Macromolecules Building Complex Molecules That Comprise Living Things

Macromolecules Building Complex Molecules That Comprise Living Things
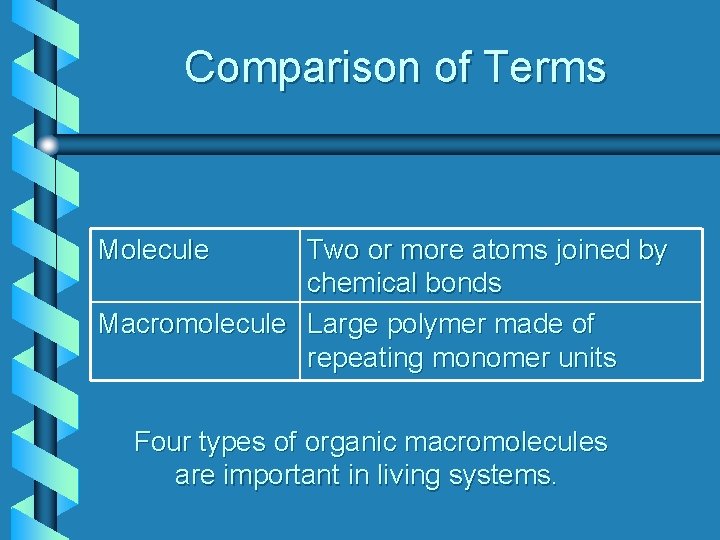
Comparison of Terms Molecule Two or more atoms joined by chemical bonds Macromolecule Large polymer made of repeating monomer units Four types of organic macromolecules are important in living systems.
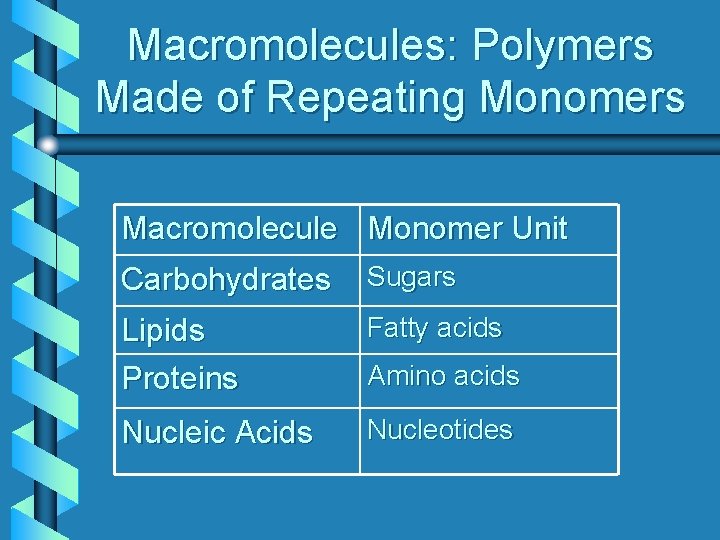
Macromolecules: Polymers Made of Repeating Monomers Macromolecule Monomer Unit Carbohydrates Sugars Lipids Proteins Fatty acids Nucleic Acids Nucleotides Amino acids
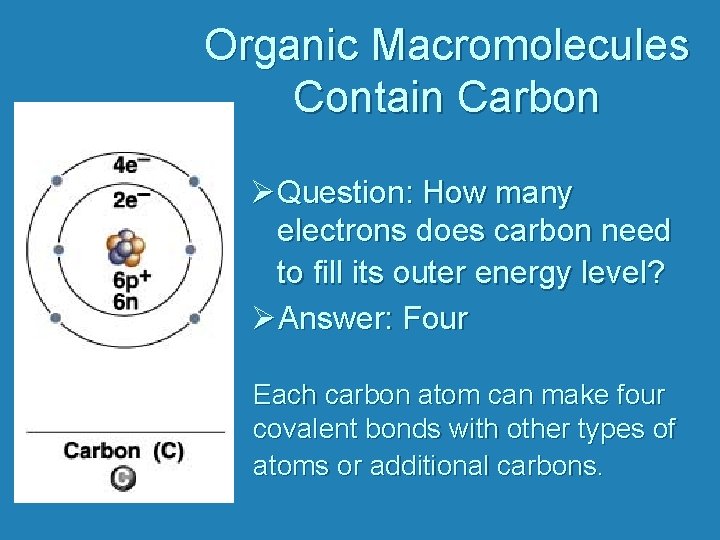
Organic Macromolecules Contain Carbon ØQuestion: How many electrons does carbon need to fill its outer energy level? ØAnswer: Four Each carbon atom can make four covalent bonds with other types of atoms or additional carbons.
