Macromolecules 1 Organic Compounds Compounds that contain CARBON

Macromolecules 1

Organic Compounds • Compounds that contain CARBON are called organic • Macromolecules are large organic molecules 2
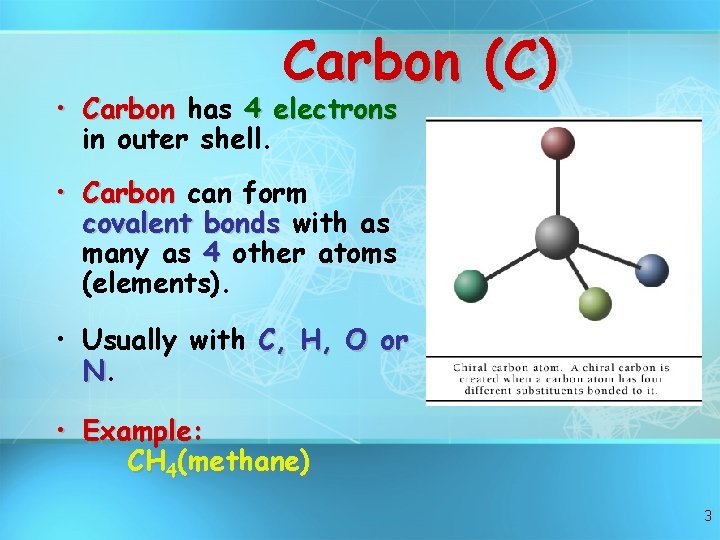
Carbon (C) • Carbon has 4 electrons in outer shell. • Carbon can form covalent bonds with as many as 4 other atoms (elements). • Usually with C, H, O or N. • Example: CH 4(methane) 3
4
Macromolecules • • • Large organic molecules. Also called POLYMERS Made up of smaller “building blocks” called MONOMERS • Examples: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids (DNA and RNA) 5

6
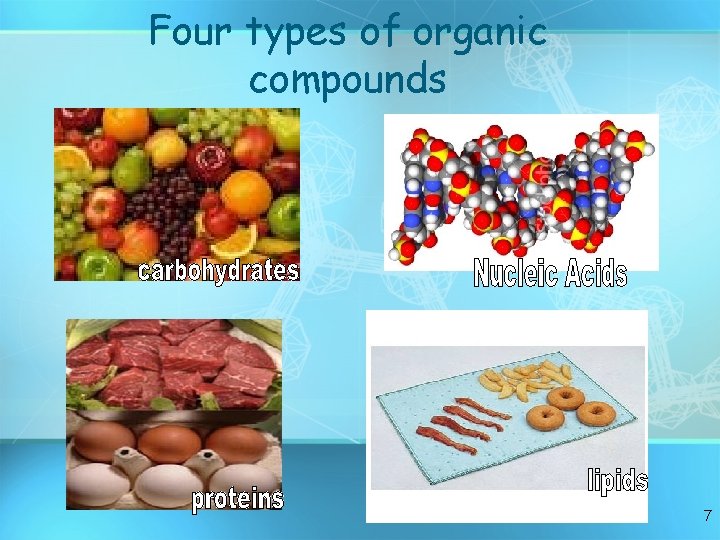
Four types of organic compounds 7

Question: How Are Macromolecules Formed? 8
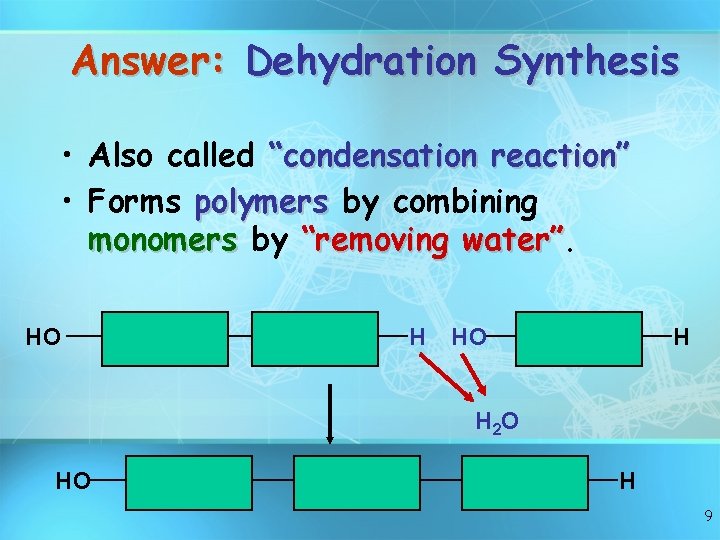
Answer: Dehydration Synthesis • Also called “condensation reaction” • Forms polymers by combining monomers by “removing water” HO H H 2 O HO H 9
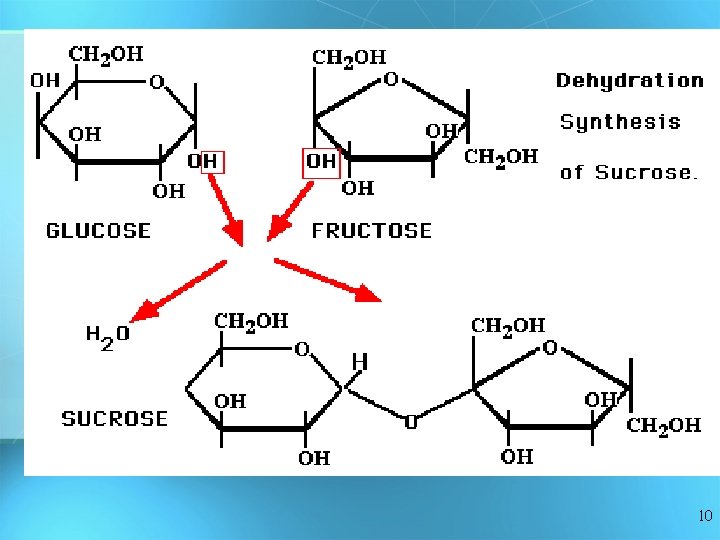
10

Question: How are Macromolecules separated or digested? 11
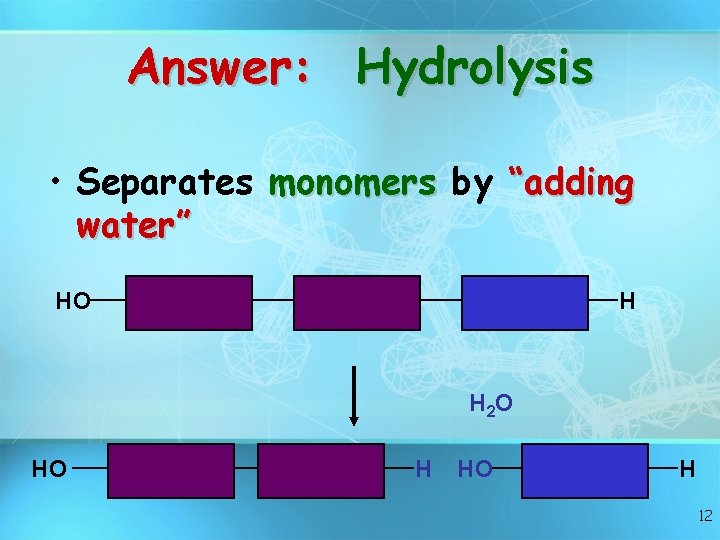
Answer: Hydrolysis • Separates monomers by “adding water” HO H H 2 O HO H 12
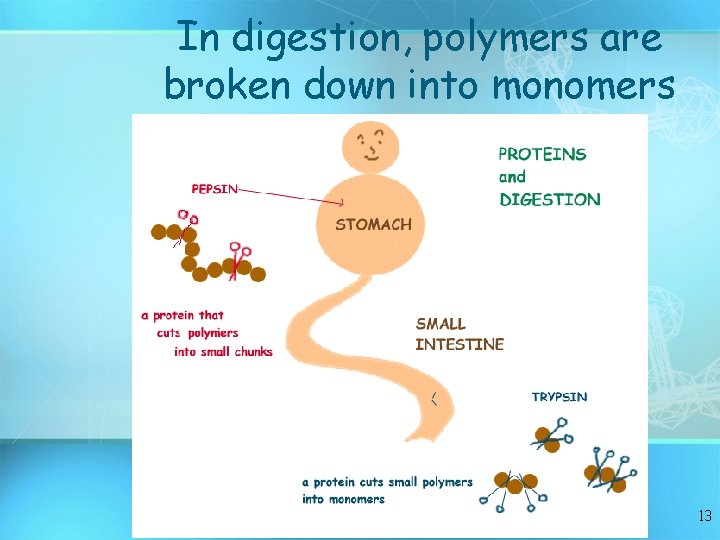
In digestion, polymers are broken down into monomers 13

