m TOR Signaling and Drug Development in Cancer






- Slides: 32

m. TOR Signaling and Drug Development in Cancer 財團法人台灣癌症臨床研究發展基金會

Ø Nature Reviews Clinical Oncology 2010; 7: 209– 19 Ø 2010 IF: 10. 787 Ø Review article

OUTLINE Ø Introduction to m. TOR inhibitors Ø m. TOR signaling pathway Ø m. TOR inhibitors and transplant Ø m. TOR inhibitors and cancer Ø Current development of m. TOR inhibitors Ø Conclusion

Background-1 Ø Rapamycin – Triene macrolide antibiotic from S. hygroscopicus in a soil sample from Easter Island (Rapa Nui) in 1975 – Originally developed as antifungal agent – Sirolimus (Rapamune®) approved by FDA in 1999 as immunosuppressant used to prevent rejection in organ transplant

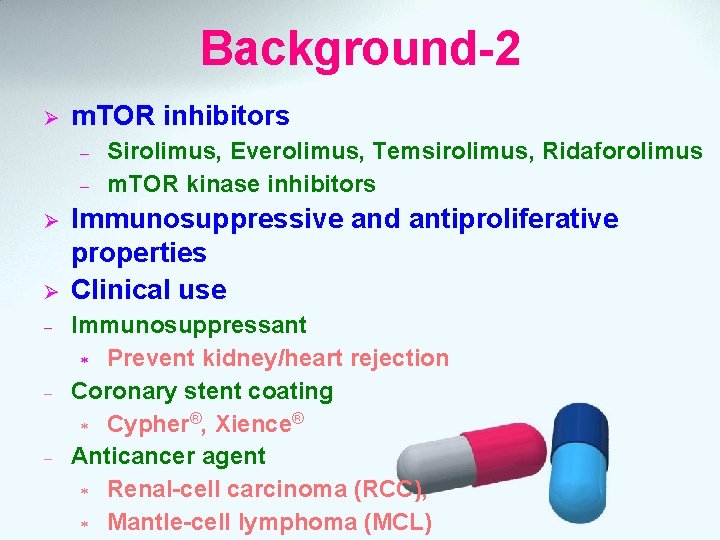
Background-2 Ø m. TOR inhibitors – – Ø Ø - - - Sirolimus, Everolimus, Temsirolimus, Ridaforolimus m. TOR kinase inhibitors Immunosuppressive and antiproliferative properties Clinical use Immunosuppressant * Prevent kidney/heart rejection Coronary stent coating * Cypher®, Xience® Anticancer agent * Renal-cell carcinoma (RCC), * Mantle-cell lymphoma (MCL)

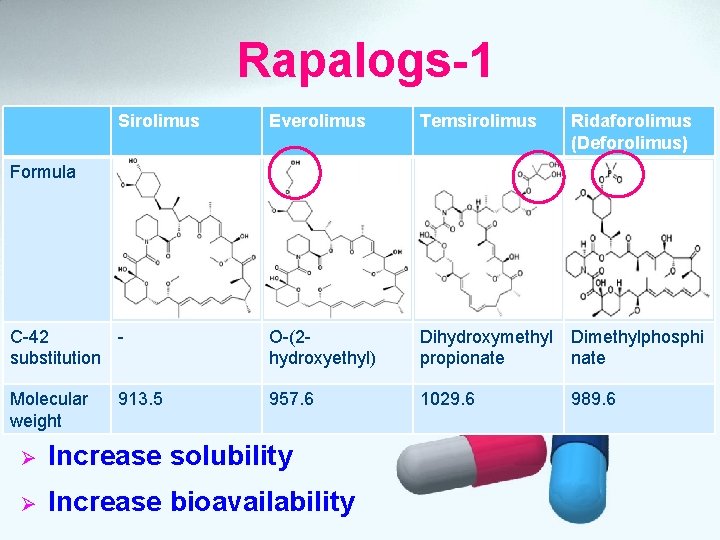
Rapalogs-1 Sirolimus Everolimus Temsirolimus Ridaforolimus (Deforolimus) C-42 substitution O-(2 hydroxyethyl) Dihydroxymethyl Dimethylphosphi propionate Molecular weight 957. 6 1029. 6 Formula 913. 5 Ø Increase solubility Ø Increase bioavailability 989. 6