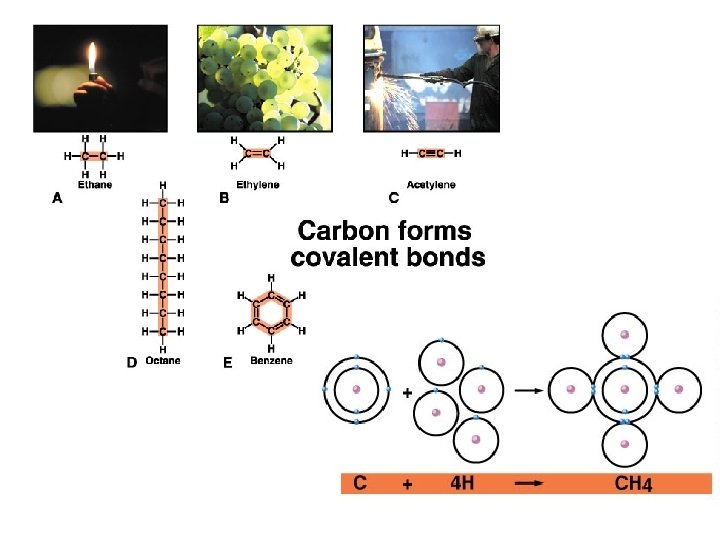
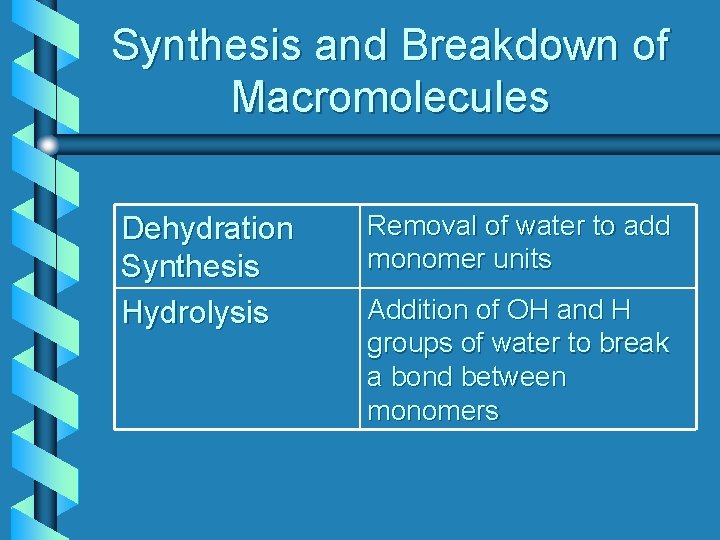
Synthesis and Breakdown of Macromolecules Dehydration Synthesis Hydrolysis Removal of water to add monomer units Addition of OH and H groups of water to break a bond between monomers
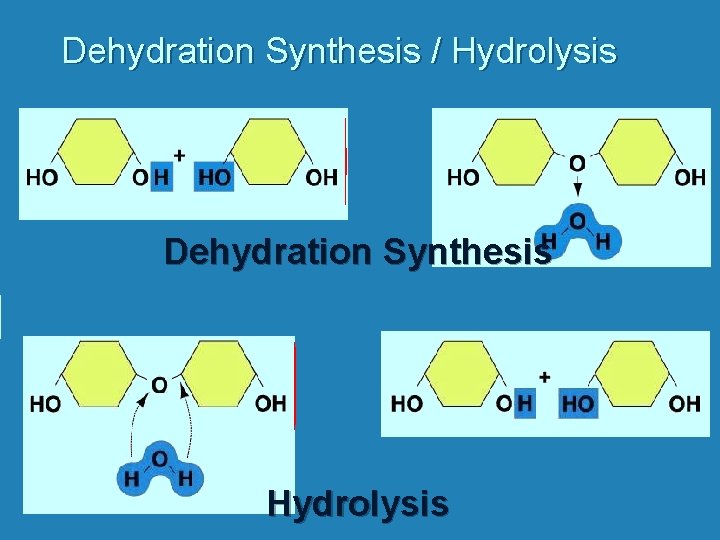
Dehydration Synthesis / Hydrolysis Dehydration Synthesis Hydrolysis
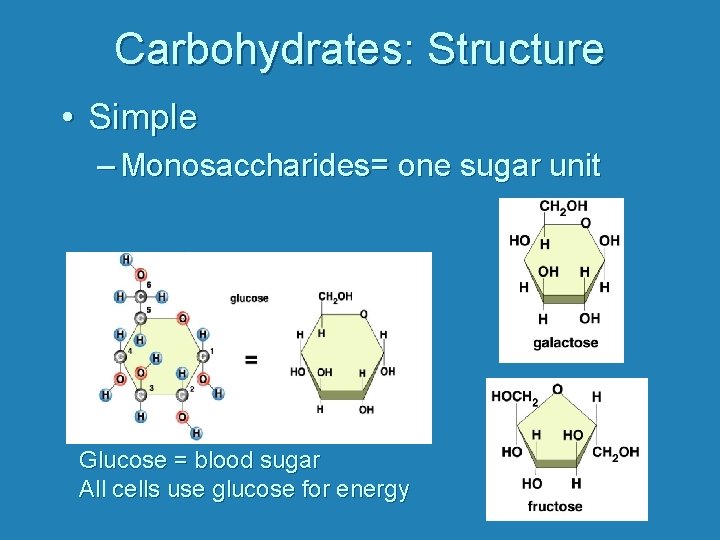
Carbohydrates: Structure • Simple – Monosaccharides= one sugar unit Glucose = blood sugar All cells use glucose for energy
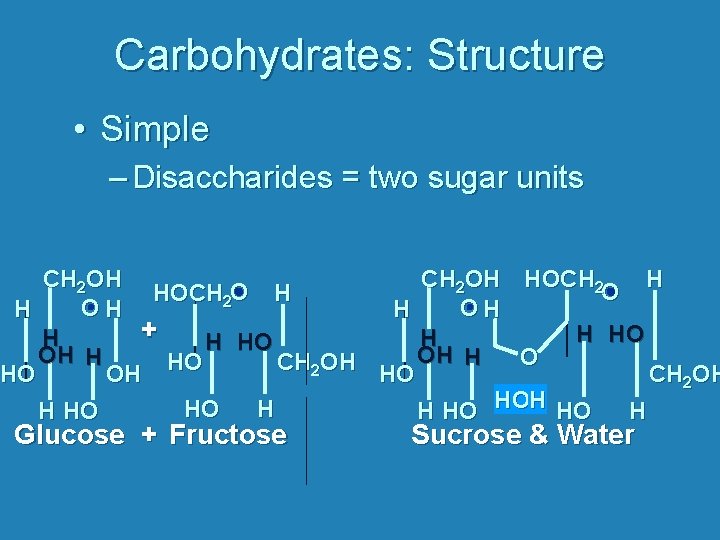
Carbohydrates: Structure • Simple – Disaccharides = two sugar units CH 2 OH HOCH 2 O HOCH 2 O H O O H H + H HO OH H O HO CH OH 2 HO OH HO CH 2 OH HO HOH HO Glucose + Fructose Sucrose & Water

Carbohydrates: Structure • Simple – Disaccharides = two sugar units • Sucrose = glucose + fructose table sugar • Lactose = glucose + galactose milk sugar • Maltose = glucose + glucose seed sugar
