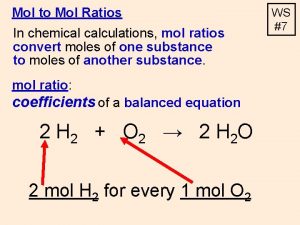
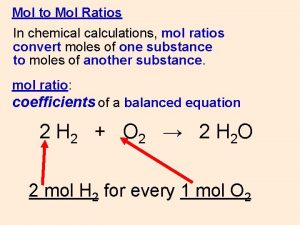
M 1 mol HCl 38 g HCl 1000


- Slides: 8




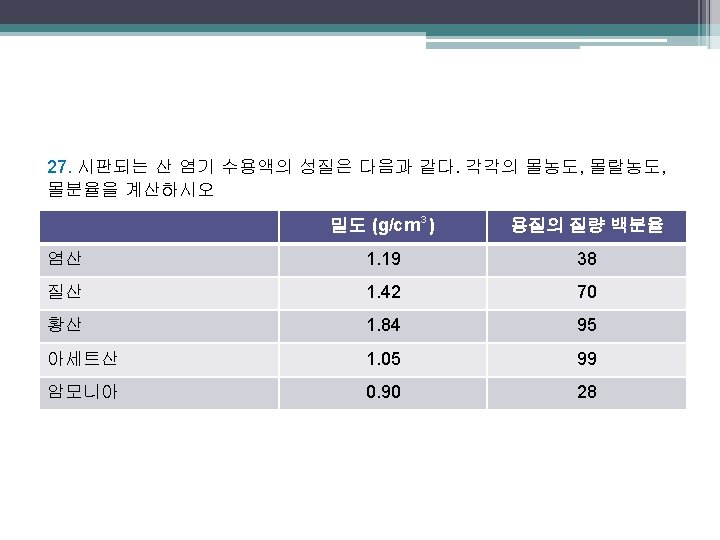
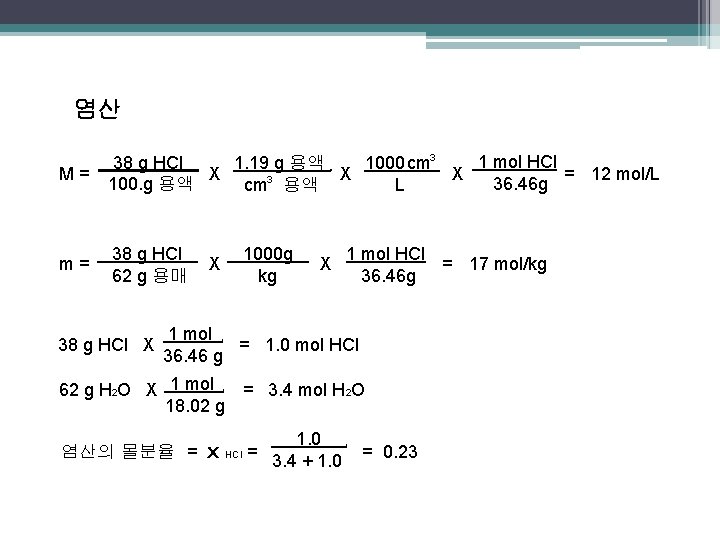
염산 M= 1 mol HCl 38 g HCl 1000 cm 3 1. 19 g 용액 X X X = 12 mol/L 36. 46 g 100. g 용액 L cm 3 용액 m= 38 g HCl 62 g 용매 . 38 g HCl X . . . 1000 g kg X . X 1 mol HCl = 17 mol/kg 36. 46 g 1 mol = 1. 0 mol HCl 36. 46 g. 62 g H 2 O X 1 mol 18. 02 g = 3. 4 mol H 2 O . 염산의 몰분율 = x HCl = 1. 0 3. 4 + 1. 0 . = 0. 23

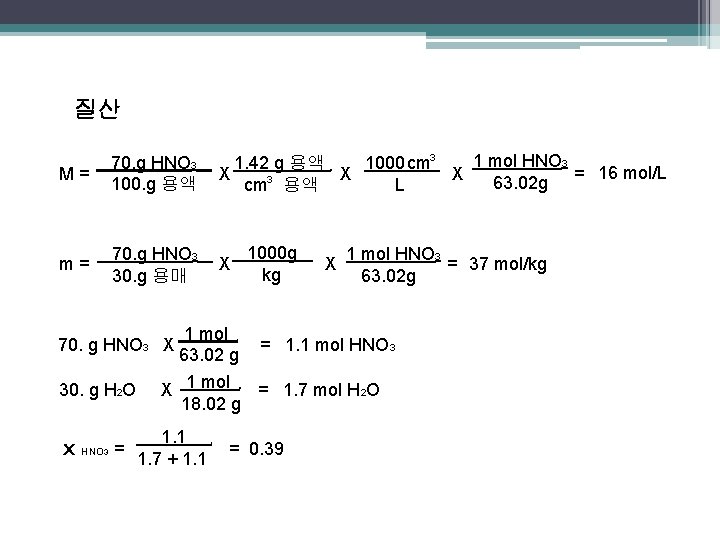
질산 M= 70. g HNO 3 100. g 용액 m= 70. g HNO 3 30. g 용매 70. g HNO 3 X 30. g H 2 O x HNO 3 = 1 mol HNO 3 1000 cm 3 1. 42 g 용액 16 mol/L X X X 63. 02 g = L cm 3 용액 . . 1000 g kg X 1 mol 63. 02 g . X 1 mol HNO 3 = 37 mol/kg 63. 02 g = 1. 1 mol HNO 3 . X 1 mol = 1. 7 mol H 2 O 18. 02 g. 1. 1 1. 7 + 1. 1 . = 0. 39