Carbohydrates 14

Characteristics of Carbohydrates • • Consist of carbon, hydrogen, & oxygen Energy containing molecules Some provide structure Basic building block is a monosaccharide (CH 2 O)n ; n = 3, 5, 6 • Two monosaccharides form a disaccharide 15
Carbohydrates • Small sugar molecules to large sugar molecules • Examples: A. monosaccharide B. disaccharide C. polysaccharide 16
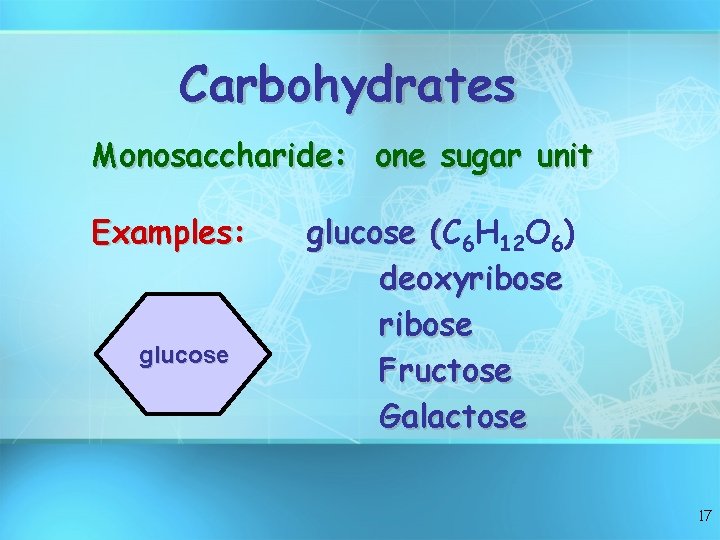
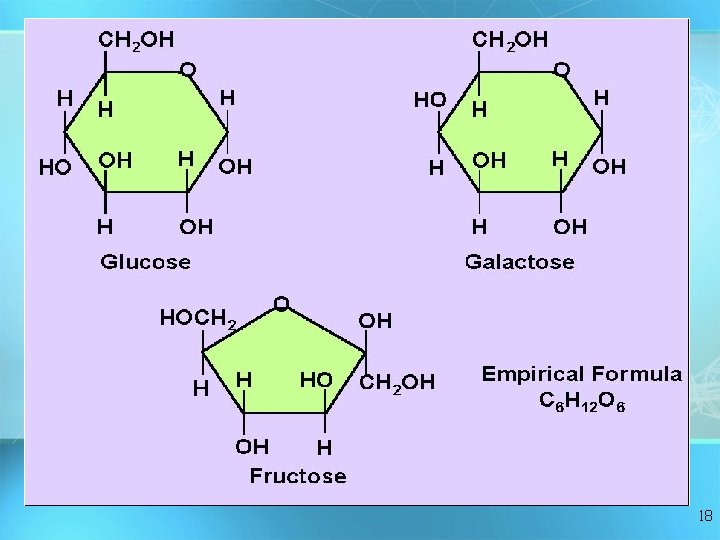
Carbohydrates Monosaccharide: one sugar unit Examples: glucose (C ( 6 H 12 O 6) deoxyribose Fructose Galactose 17
18
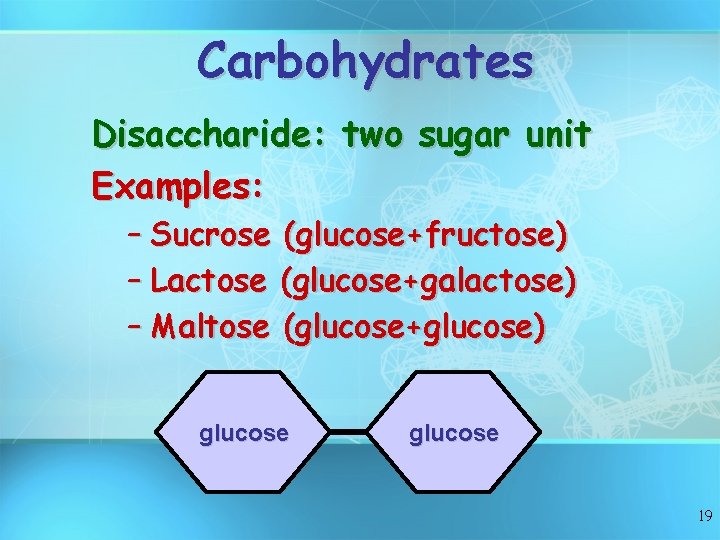
Carbohydrates Disaccharide: two sugar unit Examples: – Sucrose (glucose+fructose) – Lactose (glucose+galactose) – Maltose (glucose+glucose) glucose 19
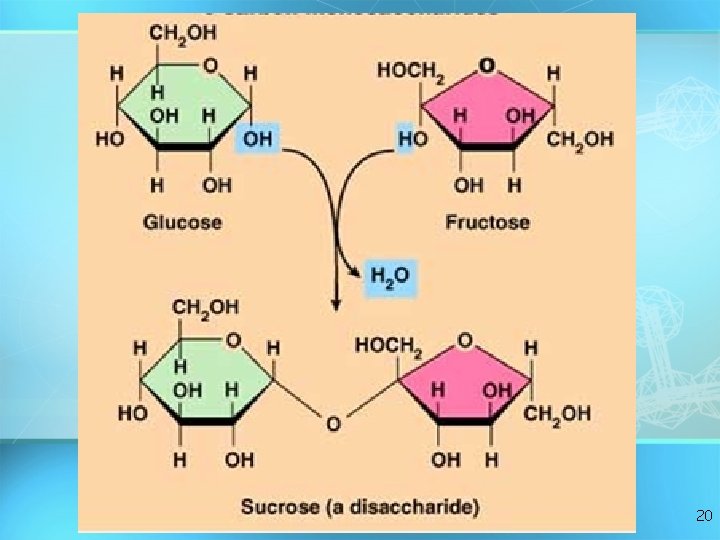
20
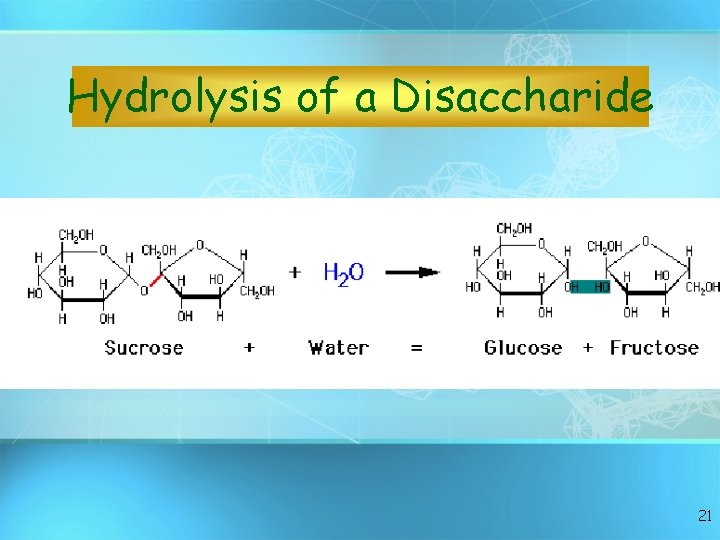
Hydrolysis of a Disaccharide 21
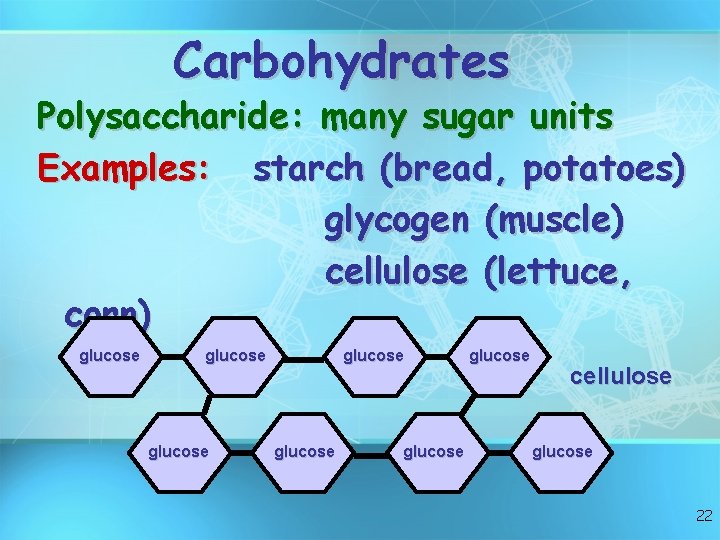
Carbohydrates Polysaccharide: many sugar units Examples: starch (bread, potatoes) glycogen (muscle) cellulose (lettuce, corn) glucose glucose cellulose glucose 22