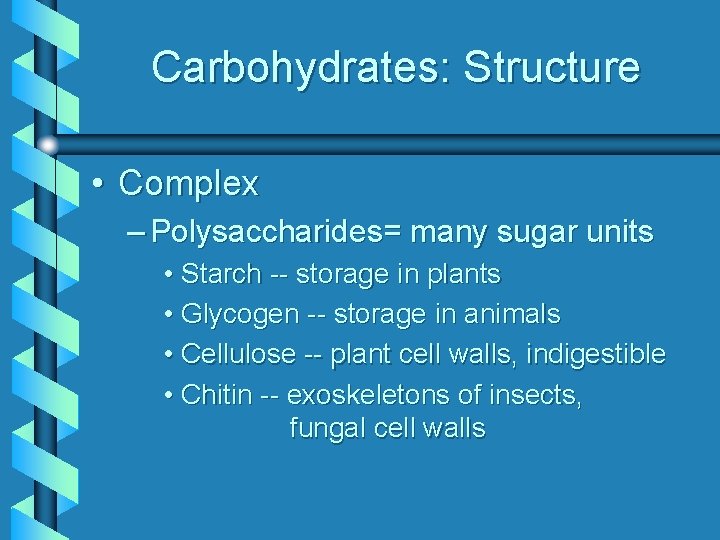
Carbohydrates: Structure • Complex – Polysaccharides= many sugar units • Starch -- storage in plants • Glycogen -- storage in animals • Cellulose -- plant cell walls, indigestible • Chitin -- exoskeletons of insects, fungal cell walls
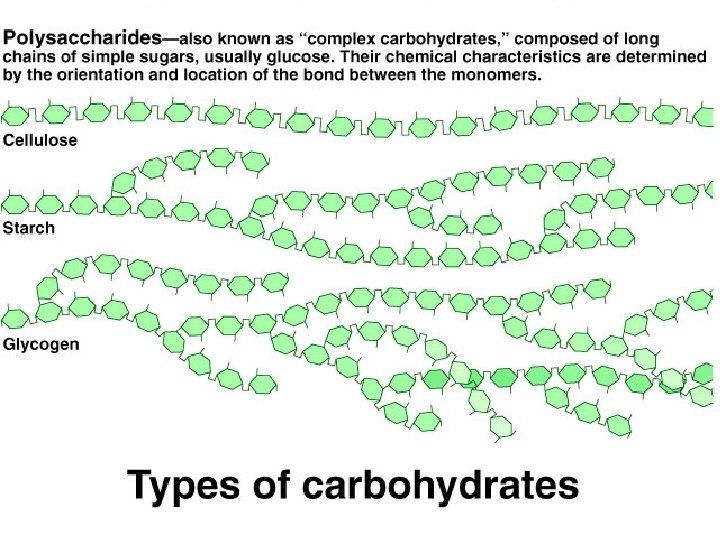
Chitin
Carbohydrates: Functions • • • Energy source Structural component Cell-cell communication

Applying Your Knowledge 1. 2. 3. Monosaccharide Polysaccharide Disacharide A. Which molecule consists of two sugar units? B. Which choice best describes glycogen? C. Which type of molecule provides the basic energy for your cells?
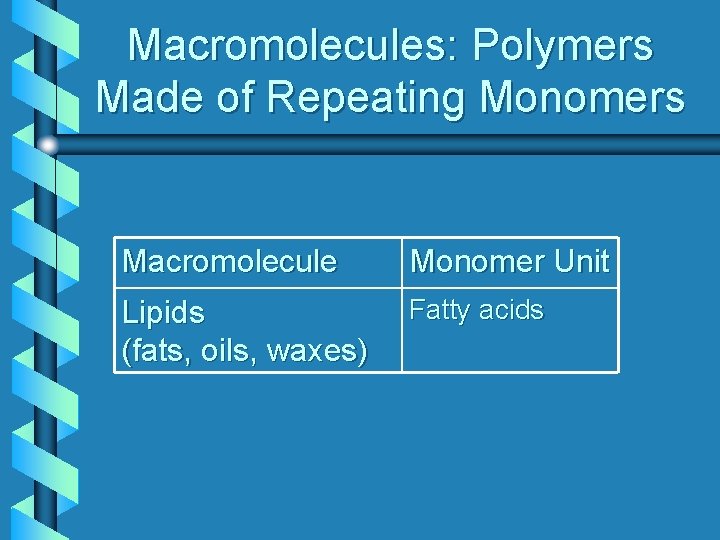
Macromolecules: Polymers Made of Repeating Monomers Macromolecule Monomer Unit Lipids (fats, oils, waxes) Fatty acids
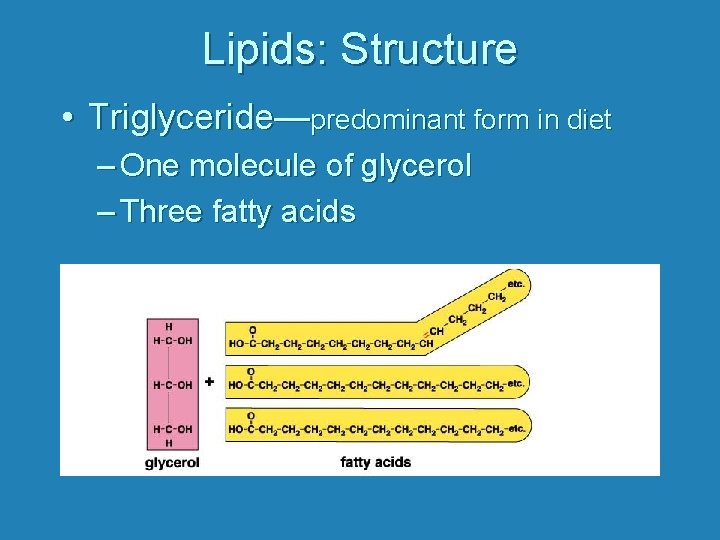
