Macromolecules 1 Organic Compounds Compounds that contain CARBON

Macromolecules 1

Organic Compounds • Compounds that contain CARBON are called organic • Macromolecules are large organic molecules 2

Carbon (C) • Carbon has 4 electrons in outer shell. • Carbon can form covalent bonds with as many as 4 other atoms (elements). • Usually with C, H, O or N. N • Example: CH 4(methane) 3
Macromolecules • Large organic molecules. • Also called POLYMERS • Made up of smaller “building blocks” called MONOMERS • Examples: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids (DNA and RNA) 4
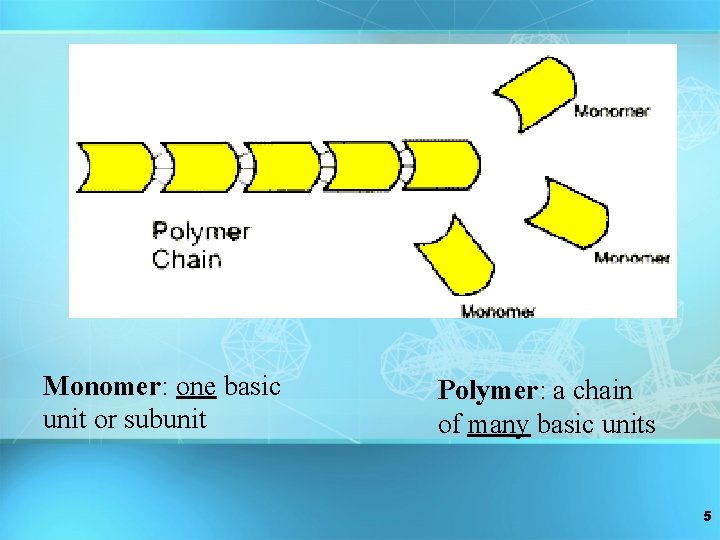
Monomer: one basic unit or subunit Polymer: a chain of many basic units 5
Question: How Are Macromolecules Formed? 6
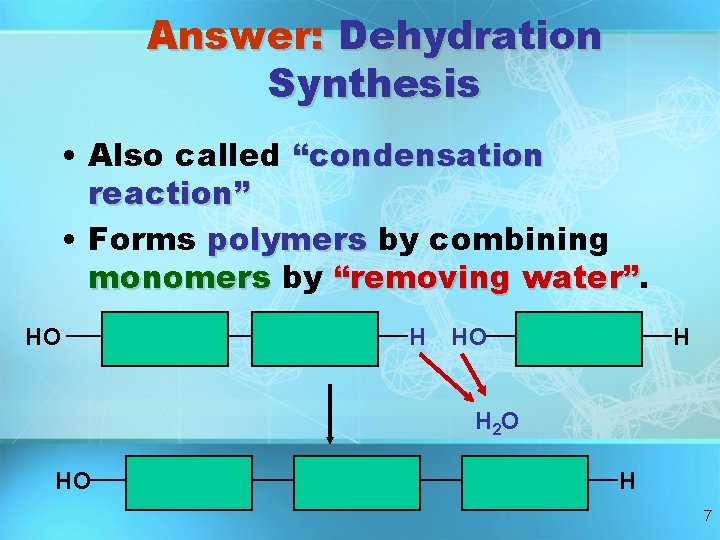