http: //www. chemistryland. com/Elementary. School/Building. Blocks/Building. Organic. htm 23
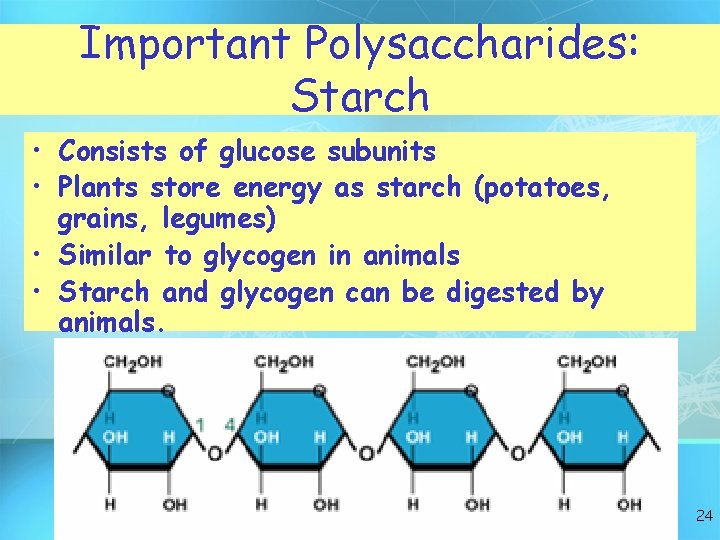
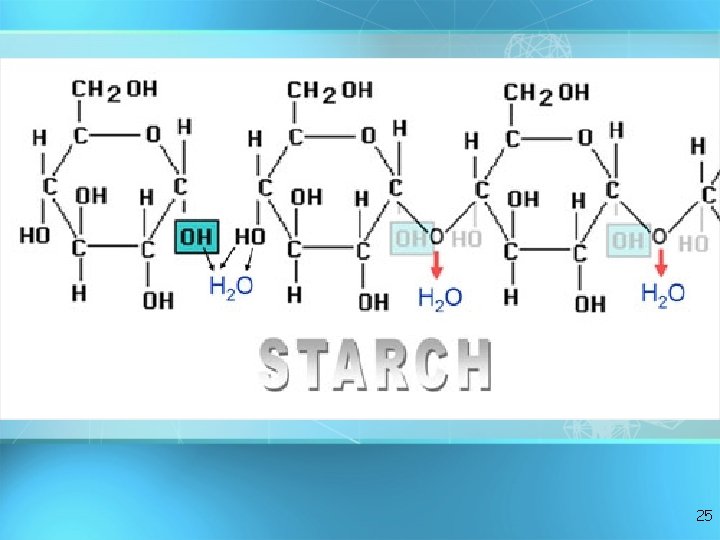
Important Polysaccharides: Starch • Consists of glucose subunits • Plants store energy as starch (potatoes, grains, legumes) • Similar to glycogen in animals • Starch and glycogen can be digested by animals. 24
25
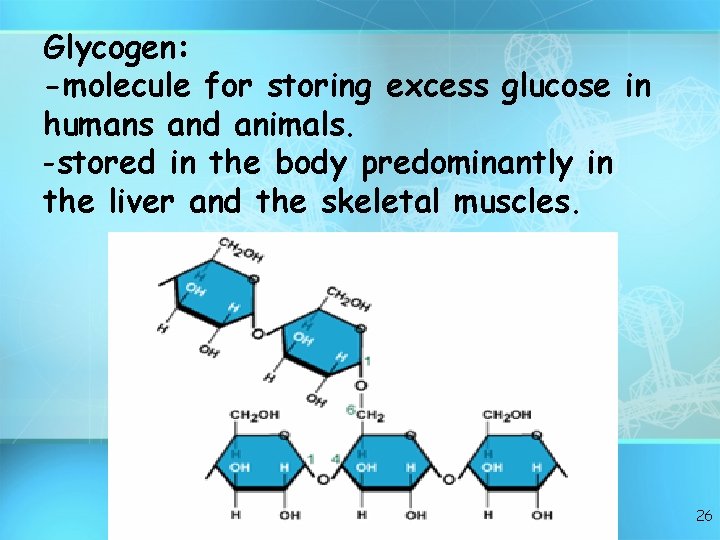
Glycogen: -molecule for storing excess glucose in humans and animals. -stored in the body predominantly in the liver and the skeletal muscles. 26

Why did the potato cross the road? Because he saw the fork up ahead. Ouch! 27
Cellulose 28
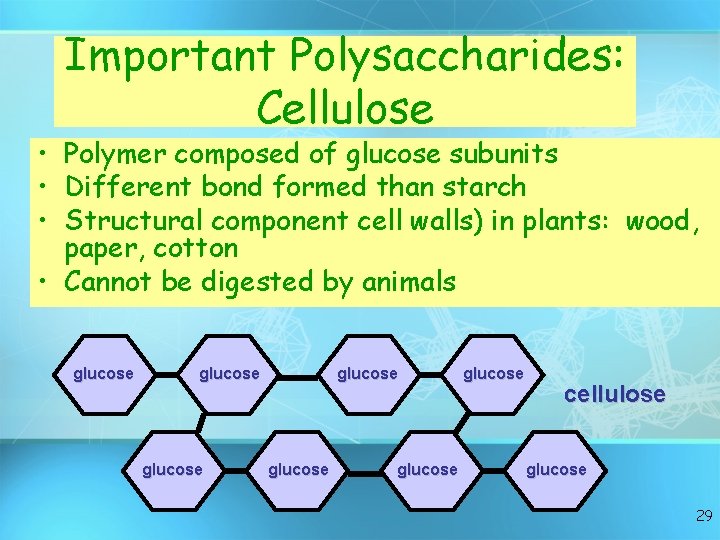
Important Polysaccharides: Cellulose • Polymer composed of glucose subunits • Different bond formed than starch • Structural component cell walls) in plants: wood, paper, cotton • Cannot be digested by animals glucose glucose cellulose glucose 29

31

LIPIDS 32

Lipids Examples: 1. 2. 3. 4. 5. 6. Fats (triglycerides) Phospholipids Oils Waxes Steroid hormones Triglycerides 33