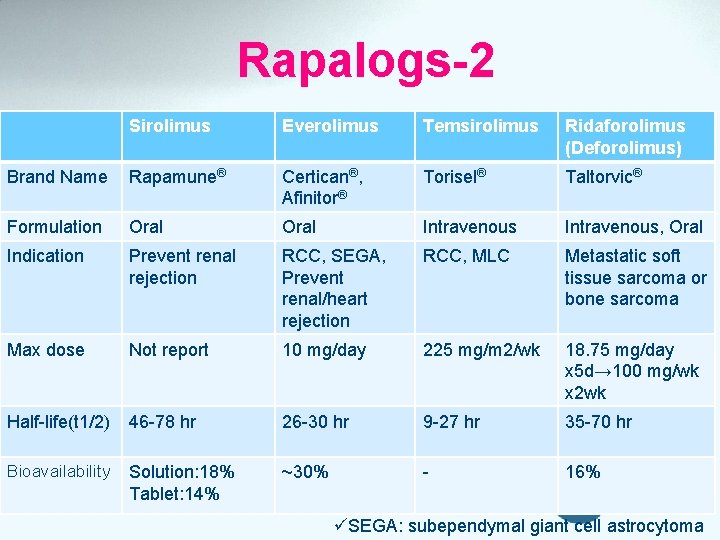
Rapalogs-2 Sirolimus Everolimus Temsirolimus Ridaforolimus (Deforolimus) Brand Name Rapamune® Certican®, Afinitor® Torisel® Taltorvic® Formulation Oral Intravenous, Oral Indication Prevent renal rejection RCC, SEGA, Prevent renal/heart rejection RCC, MLC Metastatic soft tissue sarcoma or bone sarcoma Max dose Not report 10 mg/day 225 mg/m 2/wk 18. 75 mg/day x 5 d→ 100 mg/wk x 2 wk Half-life(t 1/2) 46 -78 hr 26 -30 hr 9 -27 hr 35 -70 hr Bioavailability Solution: 18% Tablet: 14% ~30% - 16% üSEGA: subependymal giant cell astrocytoma

Pharmacologic properties Ø High blood-to-plasma ratio Ø Long plasma half-life Ø CYP 450 metabolite – Drug-drug interaction Ø P-glycoprotein modulated oral absorption – Drug-drug interaction Ø Easily pass BBB – Effective in CNS

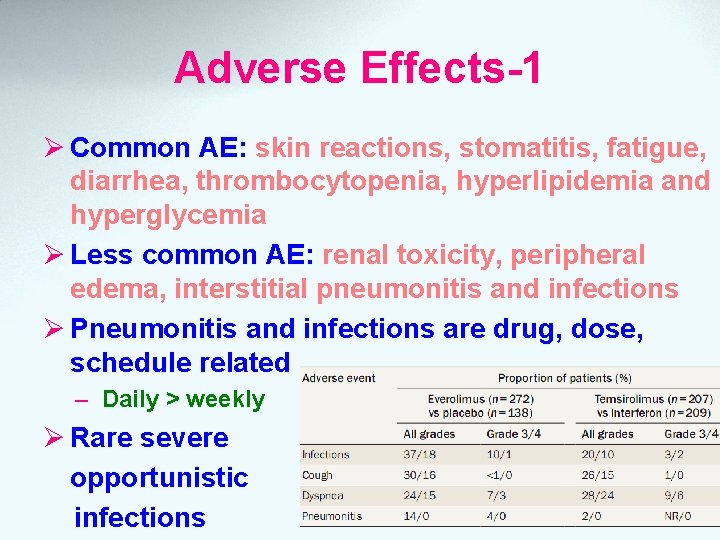
Adverse Effects-1 Ø Common AE: skin reactions, stomatitis, fatigue, diarrhea, thrombocytopenia, hyperlipidemia and hyperglycemia Ø Less common AE: renal toxicity, peripheral edema, interstitial pneumonitis and infections Ø Pneumonitis and infections are drug, dose, schedule related – Daily > weekly Ø Rare severe opportunistic infections