Answer: Dehydration Synthesis • Also called “condensation reaction” • Forms polymers by combining monomers by “removing water” HO H H 2 O HO H 7

Question: How are Macromolecules separated or digested? 8
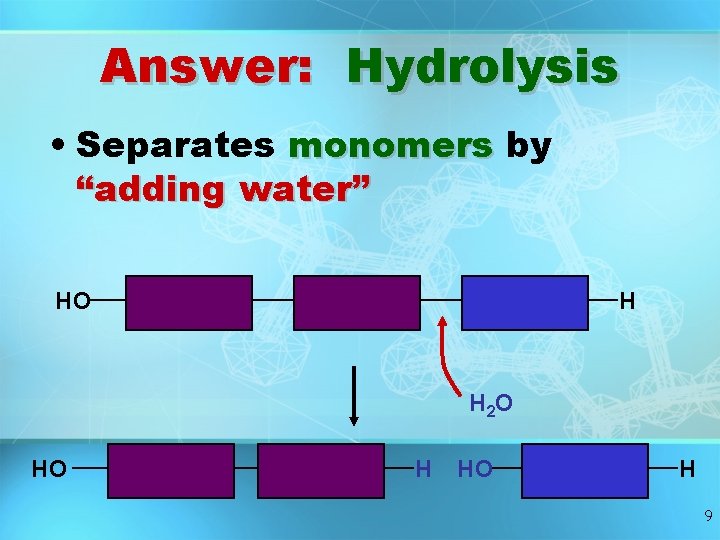
Answer: Hydrolysis • Separates monomers by “adding water” HO H H 2 O HO H 9

Carbohydrates 10

Carbohydrates • Made of Carbon, Hydrogen, and Oxygen in a 1: 2: 1 ratio. • 1 st source of energy • Found in fruits and vegetables 11
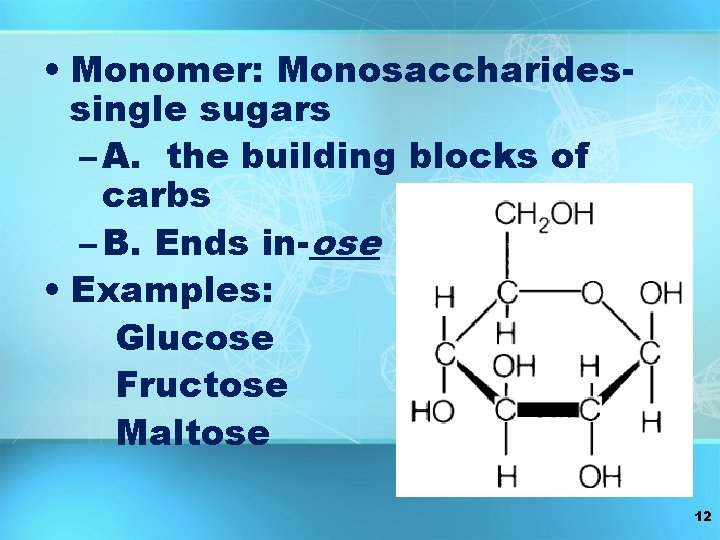
• Monomer: Monosaccharidessingle sugars – A. the building blocks of carbs – B. Ends in-ose • Examples: Glucose Fructose Maltose 12
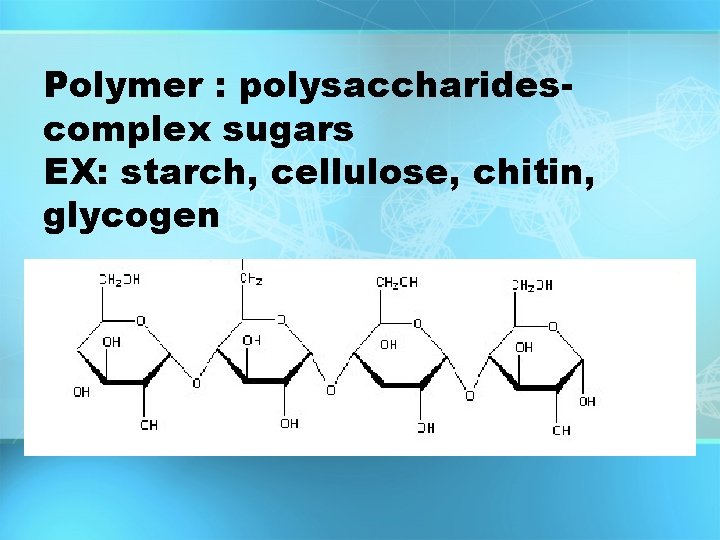
Polymer : polysaccharidescomplex sugars EX: starch, cellulose, chitin, glycogen
Polysaccharides • Starch- polysaccharide found in plants • EX: Potatoes • Glycogen- polysaccharide found in muscle cells and the liver • Cellulose- polysaccharide found in plant cell walls • Chitin- polysaccharide found in fungi cell walls and arthropod exoskeletons