Lipids Six functions of lipids: 1. Long term energy storage 2. Protection against heat loss (insulation) 3. Protection against physical shock 4. Protection against water loss 5. Chemical messengers (hormones) 6. Major component of membranes (phospholipids) 34
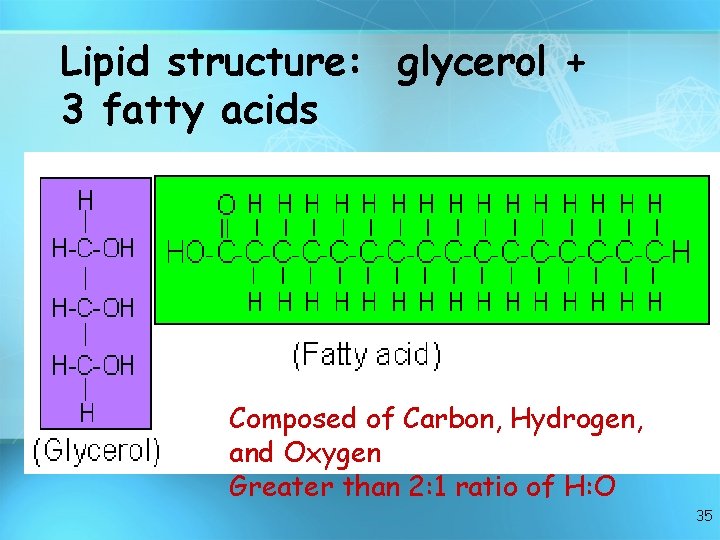
Lipid structure: glycerol + 3 fatty acids Composed of Carbon, Hydrogen, and Oxygen Greater than 2: 1 ratio of H: O 35
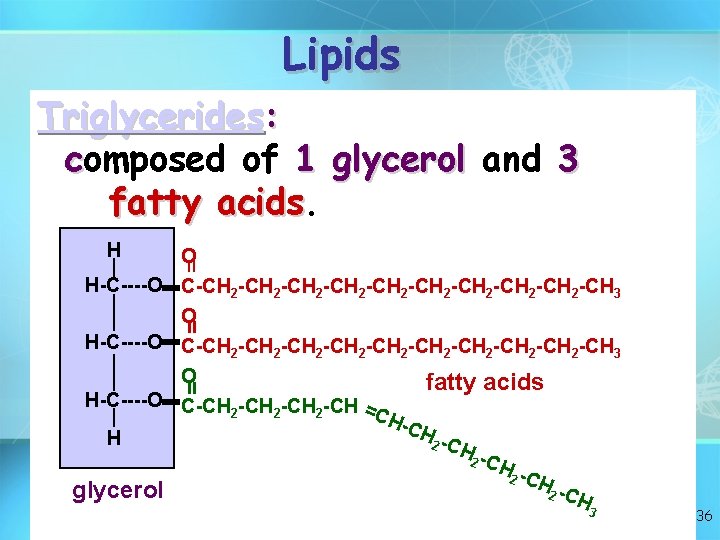
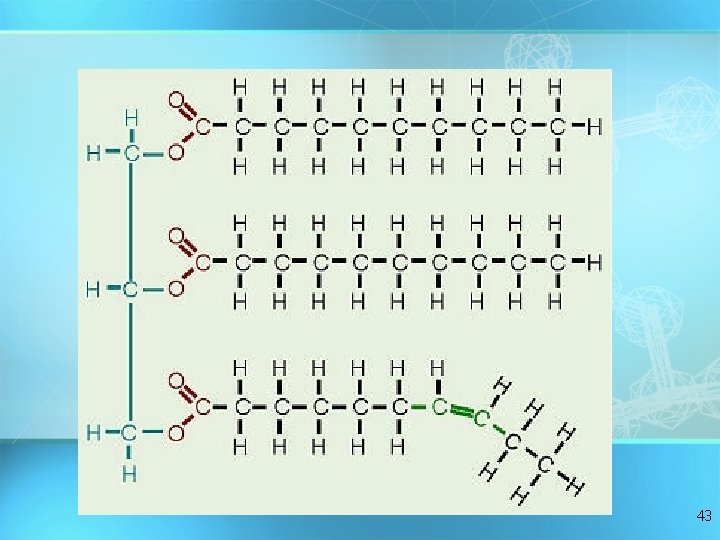
Lipids Triglycerides: composed of 1 glycerol and 3 fatty acids H = O H-C----O C-CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -CH 3 O fatty acids H-C----O C-CH -CH = 2 2 2 CH -CH H 2 -C H 2 C Hglycerol 2 C H = = 3 36
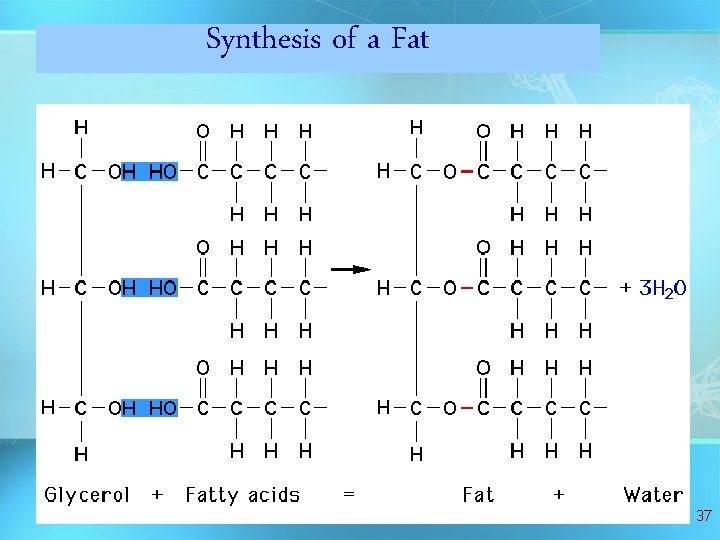
Synthesis of a Fat 37
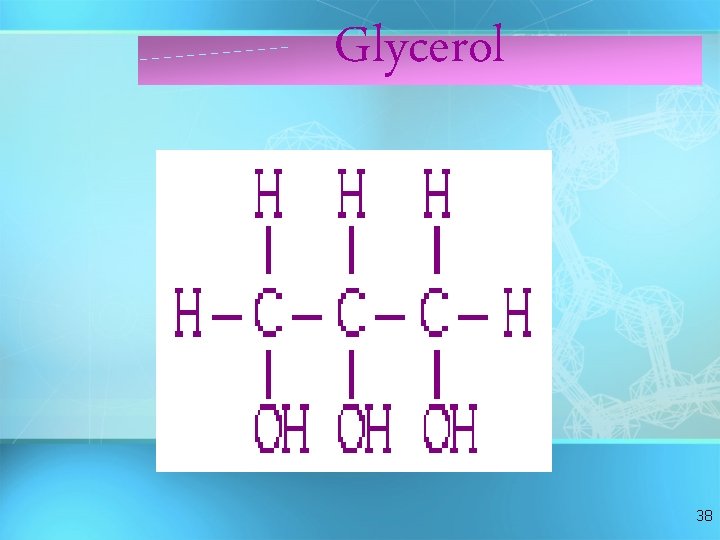
Glycerol 38
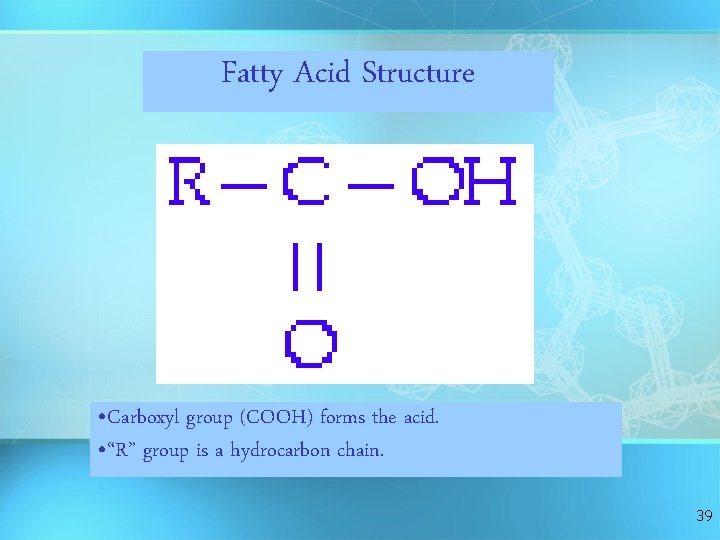
Fatty Acid Structure • Carboxyl group (COOH) forms the acid. • “R” group is a hydrocarbon chain. 39
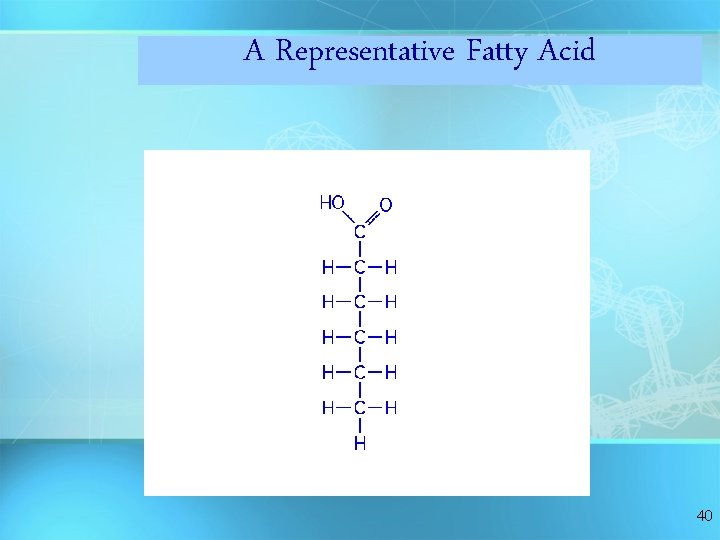
A Representative Fatty Acid 40

Fatty Acids There are two kinds of fatty acids you may see these on food labels: = 1. Saturated fatty acids: no double bonds (bad) O saturated C-CH 2 -CH 2 -CH 2 -CH 3 = 2. Unsaturated fatty acids: double bonds (good) O unsaturated C-CH 2 -CH=CH -CH 2 -C H 2 C H 3 41
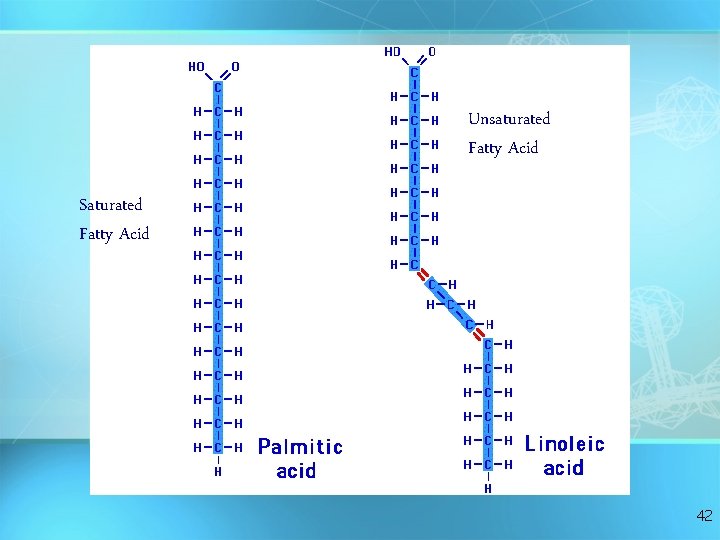
Unsaturated Fatty Acid Saturated Fatty Acid 42
43
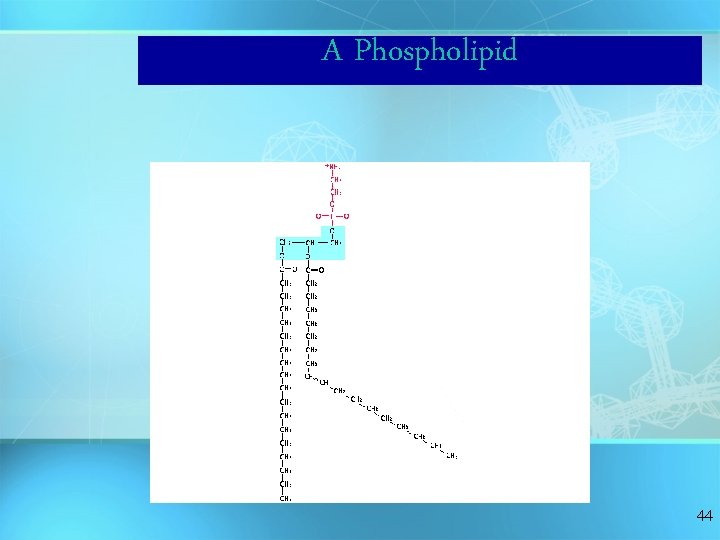
A Phospholipid 44
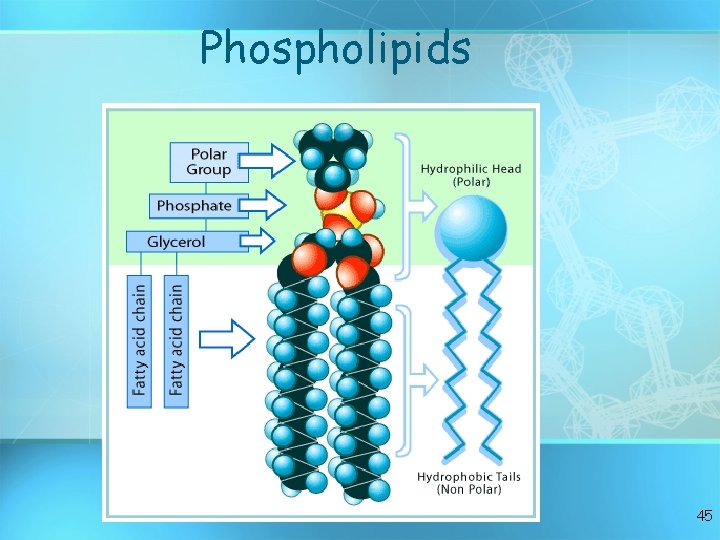
Phospholipids 45
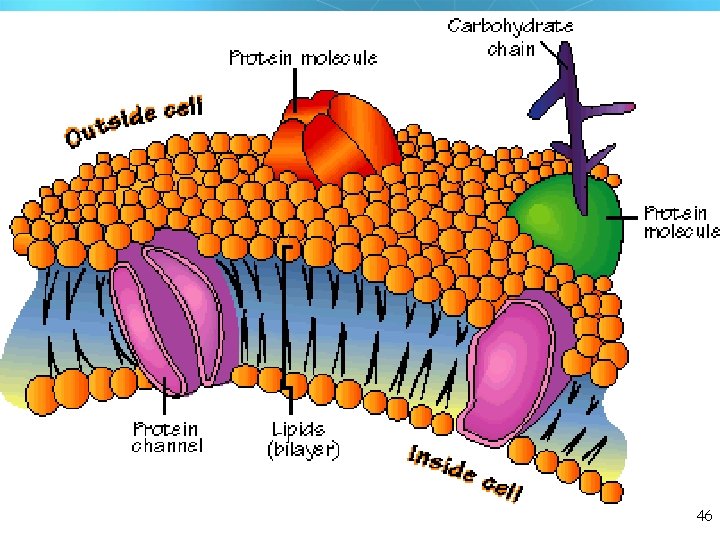
46

