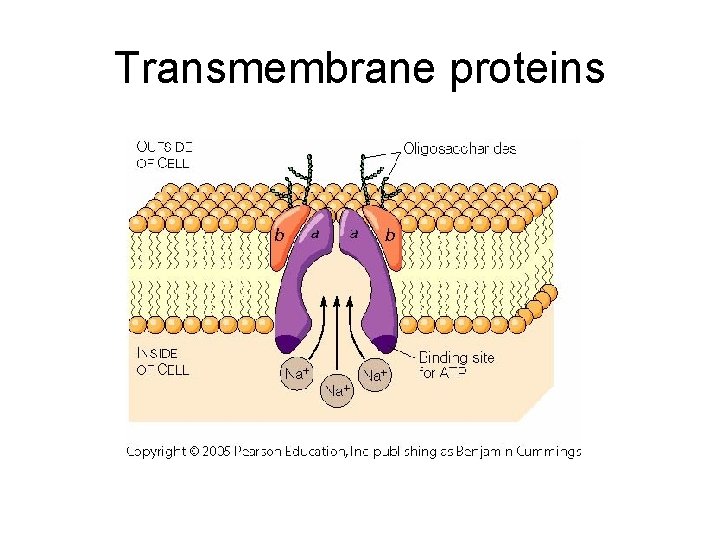
Transmembrane proteins
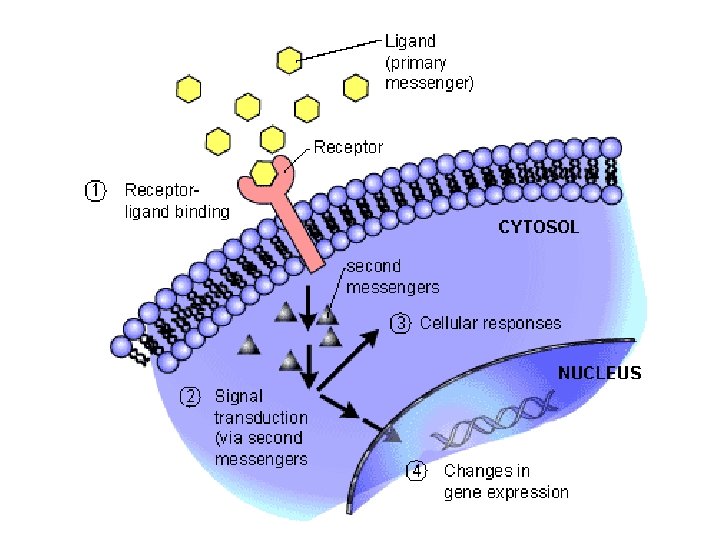
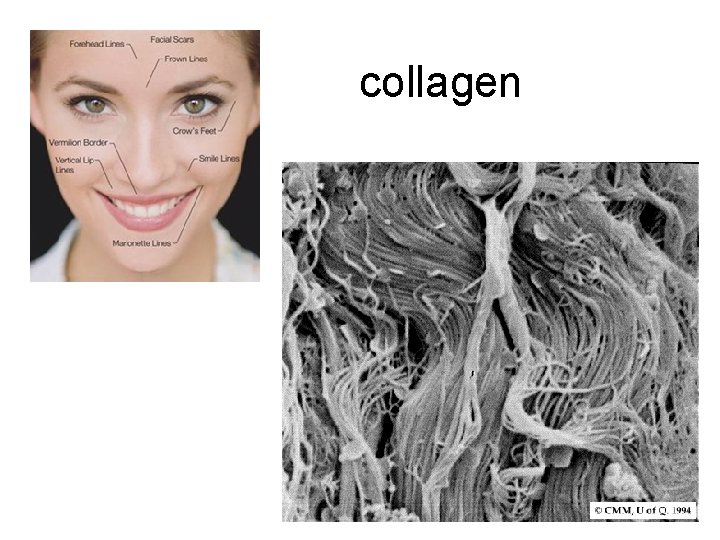
collagen
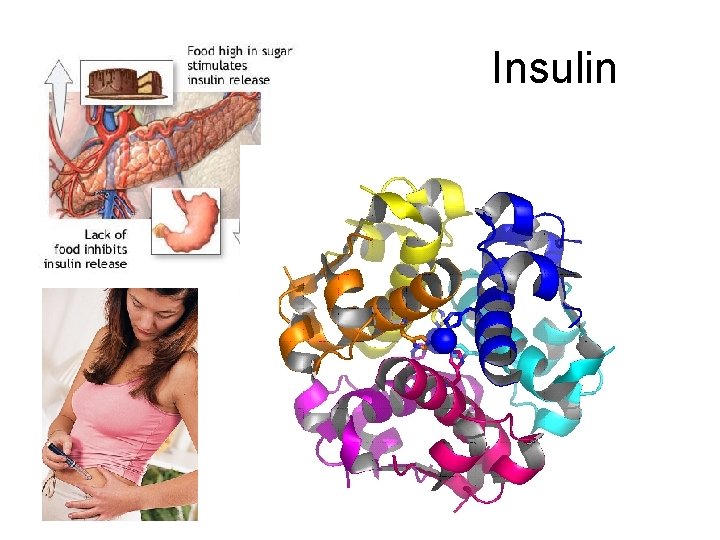
Insulin
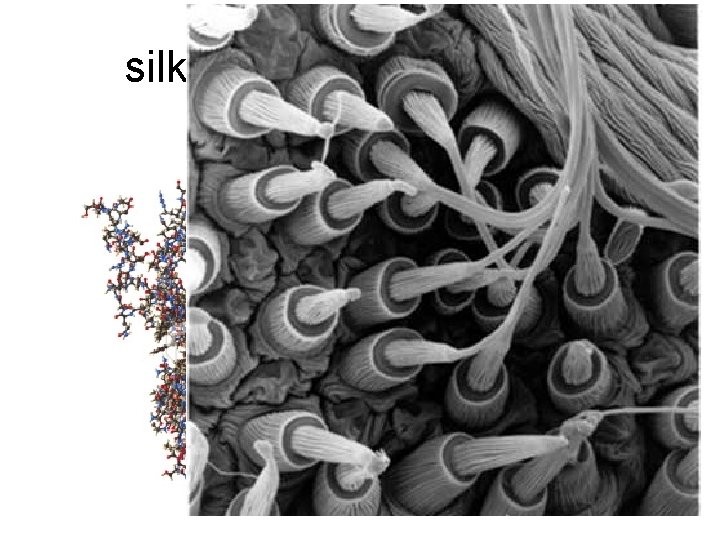
silk