Management of Adverse Effects Ø Generally mild to moderate severity Ø Reversible with DC or dose reduction Ø Specific treatment for hyperlipidemia and hyperglycemia

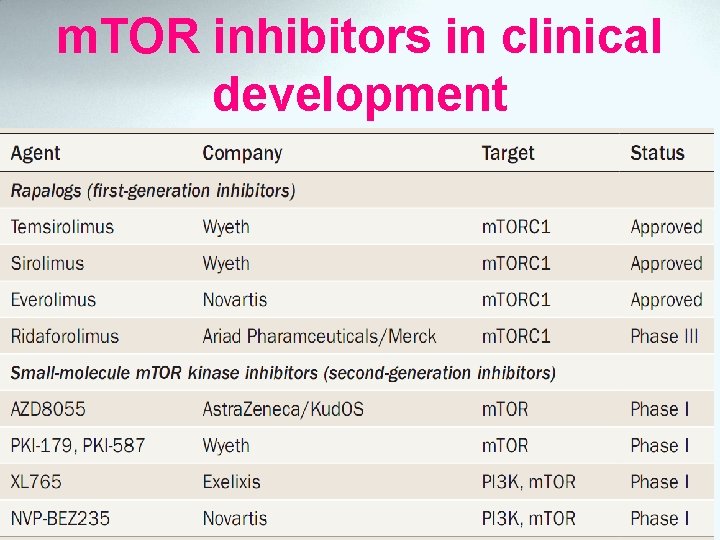
m. TOR inhibitors in clinical development

Ø Introduction to m. TOR inhibitors Ø m. TOR signaling pathway Ø m. TOR inhibitors and transplant Ø m. TOR inhibitors and cancer Ø Current development of m. TOR inhibitors Ø Conclusion

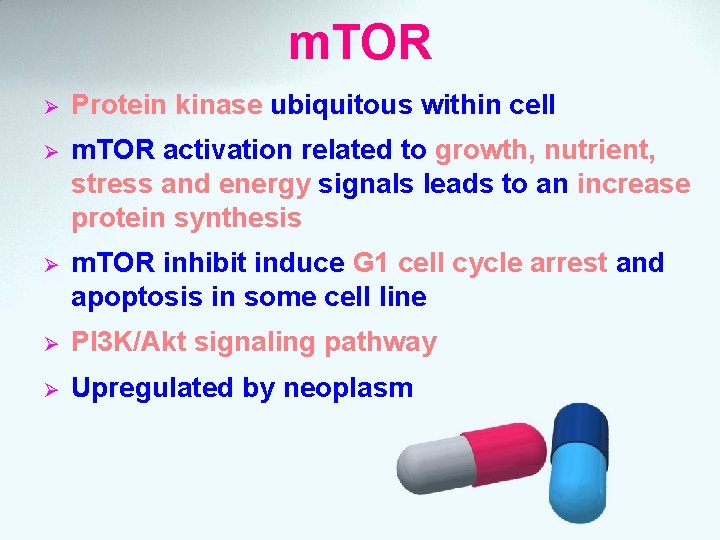
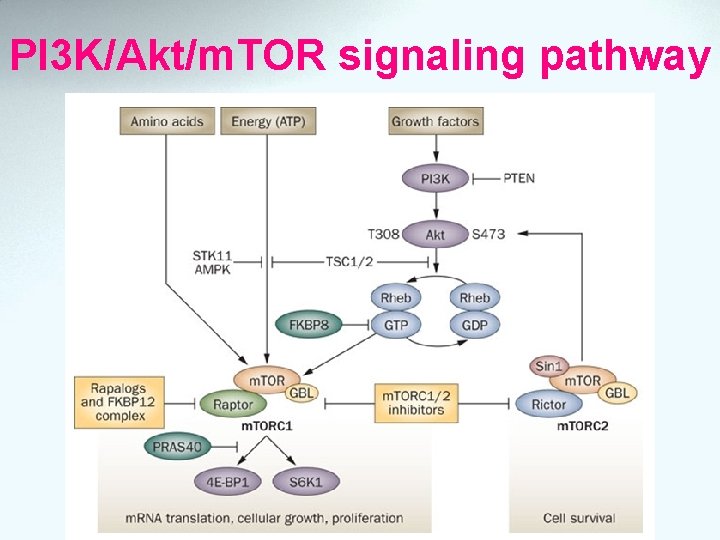
m. TOR Ø Protein kinase ubiquitous within cell Ø m. TOR activation related to growth, nutrient, stress and energy signals leads to an increase protein synthesis Ø m. TOR inhibit induce G 1 cell cycle arrest and apoptosis in some cell line Ø PI 3 K/Akt signaling pathway Ø Upregulated by neoplasm