Proteins: the body’s worker molecules 47
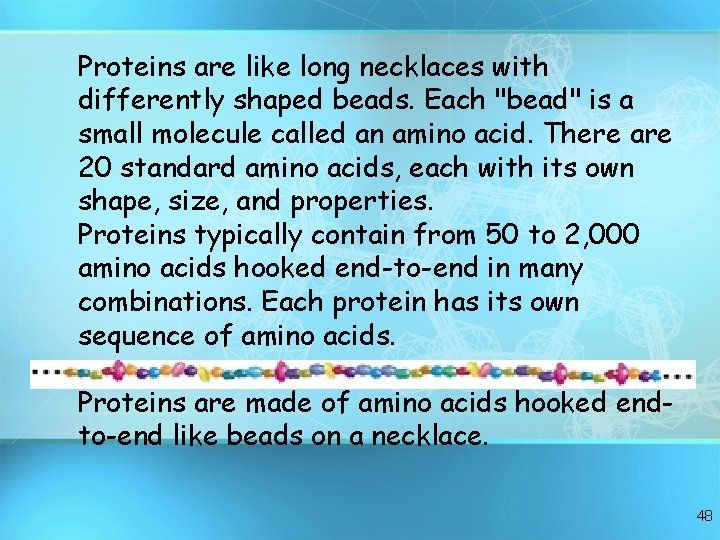
Proteins are like long necklaces with differently shaped beads. Each "bead" is a small molecule called an amino acid. There are 20 standard amino acids, each with its own shape, size, and properties. Proteins typically contain from 50 to 2, 000 amino acids hooked end-to-end in many combinations. Each protein has its own sequence of amino acids. Proteins are made of amino acids hooked endto-end like beads on a necklace. 48
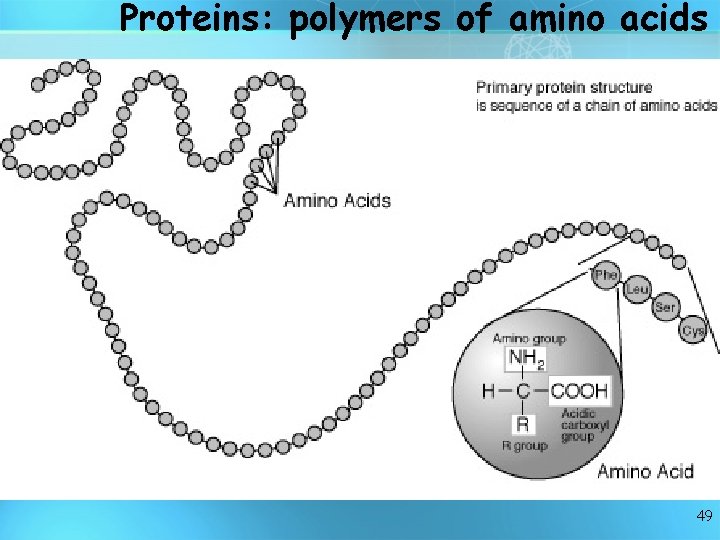
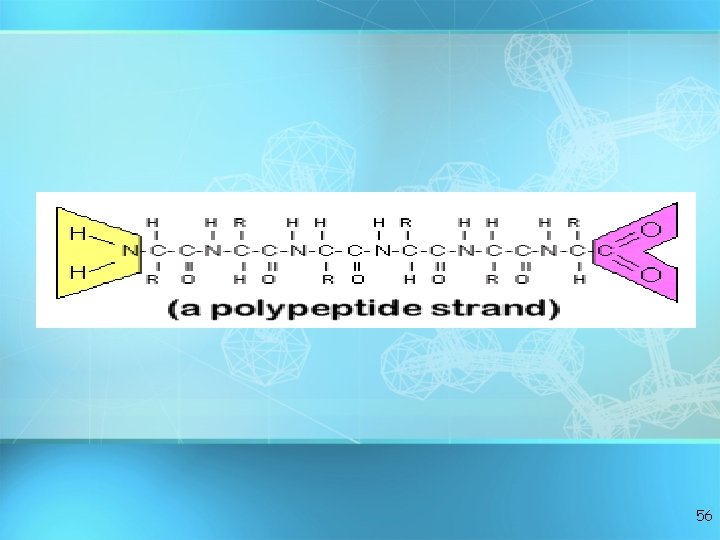
Proteins: polymers of amino acids 49
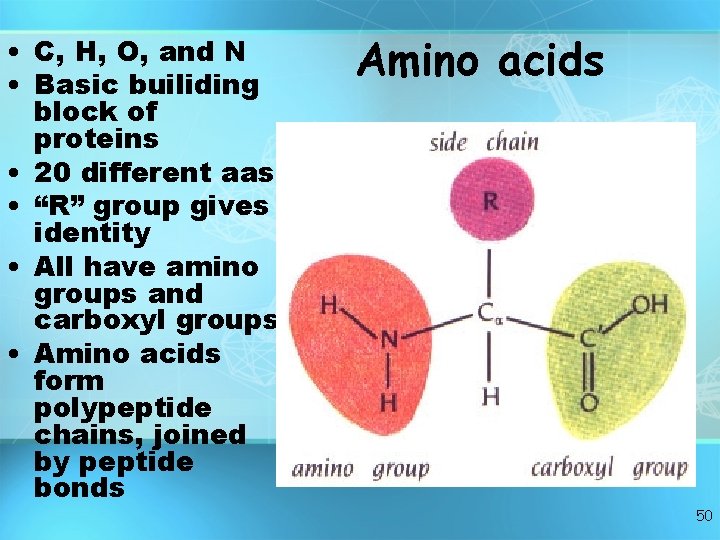
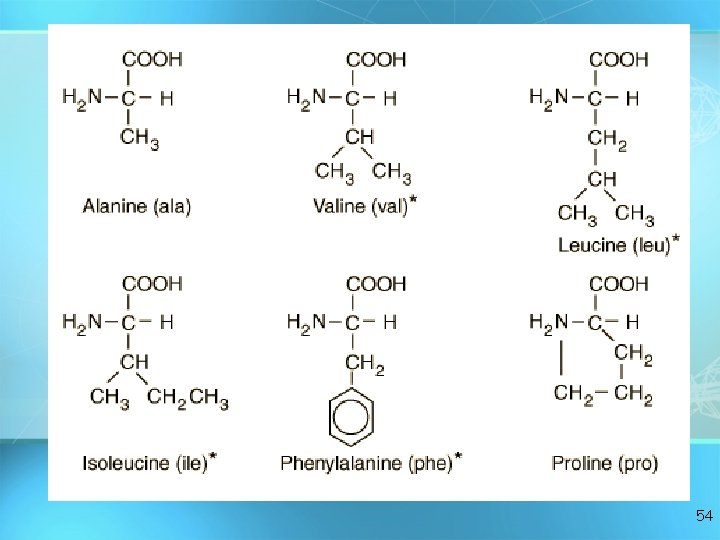
• C, H, O, and N • Basic builiding block of proteins • 20 different aas • “R” group gives identity • All have amino groups and carboxyl groups • Amino acids form polypeptide chains, joined by peptide bonds Amino acids 50
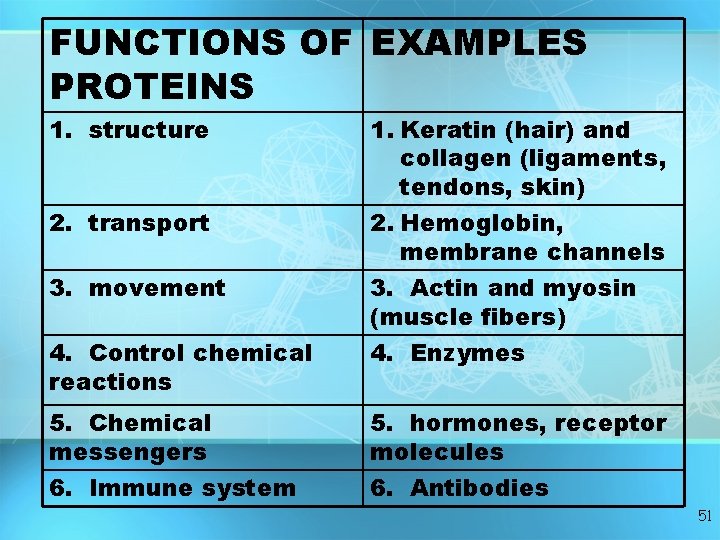
FUNCTIONS OF EXAMPLES PROTEINS 1. structure 2. transport 3. movement 4. Control chemical reactions 1. Keratin (hair) and collagen (ligaments, tendons, skin) 2. Hemoglobin, membrane channels 3. Actin and myosin (muscle fibers) 4. Enzymes 5. Chemical messengers 5. hormones, receptor molecules 6. Immune system 6. Antibodies 51
52
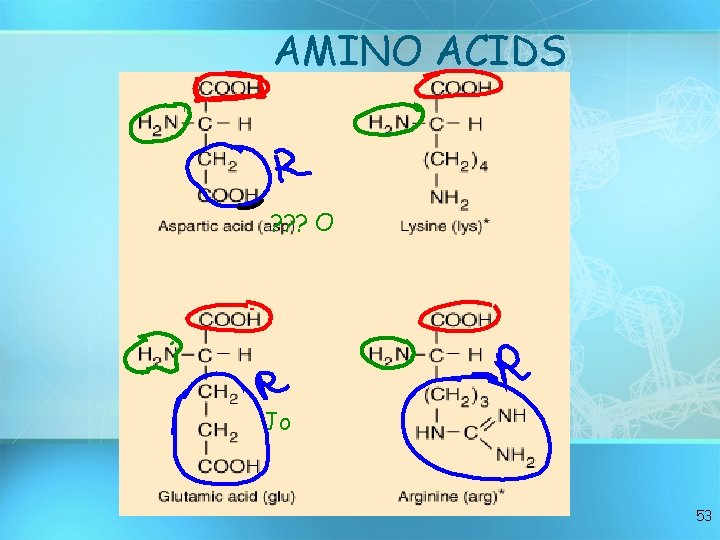
AMINO ACIDS ? ? ? O Jo 53
54
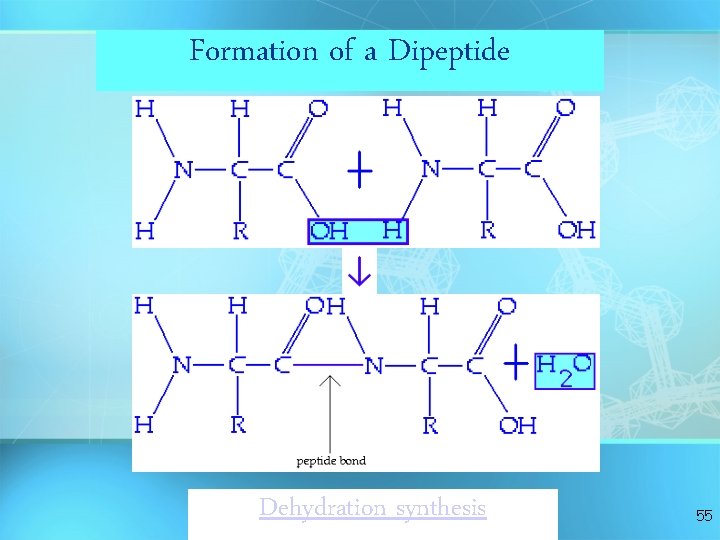
Formation of a Dipeptide Dehydration synthesis 55
56
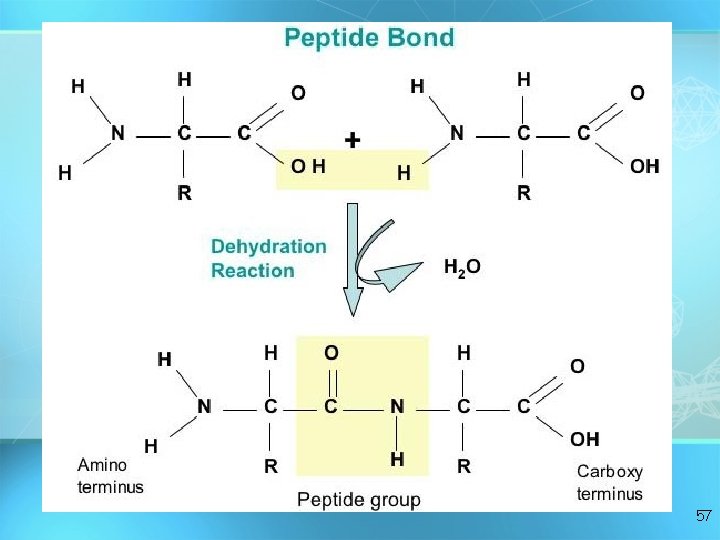
57
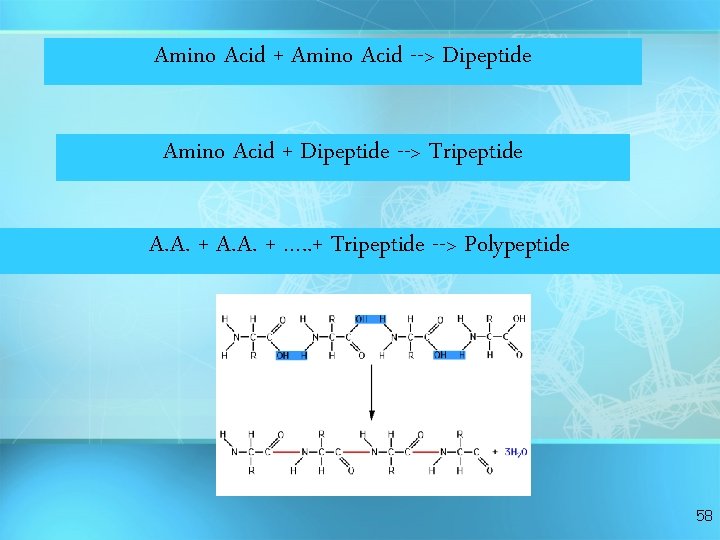
Amino Acid + Amino Acid --> Dipeptide Amino Acid + Dipeptide --> Tripeptide A. A. + …. . + Tripeptide --> Polypeptide 58
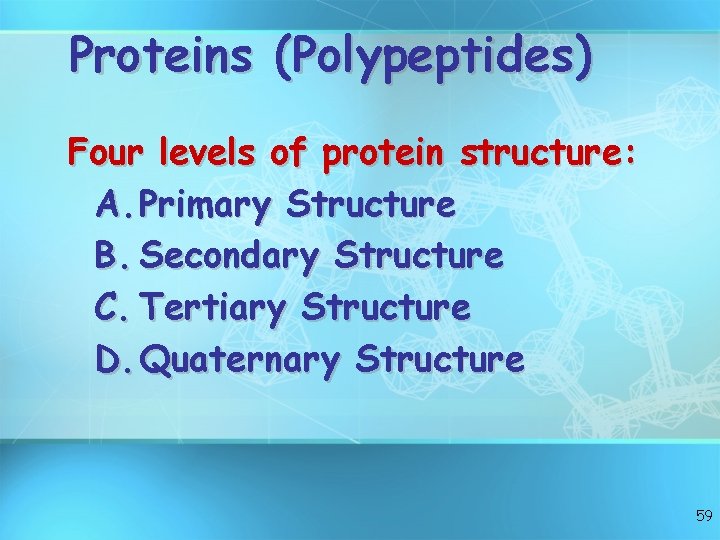
Proteins (Polypeptides) Four levels of protein structure: A. Primary Structure B. Secondary Structure C. Tertiary Structure D. Quaternary Structure 59
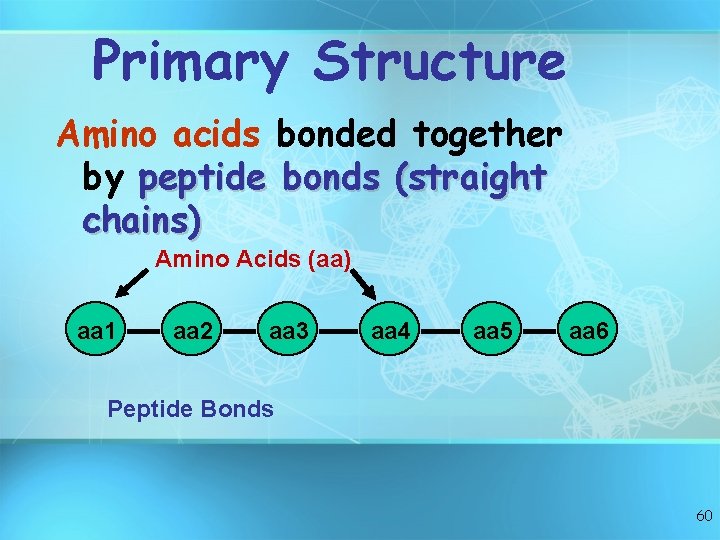
Primary Structure Amino acids bonded together by peptide bonds (straight chains) Amino Acids (aa) aa 1 aa 2 aa 3 aa 4 aa 5 aa 6 Peptide Bonds 60
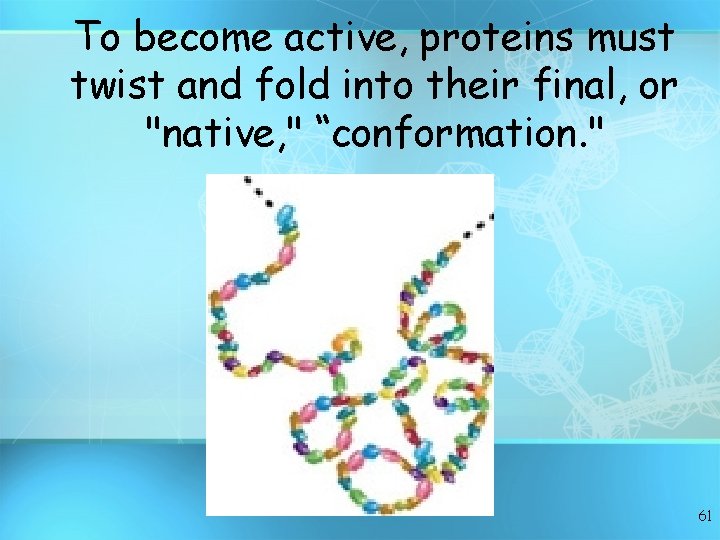
To become active, proteins must twist and fold into their final, or "native, " “conformation. " 61
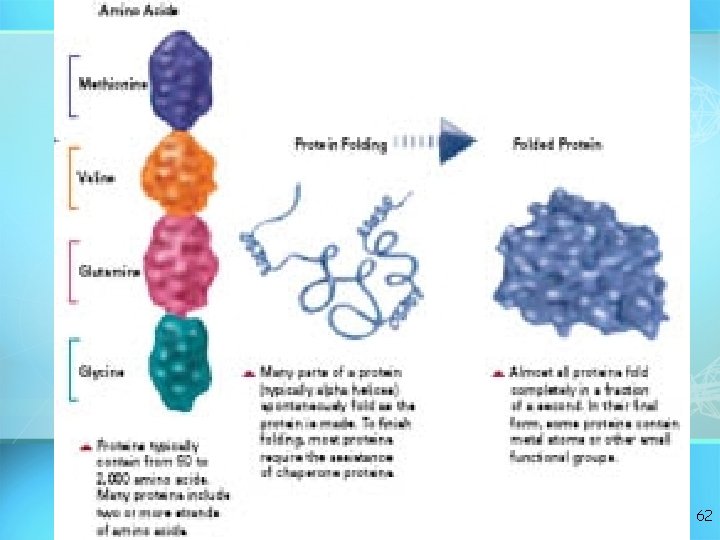
62
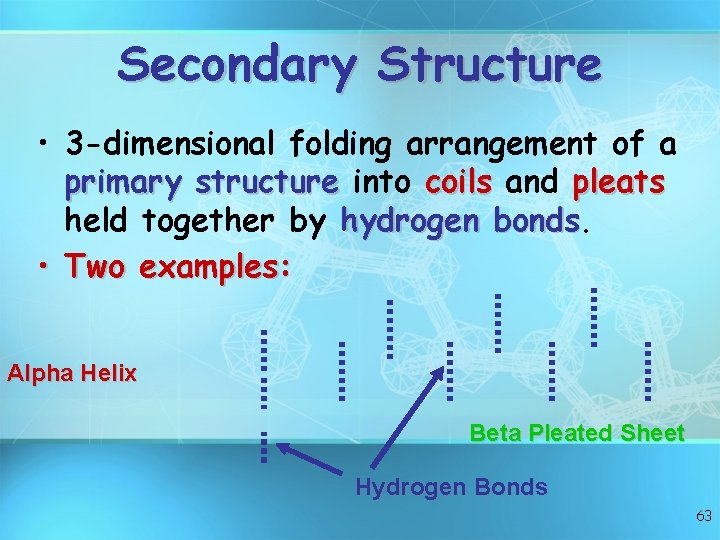
Secondary Structure • 3 -dimensional folding arrangement of a primary structure into coils and pleats held together by hydrogen bonds • Two examples: Alpha Helix Beta Pleated Sheet Hydrogen Bonds 63