Lipids: Structure • Triglyceride—predominant form in diet – One molecule of glycerol – Three fatty acids
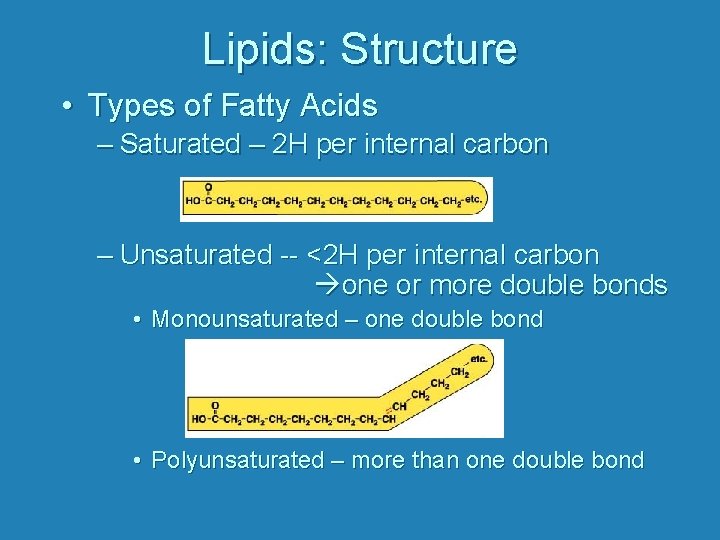
Lipids: Structure • Types of Fatty Acids – Saturated – 2 H per internal carbon – Unsaturated -- <2 H per internal carbon one or more double bonds • Monounsaturated – one double bond • Polyunsaturated – more than one double bond
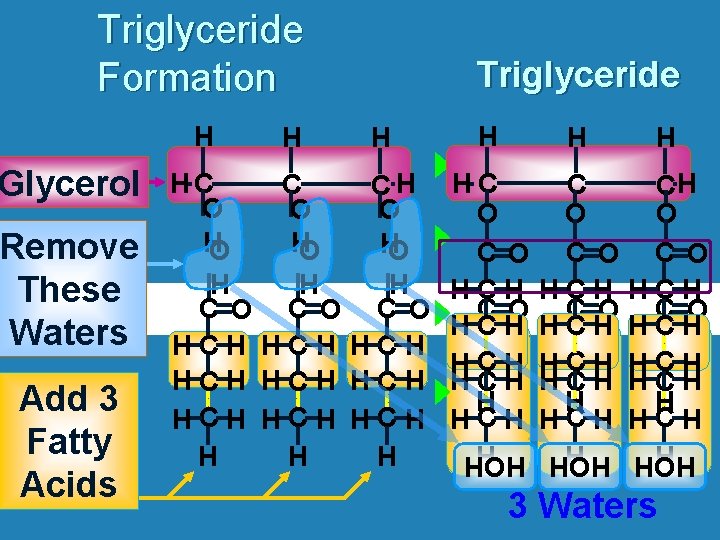
Triglyceride Formation H Glycerol Remove These Waters Add 3 Fatty Acids H Triglyceride H HC C CH O O O HO H H H C O C O HCH HCH HCH H H H HC O C O HCH HCH HCH HCH H HOH CH O C O HCH HCH HCH H HOH 3 Waters
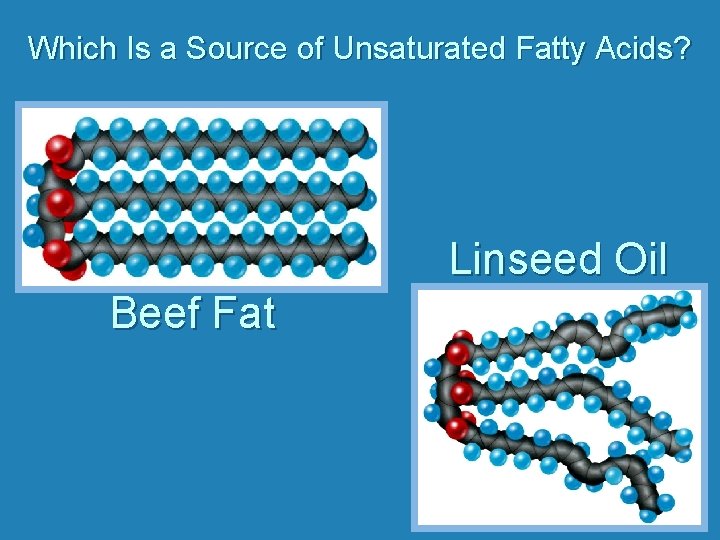
Which Is a Source of Unsaturated Fatty Acids? Linseed Oil Beef Fat
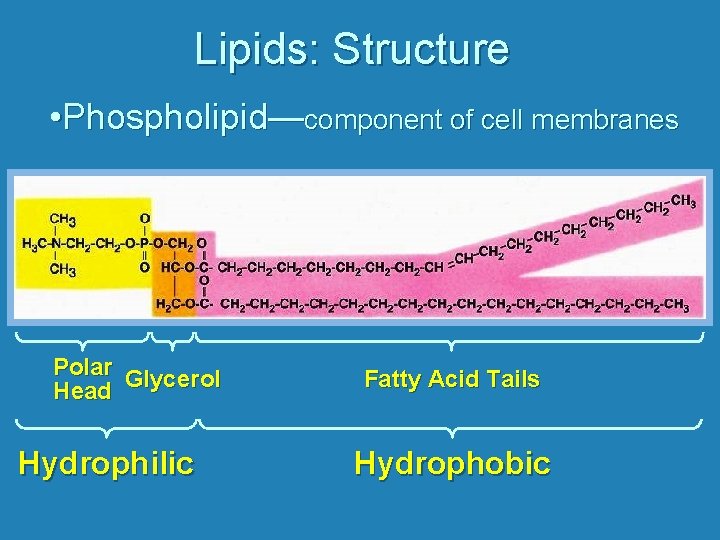
Lipids: Structure • Phospholipid—component of cell membranes Polar Glycerol Head Hydrophilic Fatty Acid Tails Hydrophobic
Lipids: Structure • Steroids – Linked carbon rings – Natural body components • Hormones • Cholesterol