Lipids 15

Lipids • Composed of Carbon, Hydrogen and Oxygen • Store the most energy • Are not soluble(do not mix) in water • Examples: 1. Fats 2. Phospholipids 3. Oils 4. Waxes 5. Steroid hormones 6. Triglycerides 16
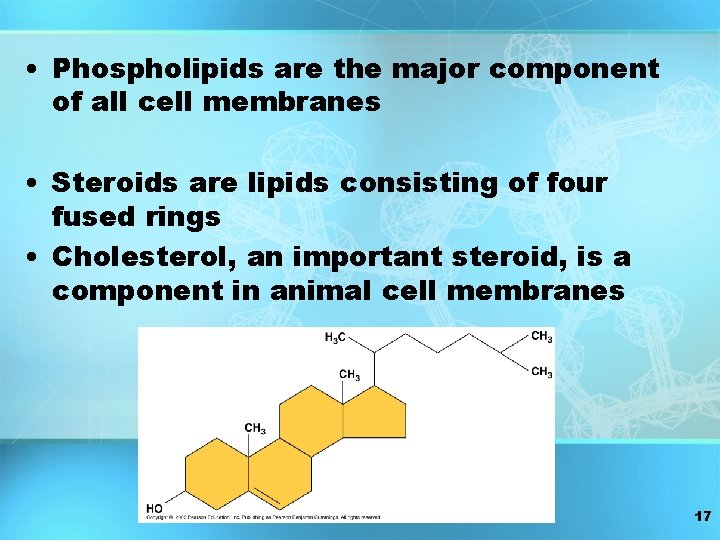
• Phospholipids are the major component of all cell membranes • Steroids are lipids consisting of four fused rings • Cholesterol, an important steroid, is a component in animal cell membranes 17

Lipids Functions of lipids: 1. Long term energy storage 2. Provide insulation 3. Protection against physical shock 4. Protection against water loss 5. Chemical messengers (hormones) 18
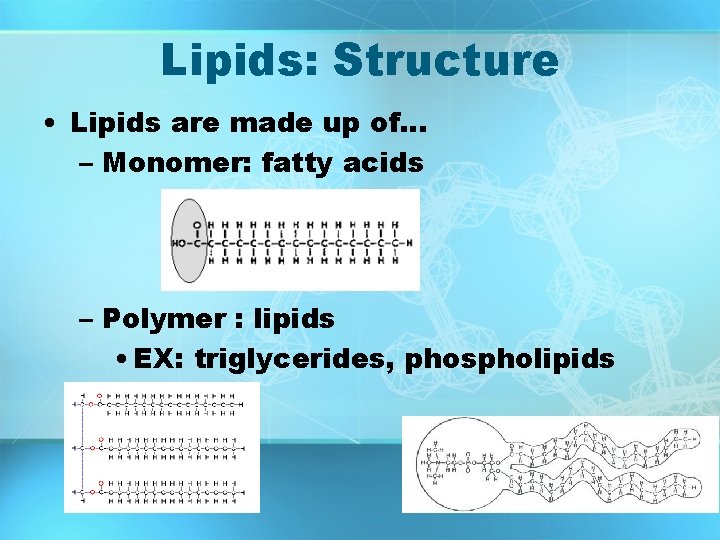
