Macro Micro Nano University of Wisconsin MRSEC Macro











- Slides: 19

Macro Micro Nano University of Wisconsin MRSEC

Macro Micro Nano Grains of sand Red blood cells DNA (width) Often requires a microscope to see Requires a highly specialized microscope, such as a Scanning Electron Microscope (SEM), to see Can see with your eyes University of Wisconsin MRSEC

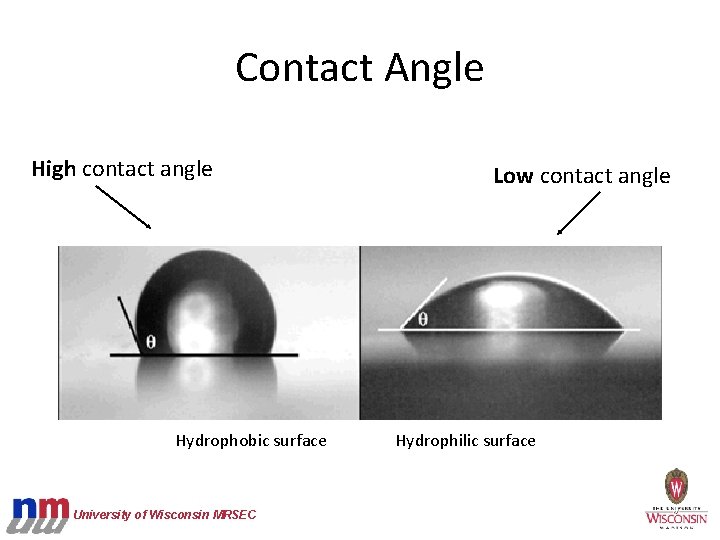
Contact Angle High contact angle Hydrophobic surface University of Wisconsin MRSEC Low contact angle Hydrophilic surface

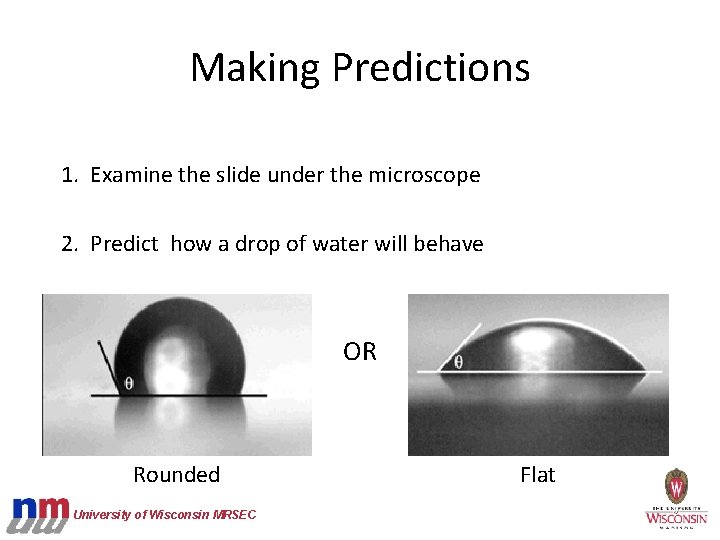
Making Predictions 1. Examine the slide under the microscope 2. Predict how a drop of water will behave OR Rounded University of Wisconsin MRSEC Flat

SEM of Soot Int. J. Electrochem. Sci. , 6 (2011) 1269 – 1276 University of Wisconsin MRSEC

What is nanotechnology? 1. The nanometer is extremely small. 2. At the nanometer scale, many things behave differently. 3. We can use this new behavior to make new technologies. University of Wisconsin MRSEC

What is nanotechnology? Nanotechnology is the understanding and control of matter 1 to 100 nanometers in size. University of Wisconsin MRSEC

Nanotechnology Anti-biofouling applications Scientists coat surfaces with nanoparticles to prevent biofouling, seen here on a submarine. University of Wisconsin MRSEC

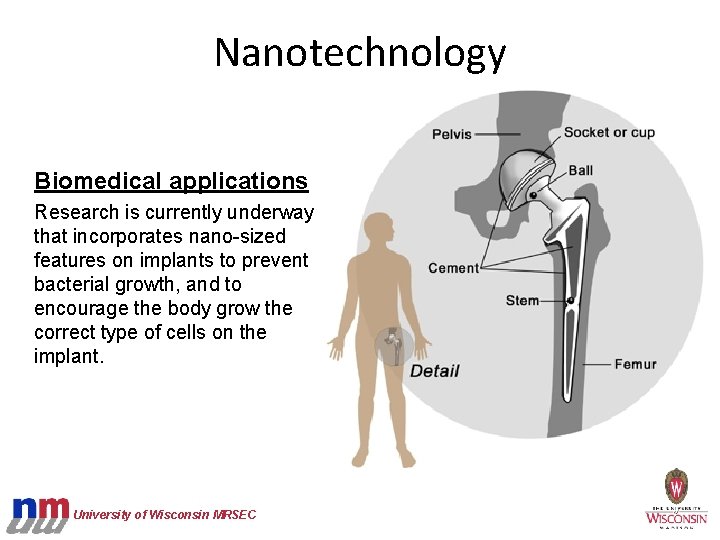
Nanotechnology Biomedical applications Research is currently underway that incorporates nano-sized features on implants to prevent bacterial growth, and to encourage the body grow the correct type of cells on the implant. University of Wisconsin MRSEC