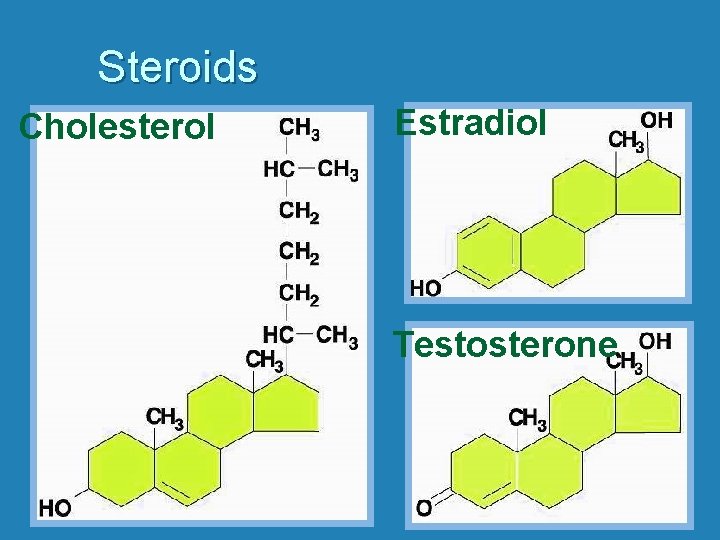
Steroids Cholesterol Estradiol Testosterone
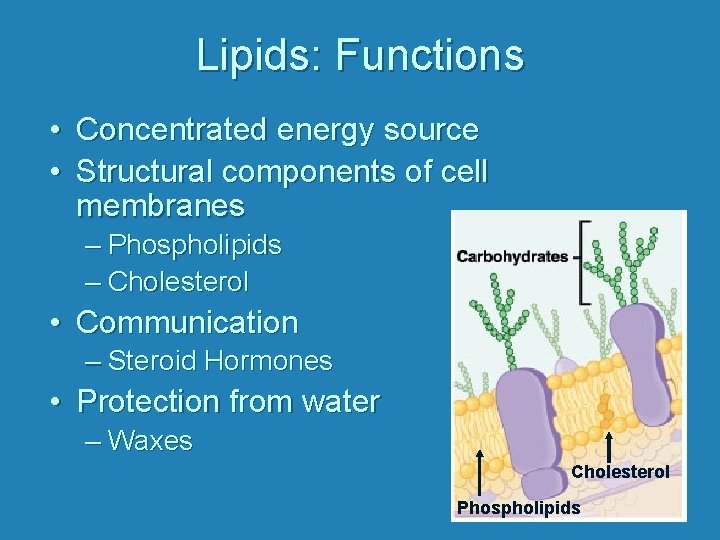
Lipids: Functions • Concentrated energy source • Structural components of cell membranes – Phospholipids – Cholesterol • Communication – Steroid Hormones • Protection from water – Waxes Cholesterol Phospholipids

Applying Your Knowledge 1. 2. 3. 4. Polyunsaturated fatty acid Cholesterol Monounsaturated fatty acid Saturated fatty acid A. Which molecule is made of a series of carbon rings? B. Which molecule has more than one double bond? C. Which molecule has 2 H for each internal carbon? D. Which molecule has one double bond?
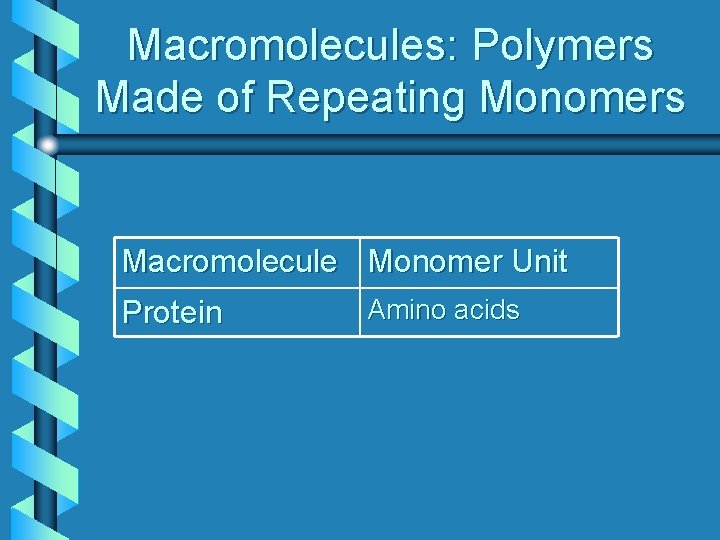
Macromolecules: Polymers Made of Repeating Monomers Macromolecule Monomer Unit Protein Amino acids
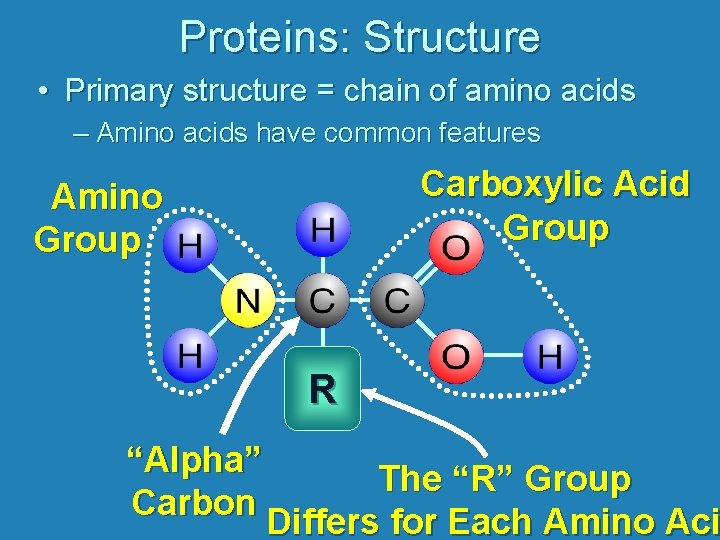