Lipids: Structure • Lipids are made up of… – Monomer: fatty acids – Polymer : lipids • EX: triglycerides, phospholipids
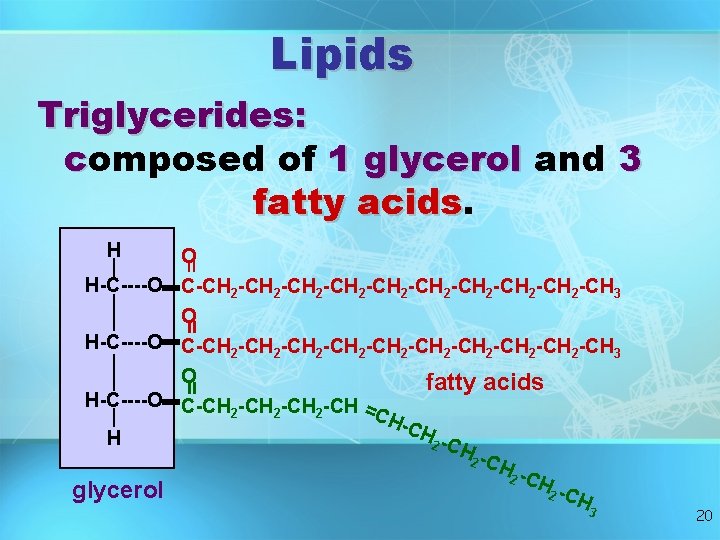
Lipids Triglycerides: composed of 1 glycerol and 3 fatty acids H = O H-C----O C-CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -CH 3 O fatty acids H-C----O C-CH -CH = 2 2 2 CH -CH H 2 -C H 2 C Hglycerol 2 C H = = 3 20

Fatty Acids There are two kinds of fatty acids you may see these on food labels: = 1. Saturated fatty acids: no double bonds, solid at room temperature(bad) O saturated C-CH 2 -CH 2 -CH 2 -CH 3 2. Unsaturated fatty acids: double bonds, liquid at room temperature(good) = O C-CH 2 -CH=C unsaturated H-C H 2 C H 3 21
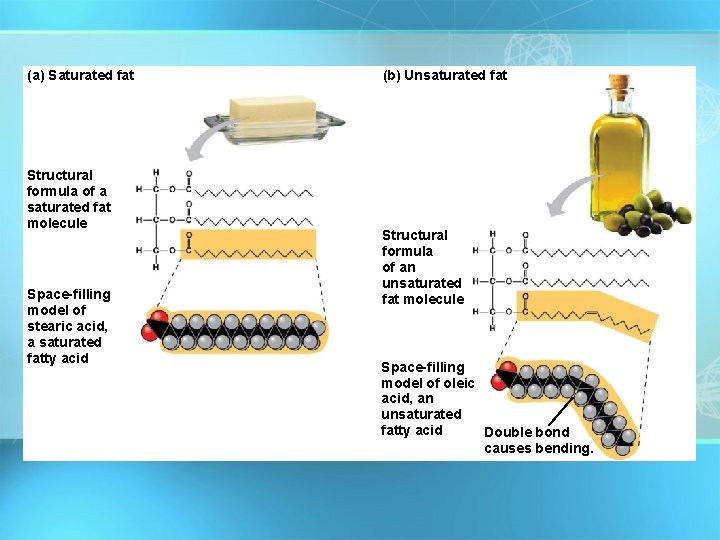
(a) Saturated fat Structural formula of a saturated fat molecule Space-filling model of stearic acid, a saturated fatty acid (b) Unsaturated fat Structural formula of an unsaturated fat molecule Space-filling model of oleic acid, an unsaturated fatty acid Double bond causes bending.