http: //www. cellsignal. com/reference/pathway/m. Tor. html

Ø Introduction to m. TOR inhibitors Ø m. TOR signaling pathway Ø m. TOR inhibitors and transplant Ø m. TOR inhibitors and cancer Ø Current development of m. TOR inhibitors Ø Conclusion

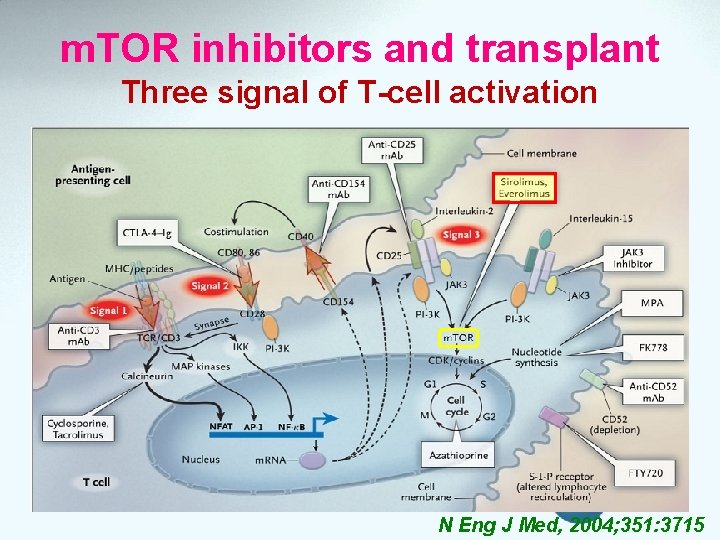
m. TOR inhibitors and transplant Three signal of T-cell activation N Eng J Med, 2004; 351: 3715

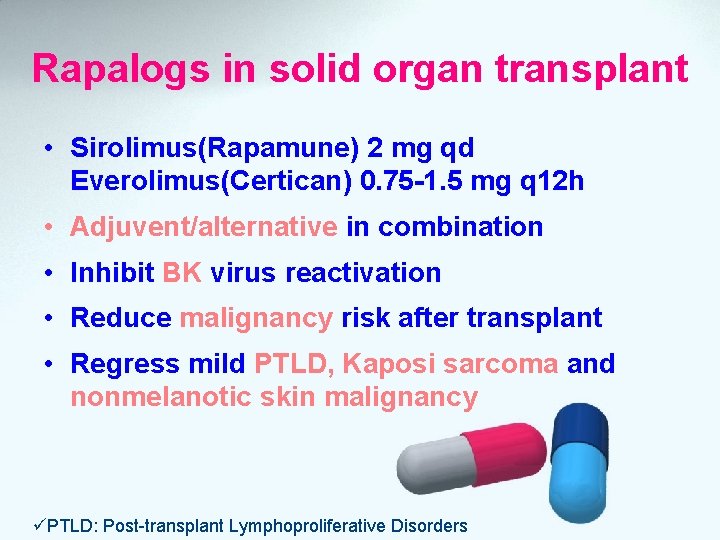
Rapalogs in solid organ transplant • Sirolimus(Rapamune) 2 mg qd Everolimus(Certican) 0. 75 -1. 5 mg q 12 h • Adjuvent/alternative in combination • Inhibit BK virus reactivation • Reduce malignancy risk after transplant • Regress mild PTLD, Kaposi sarcoma and nonmelanotic skin malignancy üPTLD: Post-transplant Lymphoproliferative Disorders

Ø Introduction to m. TOR inhibitors Ø m. TOR signaling pathway Ø m. TOR inhibitors and transplant Ø m. TOR inhibitors and cancer Ø Current development of m. TOR inhibitors Ø Conclusion