Proteins: Structure • Primary structure = chain of amino acids – Amino acids have common features Carboxylic Acid Group Amino Group R “Alpha” The “R” Group Carbon Differs for Each Amino Aci
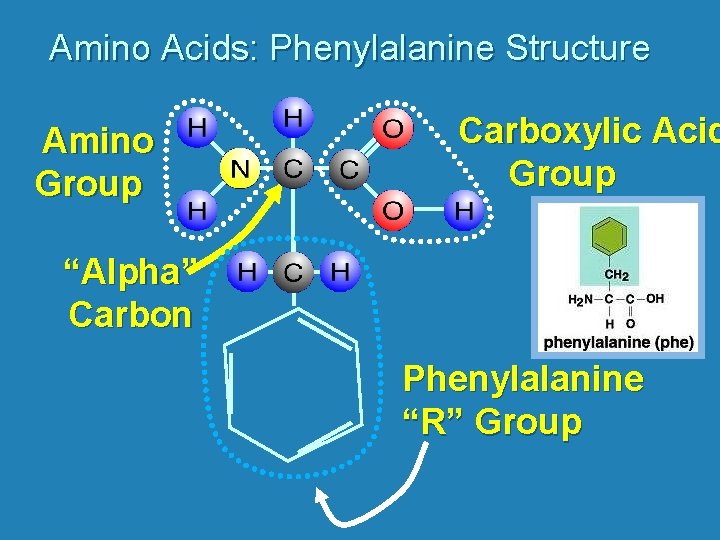
Amino Acids: Phenylalanine Structure Amino Group Carboxylic Acid Group “Alpha” Carbon Phenylalanine “R” Group
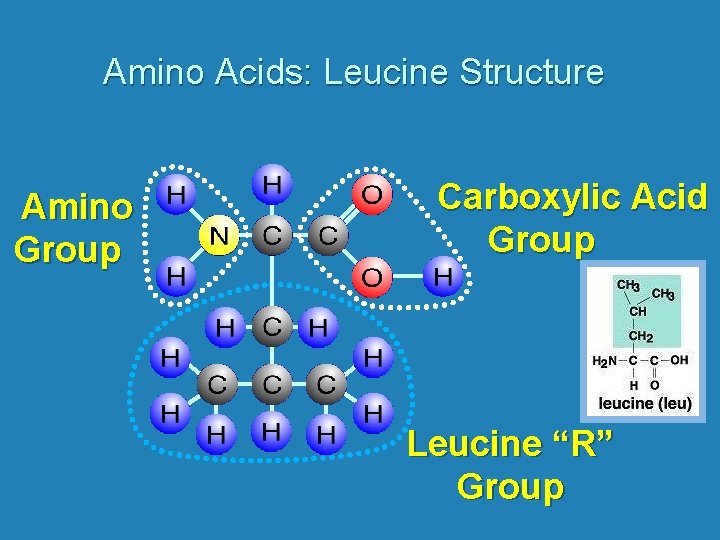
Amino Acids: Leucine Structure Amino Group Carboxylic Acid Group Leucine “R” Group
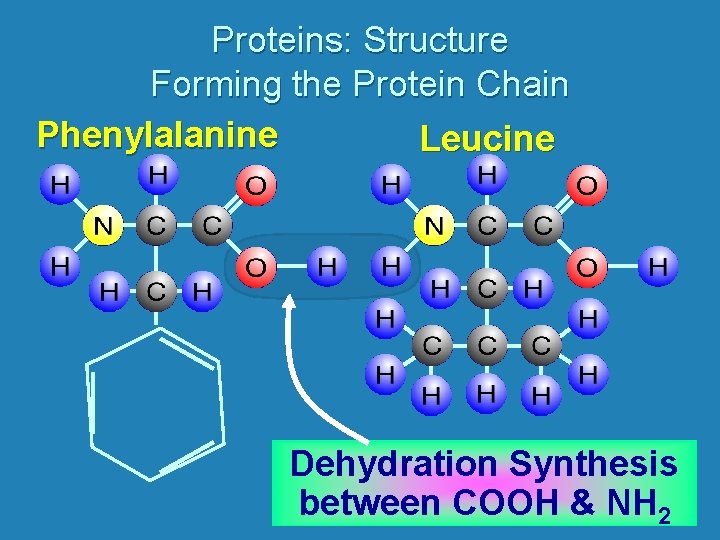
Proteins: Structure Forming the Protein Chain Phenylalanine Leucine Dehydration Synthesis between COOH & NH 2
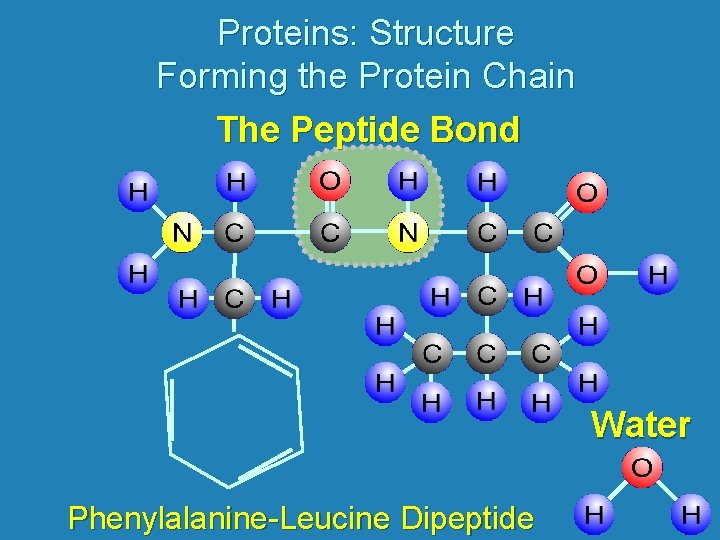
Proteins: Structure Forming the Protein Chain The Peptide Bond Water Phenylalanine-Leucine Dipeptide
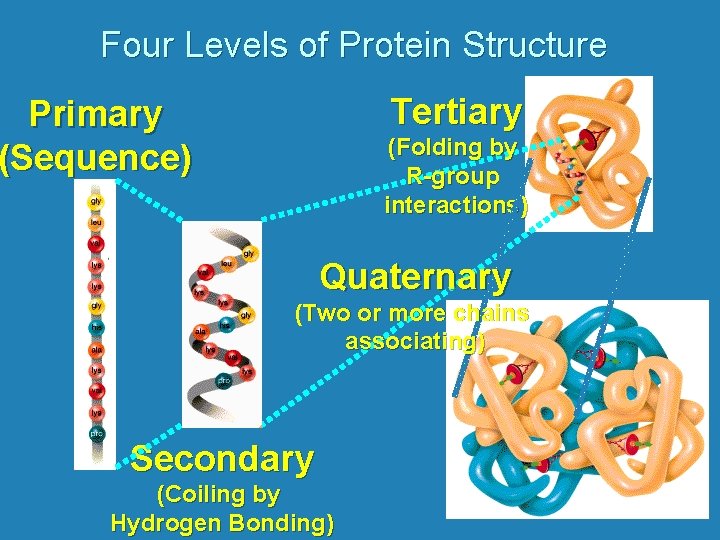
Four Levels of Protein Structure Tertiary Primary (Sequence) (Folding by R-group interactions) Quaternary (Two or more chains associating) Secondary (Coiling by Hydrogen Bonding)
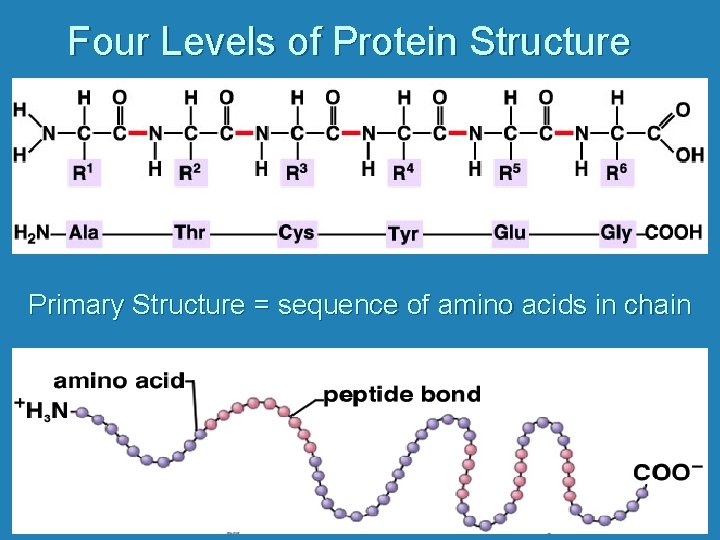