Proteins 23

Protein Function Composed of Carbon, Hydrogen, Oxygen and Nitrogen Makes up your hair, skin, muscles and nails Examples: Collagen-protein found in skin Hemoglobin-protein in blood 24
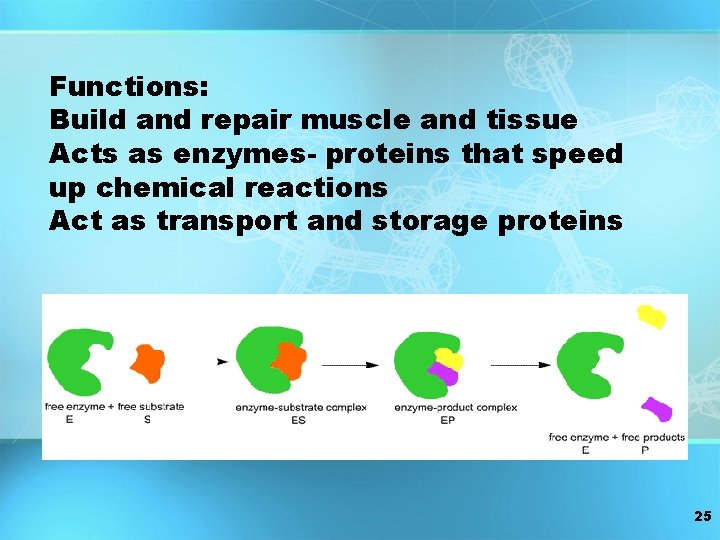
Functions: Build and repair muscle and tissue Acts as enzymes- proteins that speed up chemical reactions Act as transport and storage proteins 25
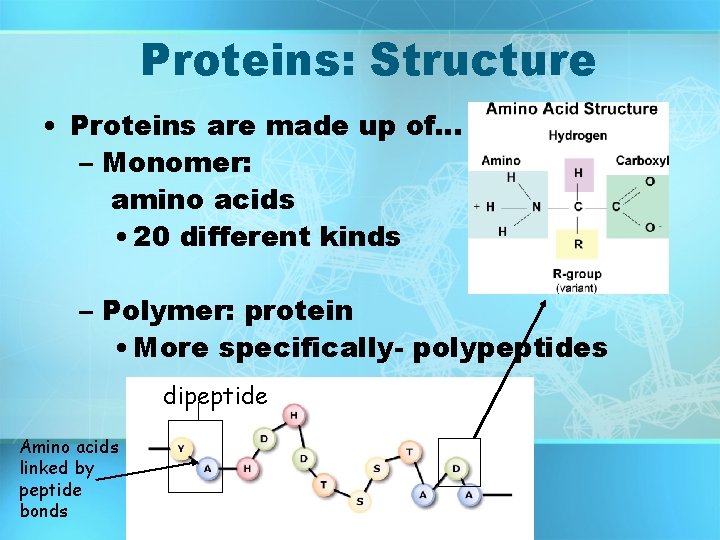
Proteins: Structure • Proteins are made up of… – Monomer: amino acids • 20 different kinds – Polymer: protein • More specifically- polypeptides dipeptide Amino acids linked by peptide bonds
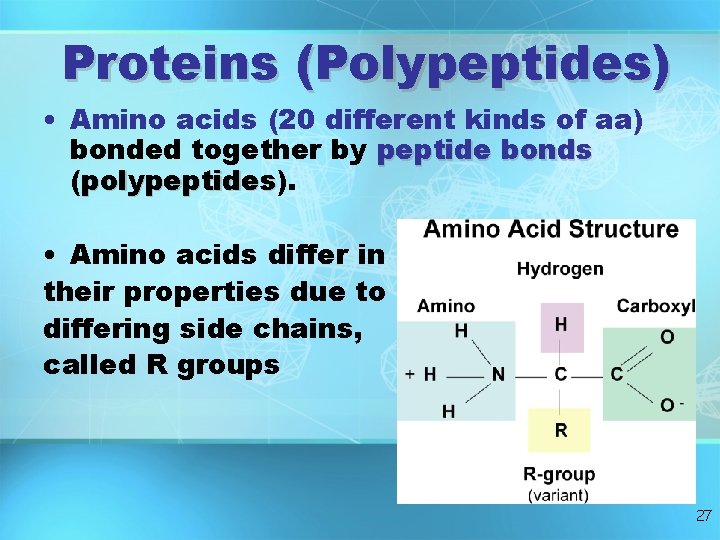
