M A T N E M E L


















- Slides: 27

M A T N E M E L E T A WH I? ? Regen ts Rev iew Ac tivity a Quest nd ions!

Let’s Play! What element am I? Element 1: __________ • I am a non-metal • I belong to the Halogen Family • I am not the largest or smallest atom in my group • My ionization energy is greater than that of Iodine • I am not a gas at room temperature

Let’s Play! What element am I? Element 1: (Br) Bromine!

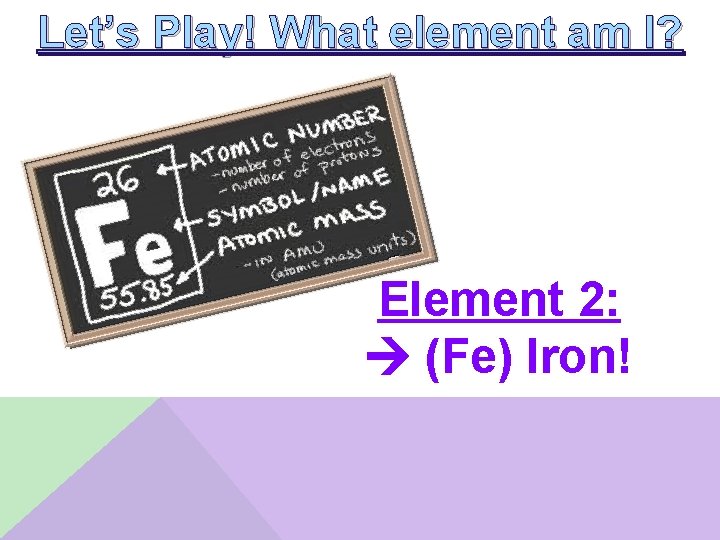
Let’s Play! What element am I? Element 2: __________ • I am a metal • I am a transition metal • I have the smallest atomic radius in my group • I have 6 electrons in the 3 d sublevel

Let’s Play! What element am I? Element 2: (Fe) Iron!

Let’s Play! What element am I? Element 3: __________ • The radius of my most common is smaller than my atomic radius • My valence shell contains only s-orbital electrons • I form an ion with a 2+ charge • I have a lower ionization energy than calcium • I have a smaller atomic radius than barium

Let’s Play! What element am I? Element 3: (Sr) Strontium!

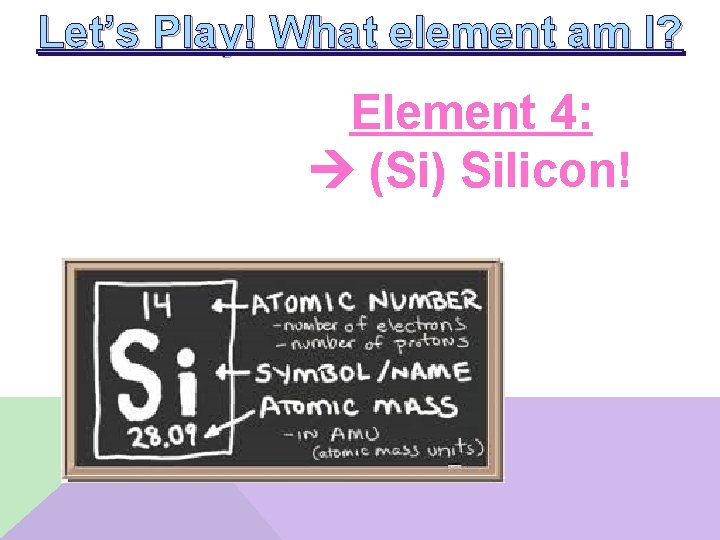
Let’s Play! What element am I? Element 4: __________ • • I am in the p-block of the Periodic Table I have 4 valence electrons I am not the smallest atom in my group My ionization energy is greater than that of germanium

Let’s Play! What element am I? Element 4: (Si) Silicon!

Let’s Play! What element am I? Element 5: __________ • I am a metalloid • I have 3 p-orbital electrons in my valence shell • I have a lower ionization energy than arsenic • I am not the largest atom in my group

Let’s Play! What element am I? Element 5: (Sb) Antimony!

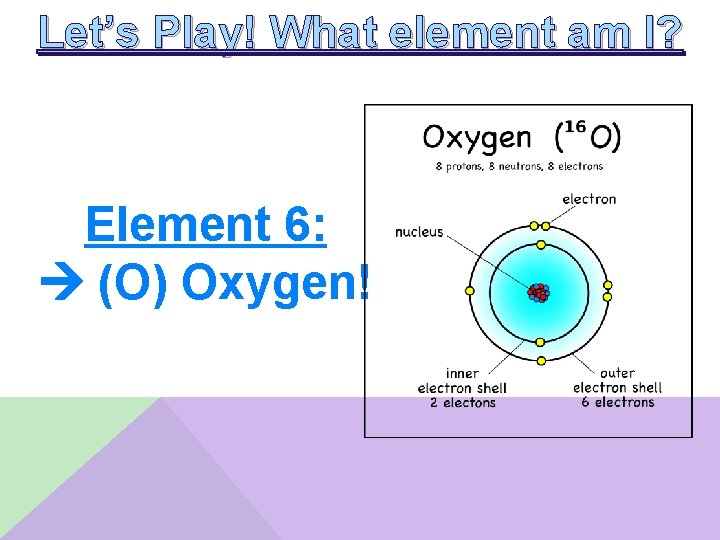
Let’s Play! What element am I? Element 6: __________ • The radius of my most common is larger than my atomic radius • I have 6 valence electrons • I have a higher ionization energy than tellurium • I am the smallest atom in my group

Let’s Play! What element am I? Element 6: (O) Oxygen!