PI 3 K/Akt/m. TOR signaling pathway

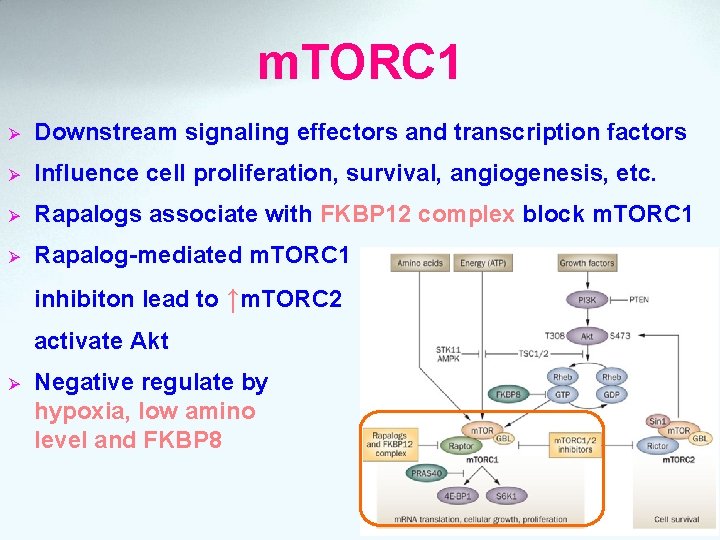
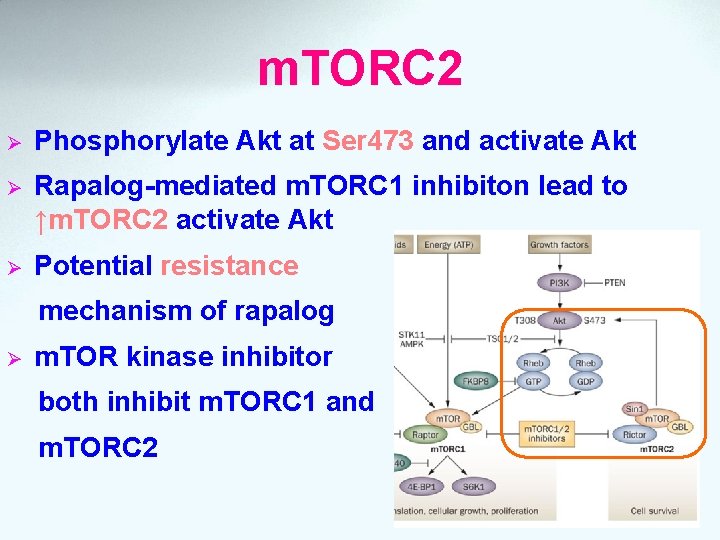
m. TORC 1 Ø Downstream signaling effectors and transcription factors Ø Influence cell proliferation, survival, angiogenesis, etc. Ø Rapalogs associate with FKBP 12 complex block m. TORC 1 Ø Rapalog-mediated m. TORC 1 inhibiton lead to ↑m. TORC 2 activate Akt Ø Negative regulate by hypoxia, low amino level and FKBP 8 acid

m. TORC 2 Ø Phosphorylate Akt at Ser 473 and activate Akt Ø Rapalog-mediated m. TORC 1 inhibiton lead to ↑m. TORC 2 activate Akt Ø Potential resistance mechanism of rapalog Ø m. TOR kinase inhibitor both inhibit m. TORC 1 and m. TORC 2