Proteins (Polypeptides) • Amino acids (20 different kinds of aa) bonded together by peptide bonds (polypeptides). polypeptides • Amino acids differ in their properties due to differing side chains, called R groups 27
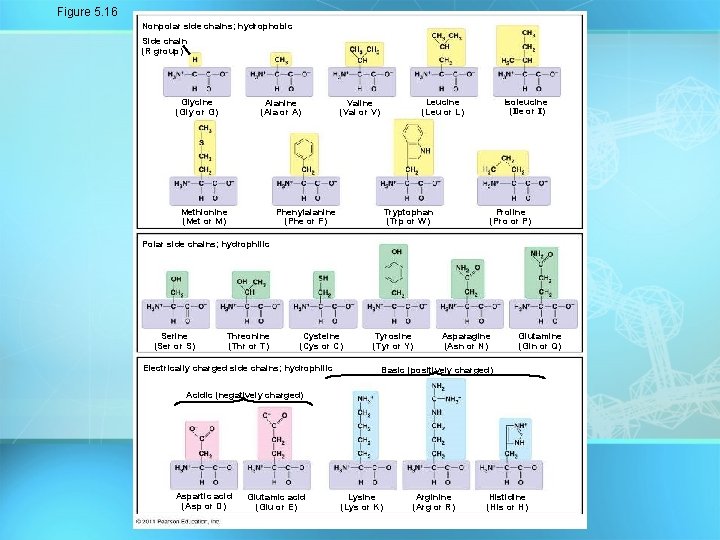
Figure 5. 16 Nonpolar side chains; hydrophobic Side chain (R group) Glycine (Gly or G) Alanine (Ala or A) Methionine (Met or M) Isoleucine (Ile or I) Leucine (Leu or L) Valine (Val or V) Phenylalanine (Phe or F) Tryptophan (Trp or W) Proline (Pro or P) Polar side chains; hydrophilic Serine (Ser or S) Threonine (Thr or T) Cysteine (Cys or C) Electrically charged side chains; hydrophilic Tyrosine (Tyr or Y) Asparagine (Asn or N) Glutamine (Gln or Q) Basic (positively charged) Acidic (negatively charged) Aspartic acid (Asp or D) Glutamic acid (Glu or E) Lysine (Lys or K) Arginine (Arg or R) Histidine (His or H)
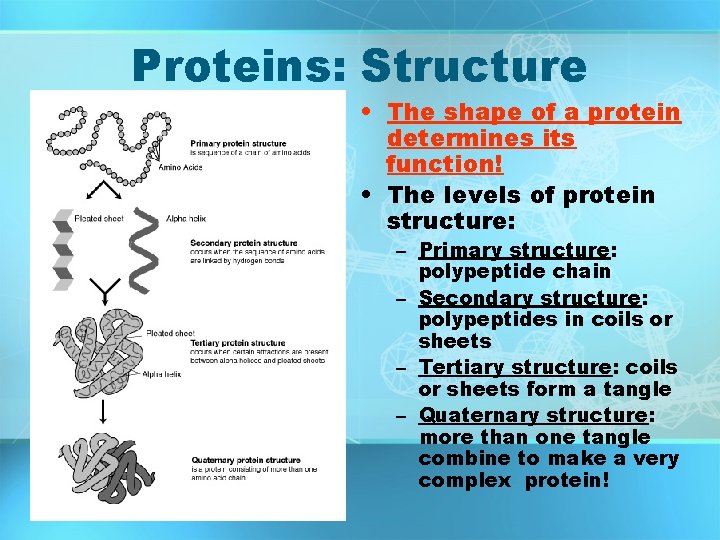