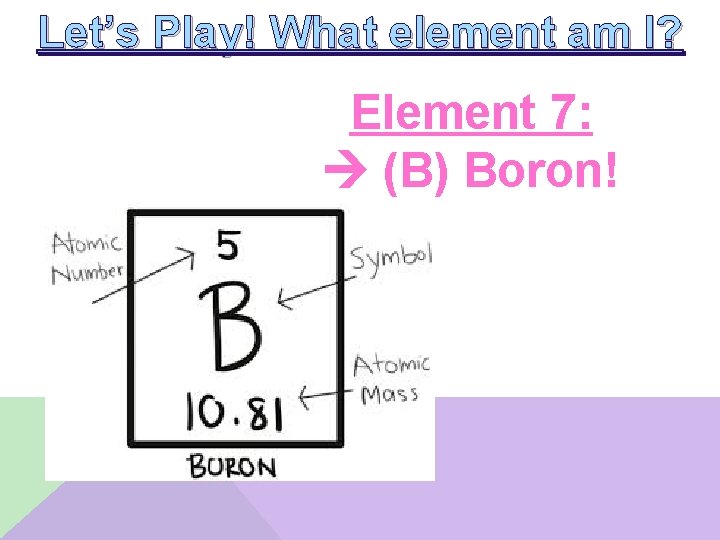
Let’s Play! What element am I? Element 7: __________ • I am a metalloid • I have one electron in the p-block • I have only 2 quantum shells

Let’s Play! What element am I? Element 7: (B) Boron!

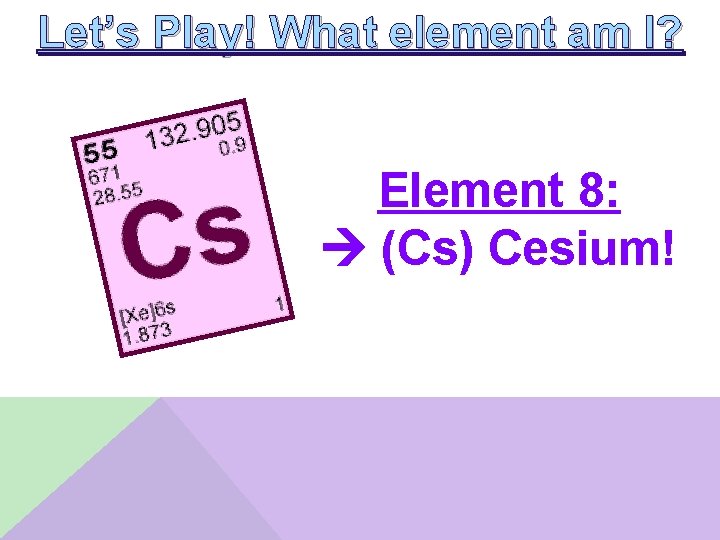
Let’s Play! What element am I? Element 8: __________ • I am a metal • I have an electronegativity less than 1 • My atomic radius is less than my ionization energy

Let’s Play! What element am I? Element 8: (Cs) Cesium!

Regents Review Questions Which element in Group 15 has the strongest metallic character? a) b) c) d) As P N Bi ANSWER d) Bi

Regents Review Questions Which compound forms a colored, aqueous solution? a) Ca. Cl 22 b) Cr. Cl 3 c) Na. OH d) KBr ANSWER b) Cr. Cl 3

Regents Review Questions Which element has the highest first ionization energy? a) Sodium b) Phosphorus c) Aluminum d) Calcium ANSWER b) Phosphorus

Regents Review Questions Which of the following elements has the smallest atomic radius? a) Nickel b) Cobalt c) Potassium d) Calcium ANSWER a) Nickel

Regents Review Questions Which set of elements contains a metalloid? a) Li, Mg, Ca, Kr b) Ba, Ag, Sn, Xe c) K, Mn, As, Ar d) Fr, F, O, Rn ANSWER c) K, Mn, As, Ar

Regents Review Questions An atom of which of the following elements has the greatest ability to attract electrons? a) Chlorine b) Silicon c) Sulfur d) Nitrogen ANSWER a) Chlorine

Regents Review Questions The elements in Period 3 all contain the same number of…. ? a) Protons b) Valence Electrons c) Occupied Principal energy levels d) Neutrons ANSWER c) Occupied Principal energy levels

Regents Review Questions Compared to atoms of metals, atoms of nonmetals generally…. . ? a) Have lower first ionization energies b) Conduct electricity more readily c) Have higher electronegativities d) Lose electrons more readily ANSWER c) Have higher electronegativities

Regents Review Questions As the elements of Group 17 are considered in order of increasing atomic number, there is an increase in…? a) First ionization energy b) Number of electrons in the first shell c) Electronegativity d) Atomic Radius ANSWER d) atomic radius

Regents Review Questions As the atoms of the elements in group 1 of the P. T. are considered from top to bottom, the number of valence electrons in the atoms of each successive element…? a) Increase b) Decrease c) Remains the same d) Returns to zero ANSWER c) Remains the same