Nucleic Acids
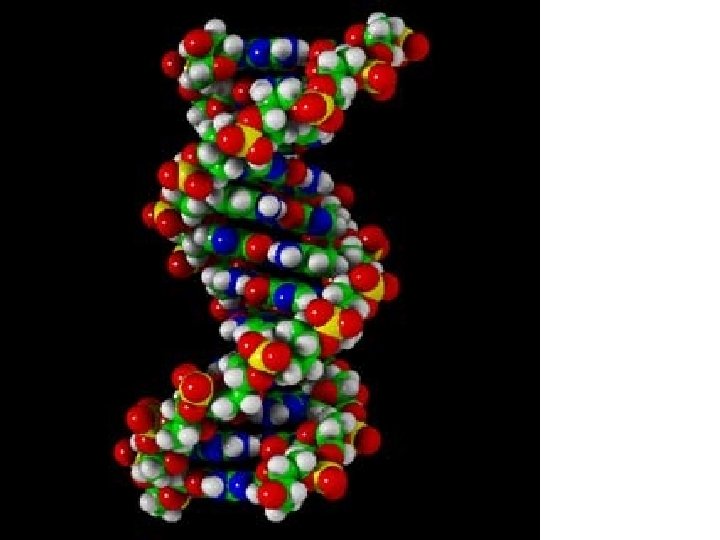

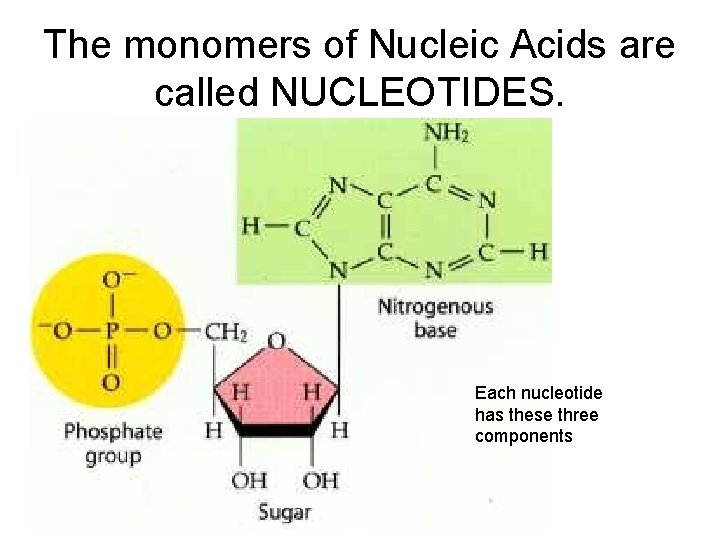
The monomers of Nucleic Acids are called NUCLEOTIDES. Each nucleotide has these three components


The rungs are composed of nitrogen base pairs

- Slides: 66