Proteins: Structure • The shape of a protein determines its function! • The levels of protein structure: – Primary structure: polypeptide chain – Secondary structure: polypeptides in coils or sheets – Tertiary structure: coils or sheets form a tangle – Quaternary structure: more than one tangle combine to make a very complex protein!
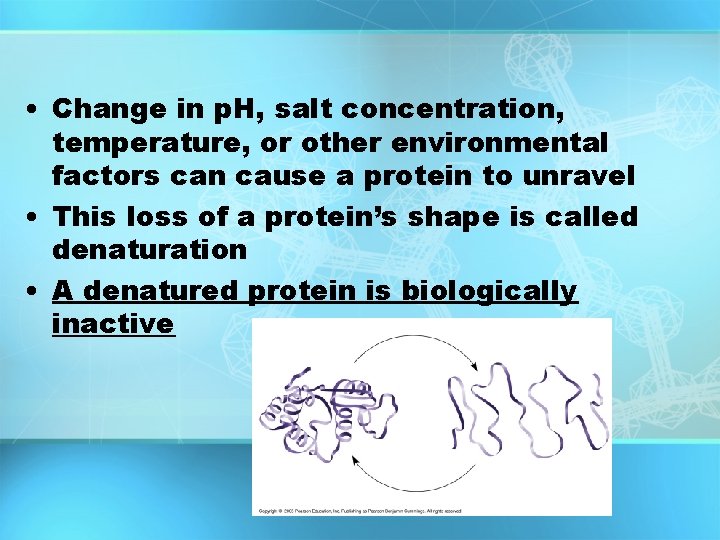
• Change in p. H, salt concentration, temperature, or other environmental factors can cause a protein to unravel • This loss of a protein’s shape is called denaturation • A denatured protein is biologically inactive

Nucleic Acids 31
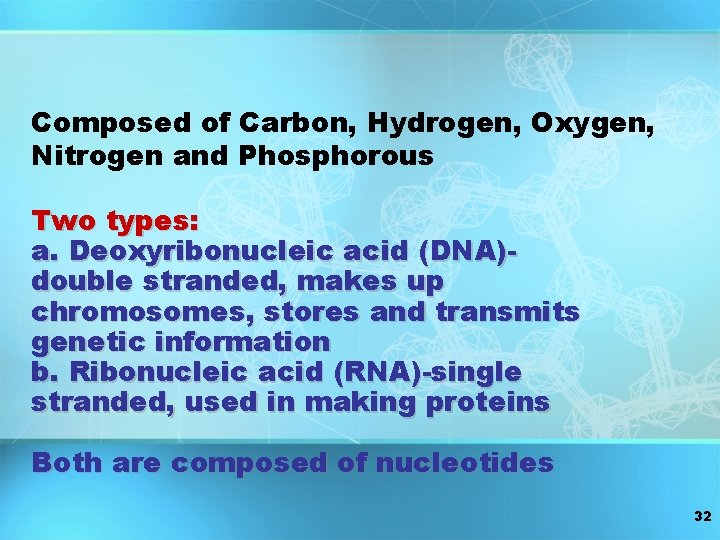
Composed of Carbon, Hydrogen, Oxygen, Nitrogen and Phosphorous Two types: a. Deoxyribonucleic acid (DNA)double stranded, makes up chromosomes, stores and transmits genetic information b. Ribonucleic acid (RNA)-single stranded, used in making proteins Both are composed of nucleotides 32