64
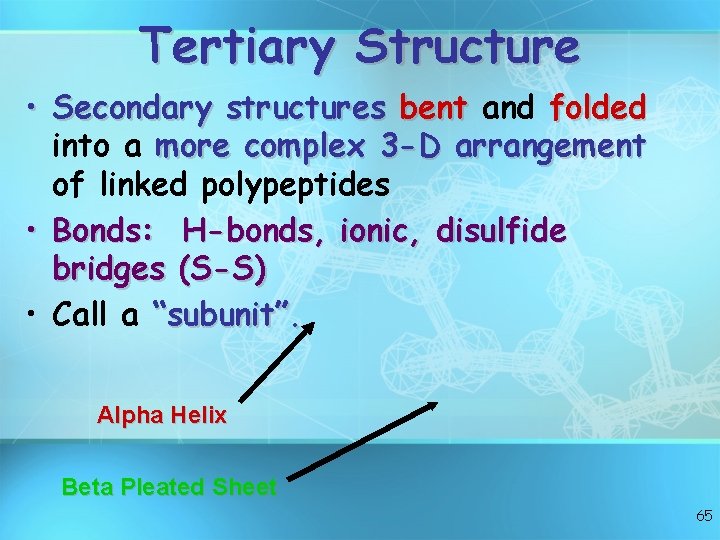
Tertiary Structure • Secondary structures bent and folded into a more complex 3 -D arrangement of linked polypeptides • Bonds: H-bonds, ionic, disulfide bridges (S-S) • Call a “subunit”. Alpha Helix Beta Pleated Sheet 65
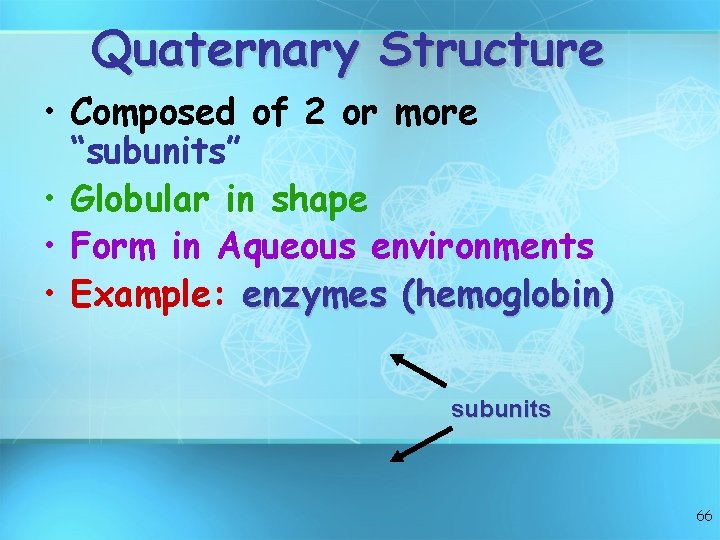
Quaternary Structure • Composed of 2 or more “subunits” • Globular in shape • Form in Aqueous environments • Example: enzymes (hemoglobin) subunits 66