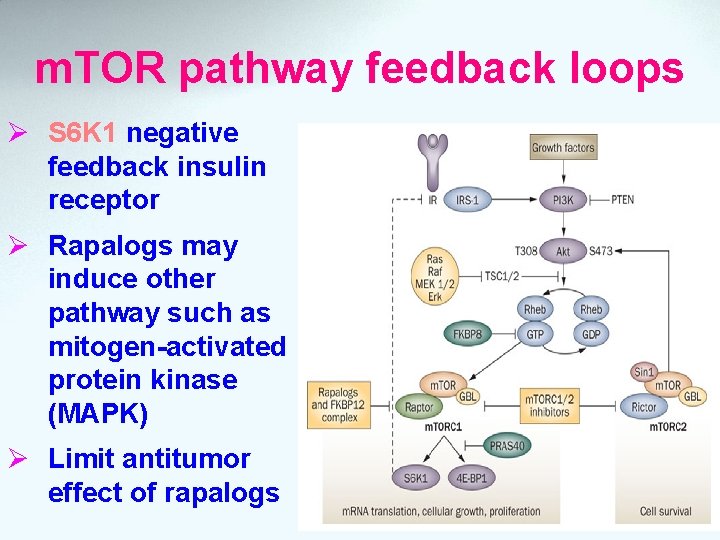
m. TOR pathway feedback loops Ø S 6 K 1 negative feedback insulin receptor Ø Rapalogs may induce other pathway such as mitogen-activated protein kinase (MAPK) Ø Limit antitumor effect of rapalogs

Ø Introduction to m. TOR inhibitors Ø m. TOR signaling pathway Ø m. TOR inhibitors and transplant Ø m. TOR inhibitors and cancer Ø Current development of m. TOR inhibitors Ø Conclusion

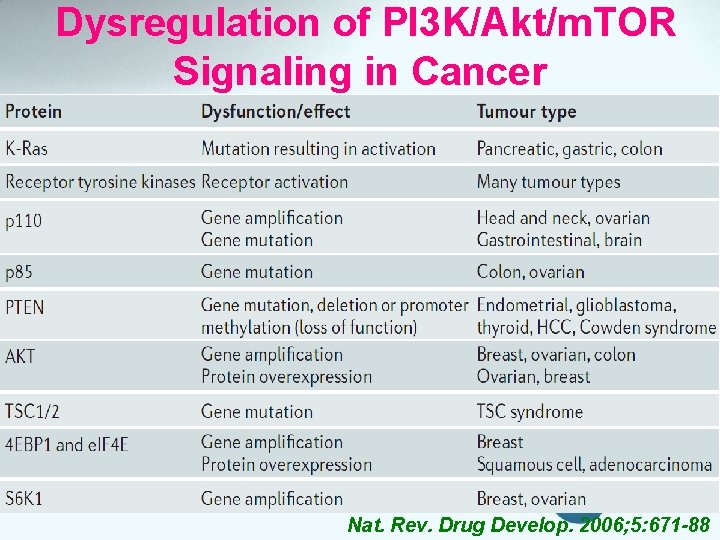
Dysregulation of PI 3 K/Akt/m. TOR Signaling in Cancer Nat. Rev. Drug Develop. 2006; 5: 671 -88

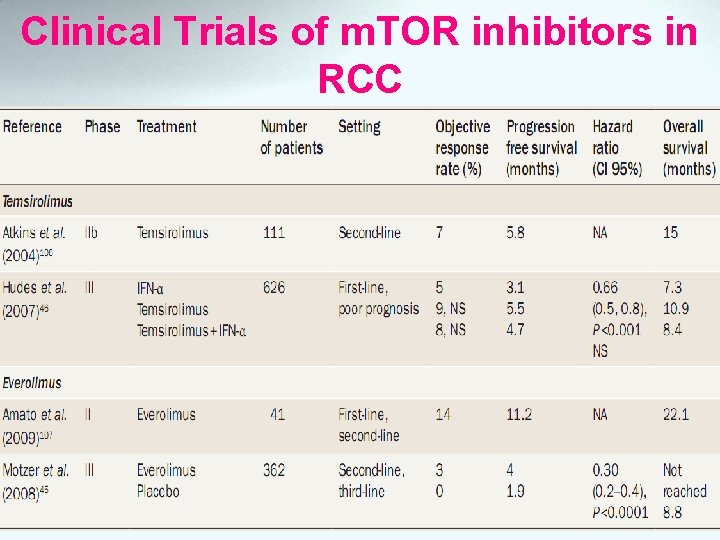
Clinical Trials of m. TOR inhibitors in RCC