Four Levels of Protein Structure Primary Structure = sequence of amino acids in chain
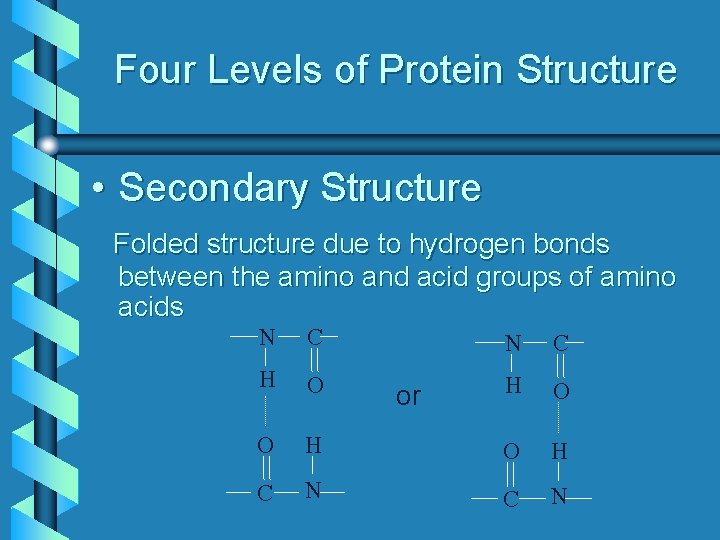
Four Levels of Protein Structure • Secondary Structure Folded structure due to hydrogen bonds between the amino and acid groups of amino acids N C H O O C N C H O H N C N or
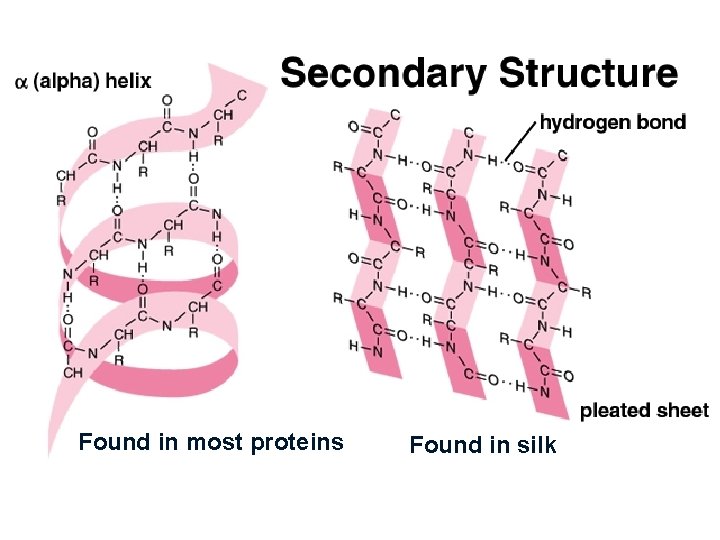
Found in most proteins Found in silk
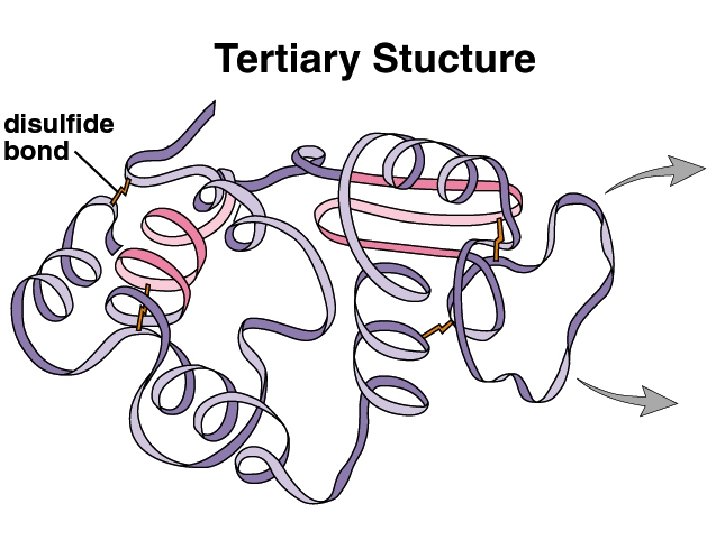
Four Levels of Protein Structure • Tertiary Structure: Three dimensional folded structure due to attractions and repulsions between R groups Can involve covalent bonding hydrogen bonding ionic interactions hydrophilic and hydrophobic interactions
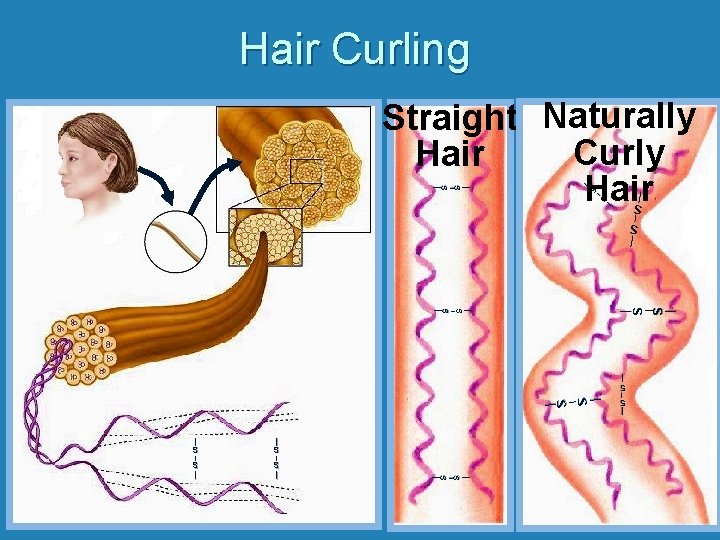
Hair Curling S | | S Straight Naturally Curly Hair | | | S | | S | S S | | S | S
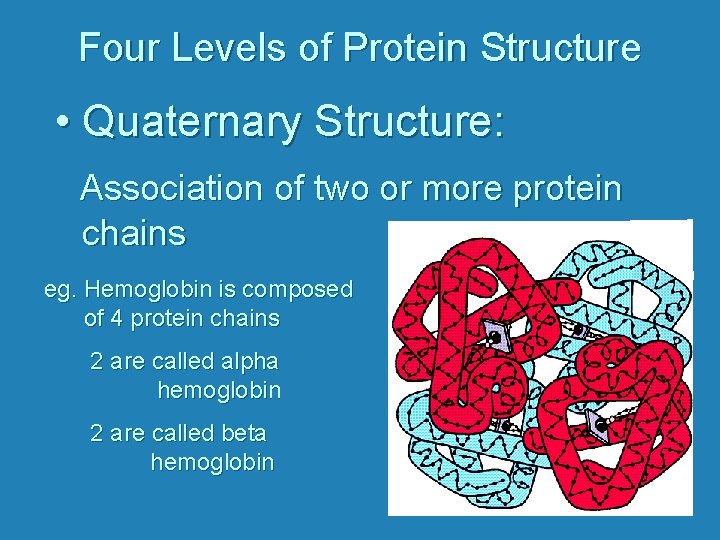