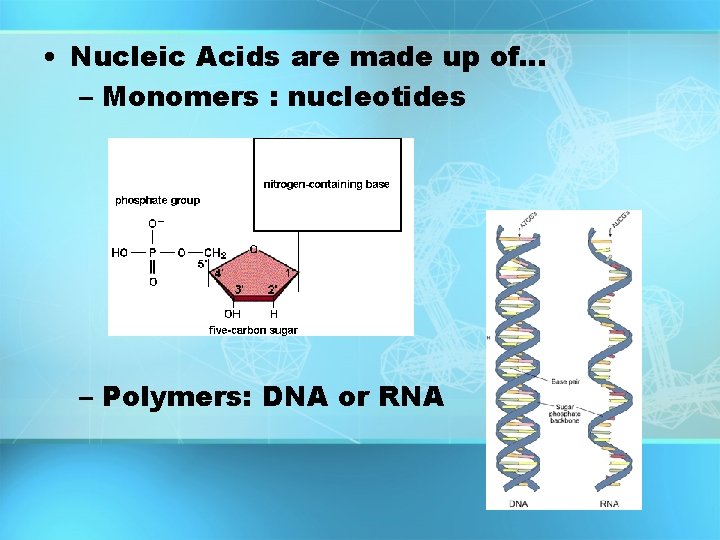
• Nucleic Acids are made up of… – Monomers : nucleotides – Polymers: DNA or RNA
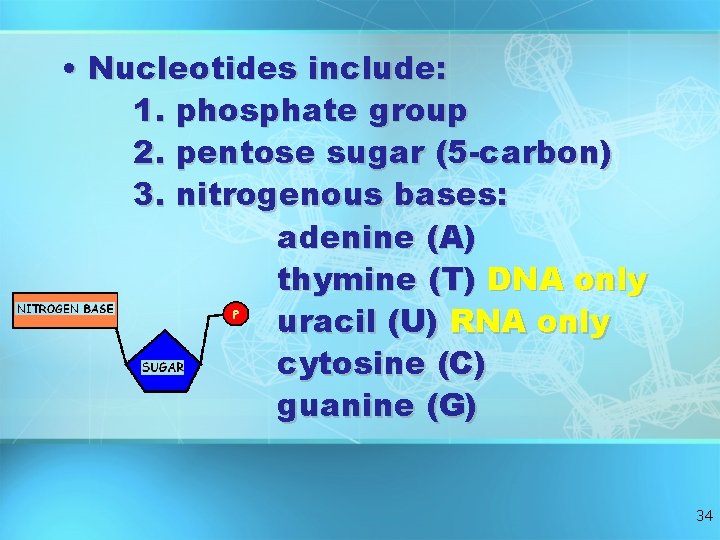
• Nucleotides include: 1. phosphate group 2. pentose sugar (5 -carbon) 3. nitrogenous bases: adenine (A) thymine (T) DNA only uracil (U) RNA only cytosine (C) guanine (G) 34
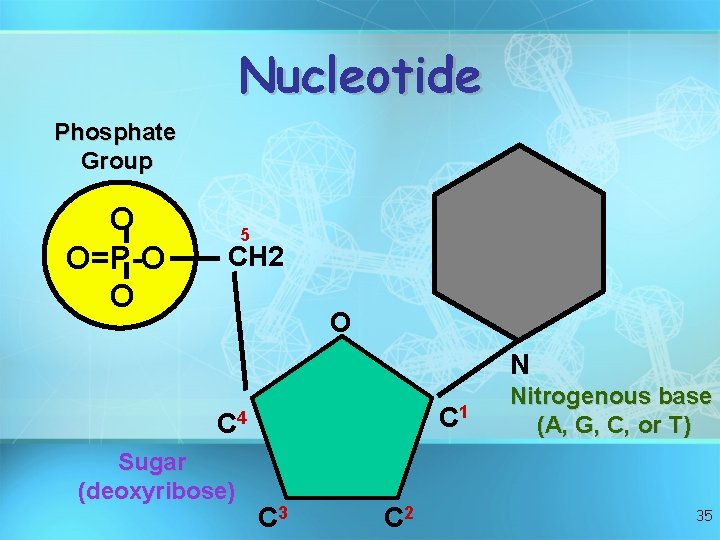
Nucleotide Phosphate Group O O=P-O O 5 CH 2 O N C 1 C 4 Sugar (deoxyribose) C 3 C 2 Nitrogenous base (A, G, C, or T) 35
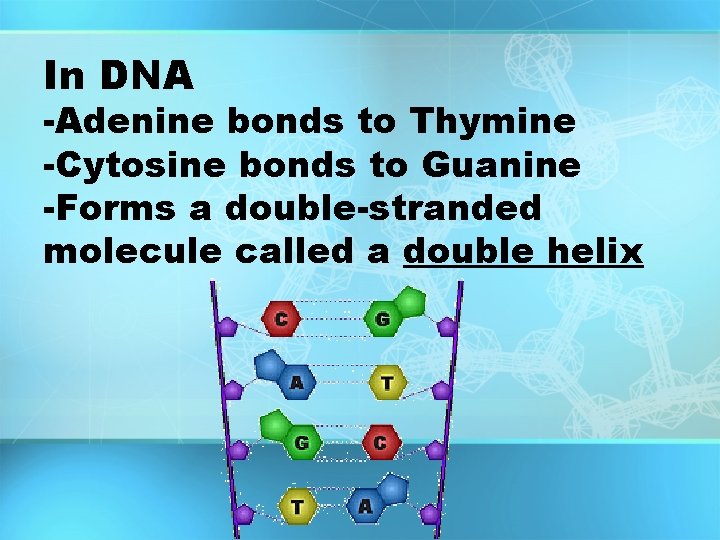
In DNA -Adenine bonds to Thymine -Cytosine bonds to Guanine -Forms a double-stranded molecule called a double helix
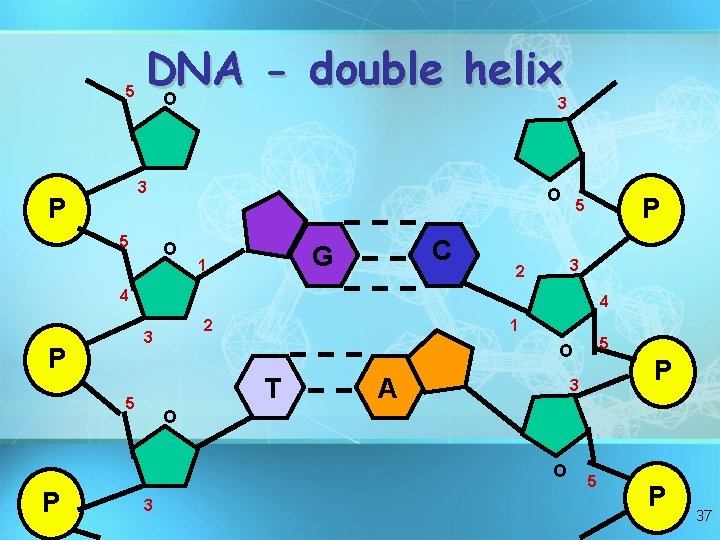
5 DNA double helix O 3 3 P 5 O O C G 1 P 5 3 2 4 4 2 3 P 1 T 5 A P 3 O O P 5 O 3 5 P 37
- Slides: 37