How heat denatures a protein 67

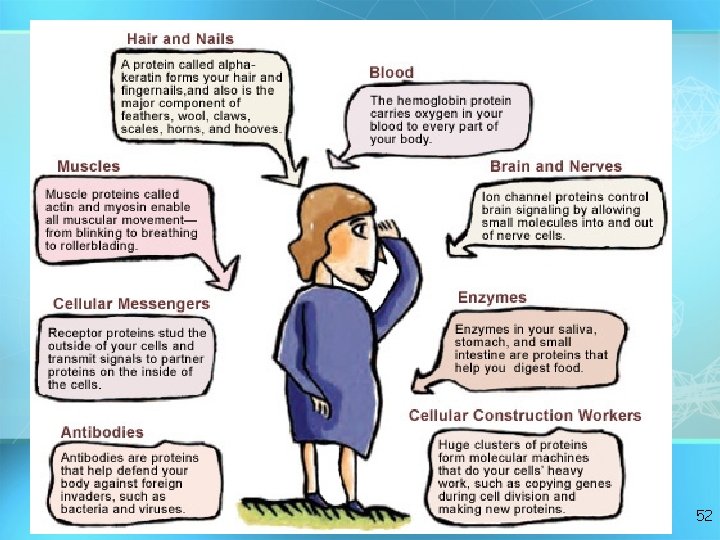
Provocative Proteins (several hundred thousand different proteins in our body) Spider webs and silk fibers are made of the strong, pliable protein fibroin. Spider silk is stronger than a steel rod of the same diameter, yet it is much more elastic, so scientists hope to use it for products as diverse as bulletproof vests and artificial joints. 68

The light of fireflies (also called lightning bugs) is made possible by a protein called luciferase. Although most predators stay away from the bittertasting insects, some frogs eat so many fireflies that they glow! 69

The deadly venoms of cobras, scorpions, and puffer fish contain small proteins that act as nerve toxins. Some sea snails stun their prey (and occasionally, unlucky humans) with up to 50 such toxins. One of these toxins has been developed into a drug called Prialt®, which is used to treat severe pain that is unresponsive even to morphine. 70

Sometimes ships in the northwest Pacific Ocean leave a trail of eerie green light. The light is produced by a protein in jellyfish when the creatures are jostled by ships. Because the trail traces the path of ships at night, this green fluorescent protein has interested the Navy for many years. 71