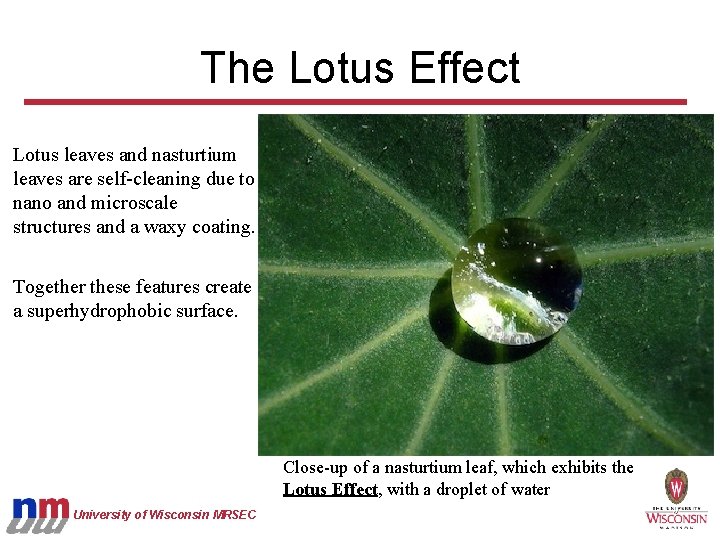
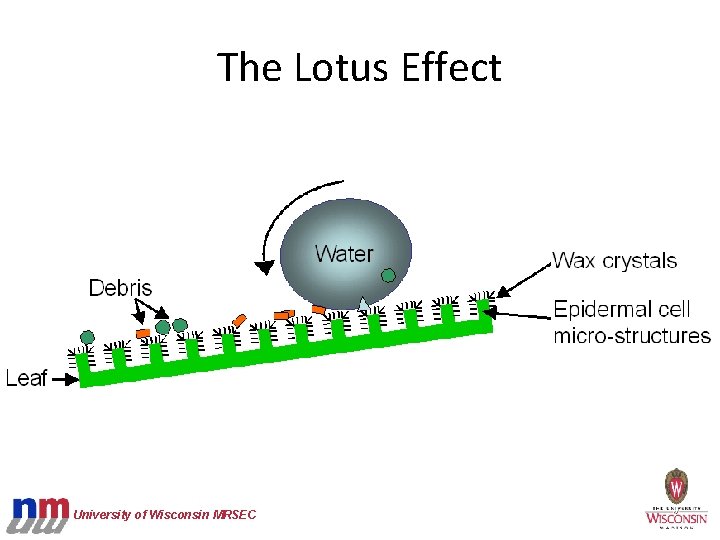
The Lotus Effect Lotus leaves and nasturtium leaves are self-cleaning due to nano and microscale structures and a waxy coating. Together these features create a superhydrophobic surface. Close-up of a nasturtium leaf, which exhibits the Lotus Effect, with a droplet of water University of Wisconsin MRSEC

The Lotus Effect University of Wisconsin MRSEC

Hydrophobic Surfaces • Water “fearing” • Repel water • Non-polar • Rounded water drop University of Wisconsin MRSEC

Superhydrophobic Surfaces • VERY water “fearing” • Non-polar • Nano-scale surface features • Water rolls off when surface is held at an angle • Water drop is very rounded (contact angle greater than 150°) University of Wisconsin MRSEC

Hydrophilic Surfaces • Water “loving” • Polar • Flat water drop • Water drop may absorb into material • Leaves a streak of water when rolling off University of Wisconsin MRSEC

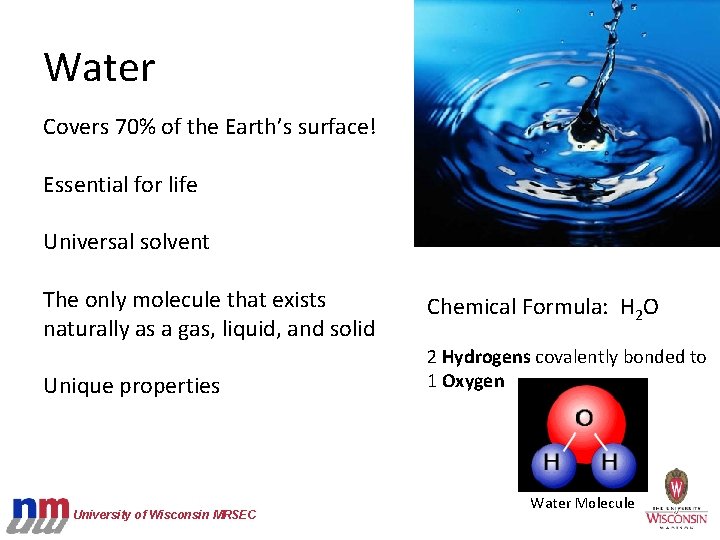
Water Covers 70% of the Earth’s surface! Essential for life Universal solvent The only molecule that exists naturally as a gas, liquid, and solid Unique properties University of Wisconsin MRSEC Chemical Formula: H 2 O 2 Hydrogens covalently bonded to 1 Oxygen Water Molecule