Four Levels of Protein Structure • Quaternary Structure: Association of two or more protein chains eg. Hemoglobin is composed of 4 protein chains 2 are called alpha hemoglobin 2 are called beta hemoglobin
Proteins: Functions • • Structural Component of Cells Control of Metabolic Reactions: enzymes Growth and Repair Communication – Protein Hormones – Cell Receptors • Energy source Protein

Applying Your Knowledge 1. 2. 3. 4. Primary Secondary Tertiary Quaternary A. Which structure results from hydrogen bonding? B. Which structure involves an association of two or more protein chains? C. Which structure describes the linear sequence of amino acids? D. Which structure depends upon interactions between the R groups of the amino acids?
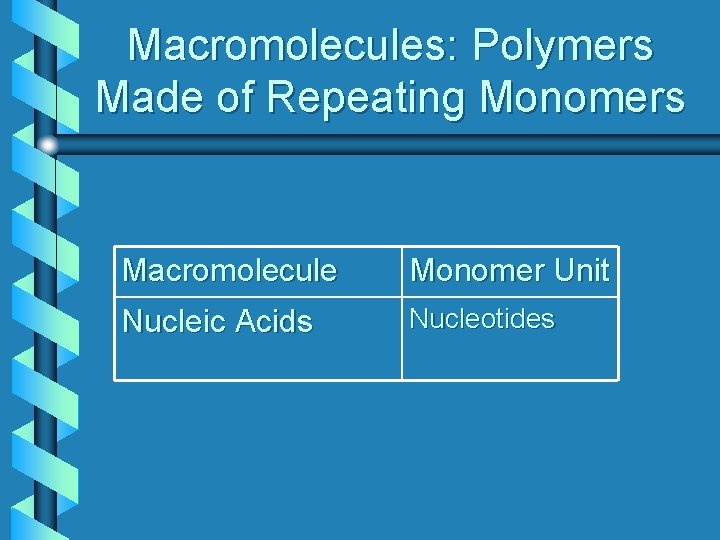
Macromolecules: Polymers Made of Repeating Monomers Macromolecule Monomer Unit Nucleic Acids Nucleotides
Nucleic Acids Function Use: To code genetic instructions. Examples: DNA, RNA
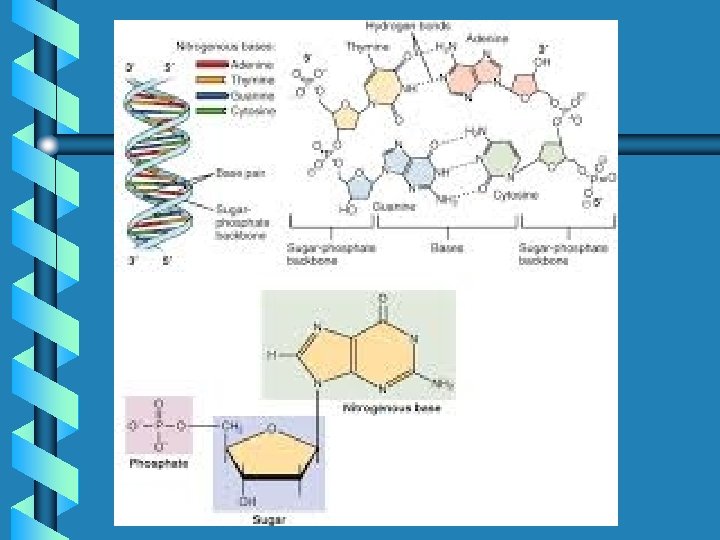
- Slides: 44