If a recipe calls for rhino horn, ibis feathers, and porcupine quills, try substituting your own hair or fingernails. It's all the same stuff— alpha-keratin, a tough, waterresistant protein that is also the main component of wool, scales, hooves, tortoise shells, and the outer layer of your skin. 72

Nucleic Acids 73
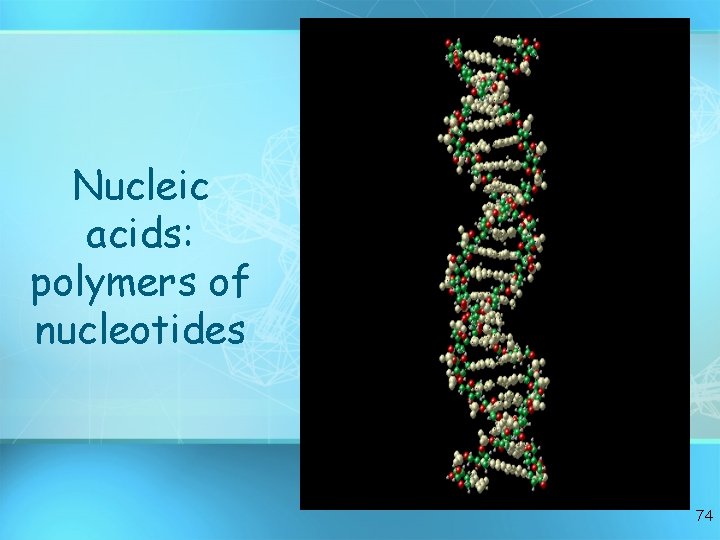
Nucleic acids: polymers of nucleotides 74

What are they made of ? • Simple units called nucleotides, connected in long chains • Nucleotides have 3 parts: 1 - 5 -Carbon sugar (pentose) 2 - Nitrogen containing base (made of C, H and N) 3 - A phosphate group ( P ) • The P groups make the links that unite the sugars (hence a “sugarphosphate backbone” 75
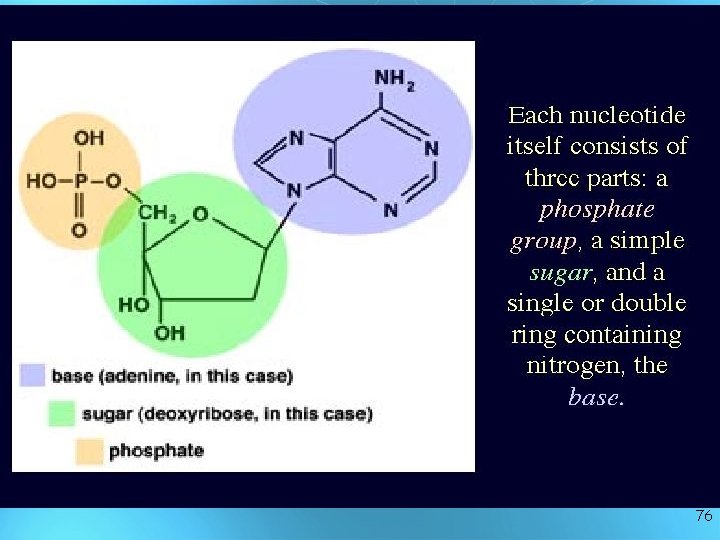
76

What do they do ? • Dictate amino-acid sequence in proteins • Give information to chromosomes, which is then passed from parent to offspring 77
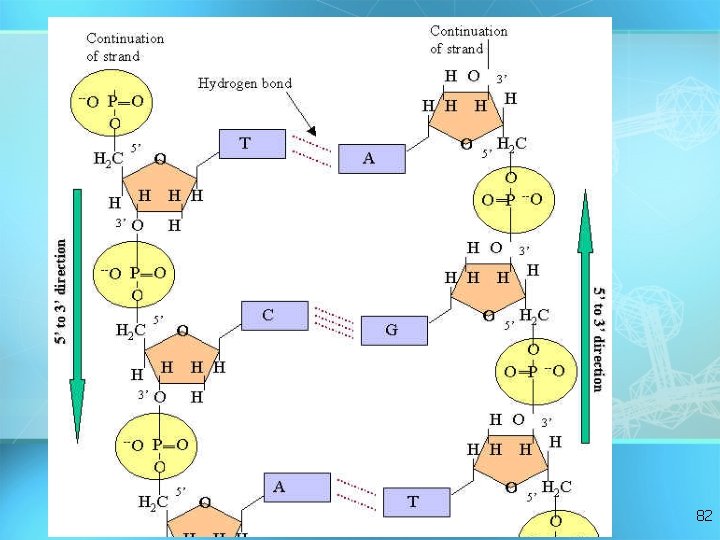
Nucleic acids • Two types: a. Deoxyribonucleic acid (DNAdouble helix) b. Ribonucleic acid (RNA-single strand) • Nucleic acids are composed of long chains of nucleotides linked by dehydration synthesis 78
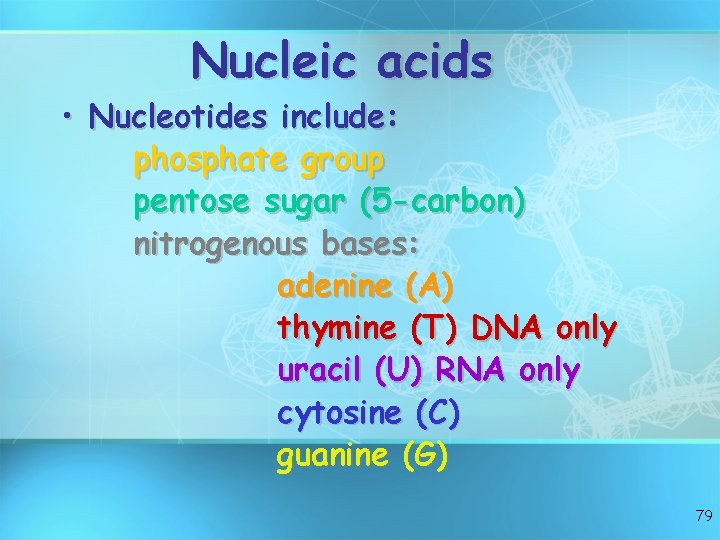
Nucleic acids • Nucleotides include: phosphate group pentose sugar (5 -carbon) nitrogenous bases: adenine (A) thymine (T) DNA only uracil (U) RNA only cytosine (C) guanine (G) 79
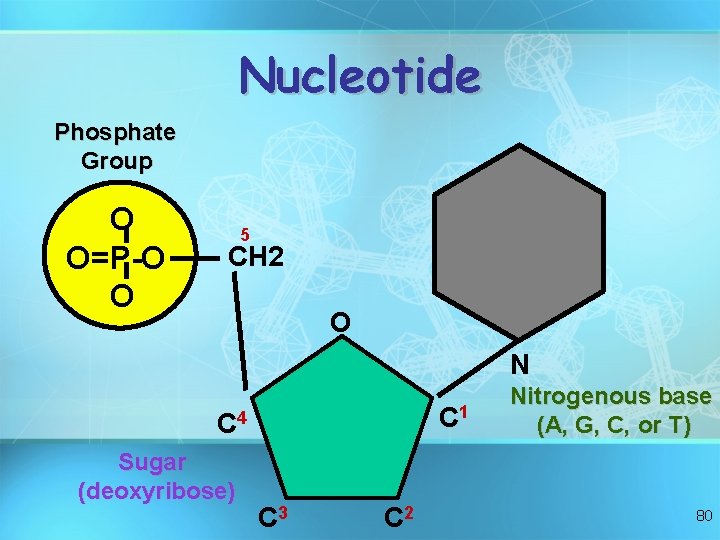
Nucleotide Phosphate Group O O=P-O O 5 CH 2 O N C 1 C 4 Sugar (deoxyribose) C 3 C 2 Nitrogenous base (A, G, C, or T) 80
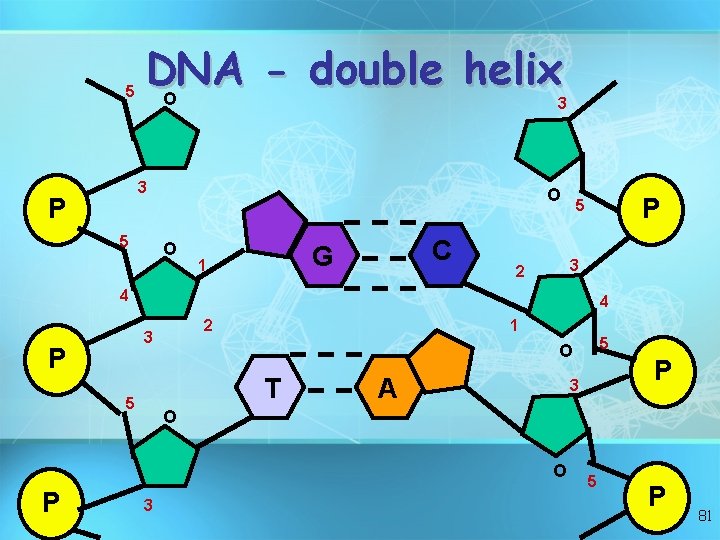
5 DNA double helix O 3 3 P 5 O O C G 1 P 5 3 2 4 4 2 3 P 1 T 5 A P 3 O O P 5 O 3 5 P 81
82

Review film: compounds of carbon 83
- Slides: 82