Properties of Water 1. Polarity 2. Hydrogen Bonding 3. High Surface Tension University of Wisconsin MRSEC

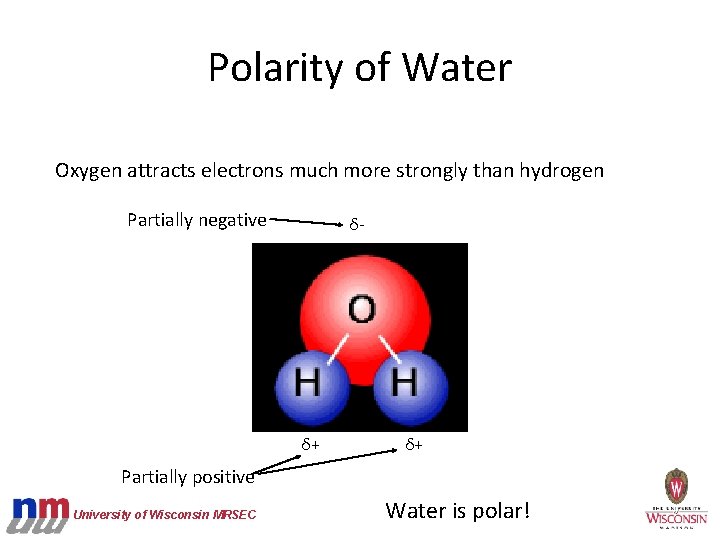
Polarity of Water Oxygen attracts electrons much more strongly than hydrogen Partially negative δ- δ+ δ+ Partially positive University of Wisconsin MRSEC Water is polar!

Hydrogen Bonding Attraction between water molecules University of Wisconsin MRSEC

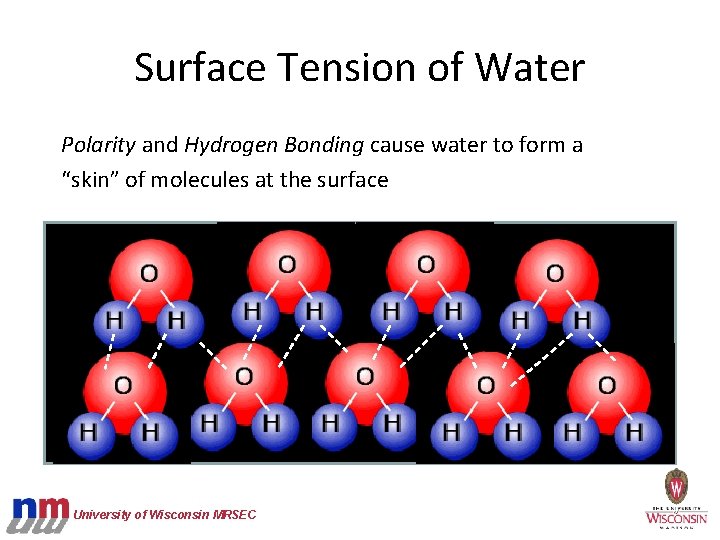
Surface Tension of Water Polarity and Hydrogen Bonding cause water to form a “skin” of molecules at the surface University of Wisconsin MRSEC
 Macro micro nano scale
Macro micro nano scale Mrsec science slam
Mrsec science slam Milli micro nano
Milli micro nano Notacion cientifica mili micro nano pico
Notacion cientifica mili micro nano pico John summerscales
John summerscales Giga mega tera
Giga mega tera Milli centi deci chart
Milli centi deci chart Law of definite proportion class 11
Law of definite proportion class 11 Milli chemistry
Milli chemistry Mm micro nano pico
Mm micro nano pico Mega kilo deci centi milli micro nano
Mega kilo deci centi milli micro nano Ecuaciones dimensionales
Ecuaciones dimensionales Uw integrative medicine
Uw integrative medicine University of wisconsin madison chemical engineering
University of wisconsin madison chemical engineering University of wisconsin-madison biomedical engineering
University of wisconsin-madison biomedical engineering University of wisconsin nickname
University of wisconsin nickname Oshkosh employee discount
Oshkosh employee discount Macro y micro filtro
Macro y micro filtro Macro e micro contesto
Macro e micro contesto Micro market and macro environment
Micro market and macro environment