Phase II Trials with Rapalogs

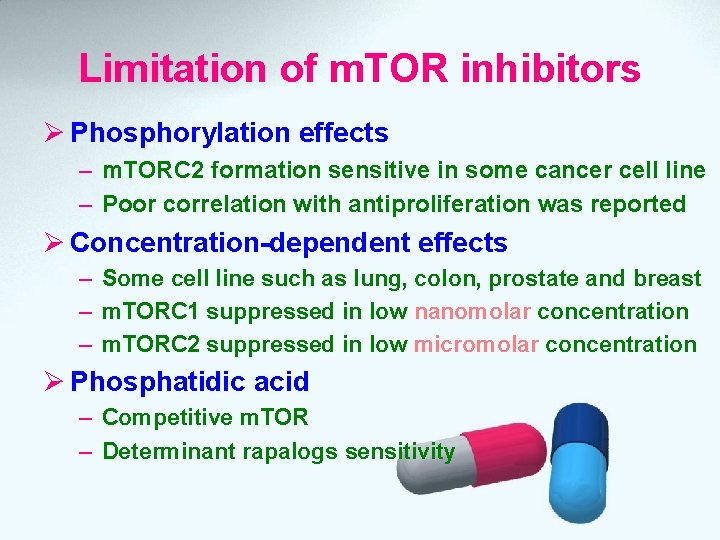
Limitation of m. TOR inhibitors Ø Phosphorylation effects – m. TORC 2 formation sensitive in some cancer cell line – Poor correlation with antiproliferation was reported Ø Concentration-dependent effects – Some cell line such as lung, colon, prostate and breast – m. TORC 1 suppressed in low nanomolar concentration – m. TORC 2 suppressed in low micromolar concentration Ø Phosphatidic acid – Competitive m. TOR – Determinant rapalogs sensitivity

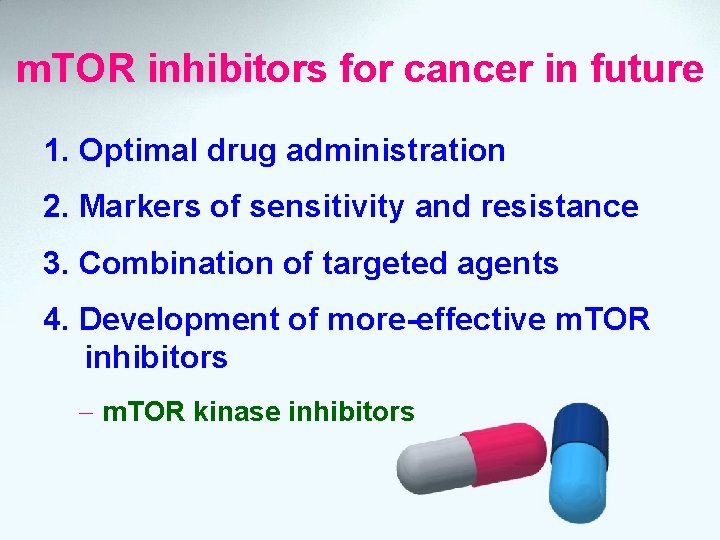
m. TOR inhibitors for cancer in future 1. Optimal drug administration 2. Markers of sensitivity and resistance 3. Combination of targeted agents 4. Development of more-effective m. TOR inhibitors - m. TOR kinase inhibitors

Ø Introduction to m. TOR inhibitors Ø m. TOR signaling pathway Ø m. TOR inhibitors and transplant Ø m. TOR inhibitors and cancer Ø Current development of m. TOR inhibitors Ø Conclusion

Conclusion-1 Ø m. TOR is a central regulator of cell proliferation Ø In some tumor types, such as RCC and certain lymphomas, m. TOR as key role in tumor cell proliferation and angiogenesis Ø Temsirolimus and everolimus are approved as monotherapy in advanced RCC

Conclusion-2 Ø Temsirolimus also approved in MCL with notable improvement in PFS Ø Biomarkers to identify tumor types that are sensitive to m. TOR inhibition Ø Combination target therapy augment anti-tumor activity and overcoming resistance üPFS: progression-free survival

Recommendations • In vivo concentration of endoxifen needed to maximally inhibit breast cancer proliferation is unknown • Potent CYP 2 D 6 inhibitors be avoided in women receiving tamoxifen (Strong) • When the use of a drug known to potently inhibit CYP 2 D 6 is necessary, consideration should be given to treat with the inhibitor for Thank you for the shortest period of time possible. (Weak) your attention !!