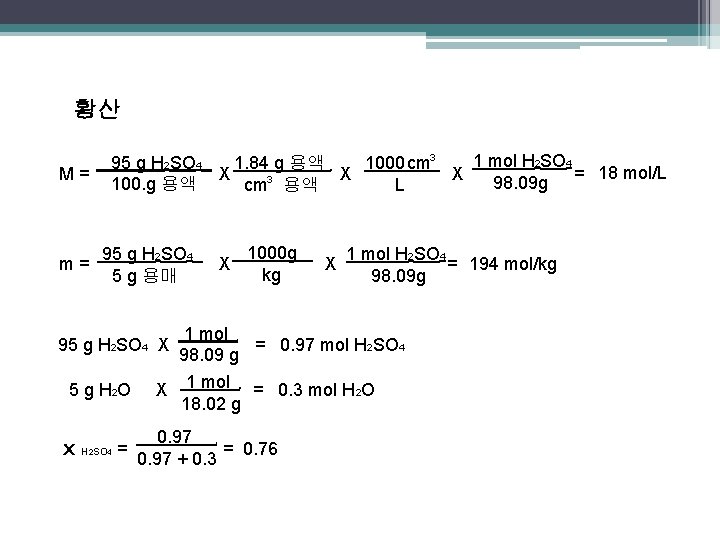
황산 M= m= 1 mol H 2 SO 4 95 g H 2 SO 4 1000 cm 3 1. 84 g 용액 18 mol/L X X X 98. 09 g = 100. g 용액 L cm 3 용액. 95 g H 2 SO 4 5 g 용매 95 g H 2 SO 4 X 5 g H 2 O x H 2 SO 4 = . . 1000 g kg X . . X 1 mol H 2 SO 4 = 194 mol/kg 98. 09 g 1 mol = 0. 97 mol H 2 SO 4 98. 09 g. X 1 mol = 0. 3 mol H 2 O 18. 02 g. 0. 97 = 0. 76 0. 97 + 0. 3.

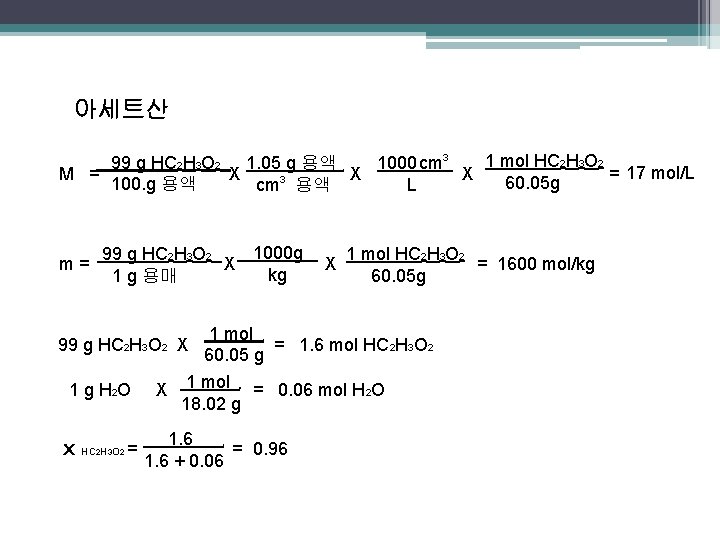
아세트산 1 mol HC 2 H 3 O 2 99 g HC 2 H 3 O 2 1. 05 g 용액 1000 cm 3 M = X X X = 17 mol/L 60. 05 g 100. g 용액 L cm 3 용액. m= 1000 g 99 g HC 2 H 3 O 2 X kg 1 g 용매. 99 g HC 2 H 3 O 2 X 1 g H 2 O x HC 2 H 3 O 2 = . . . X 1 mol HC 2 H 3 O 2 = 1600 mol/kg 60. 05 g 1 mol = 1. 6 mol HC 2 H 3 O 2 60. 05 g. X 1 mol = 0. 06 mol H 2 O 18. 02 g. 1. 6 = 0. 96 1. 6 + 0. 06.

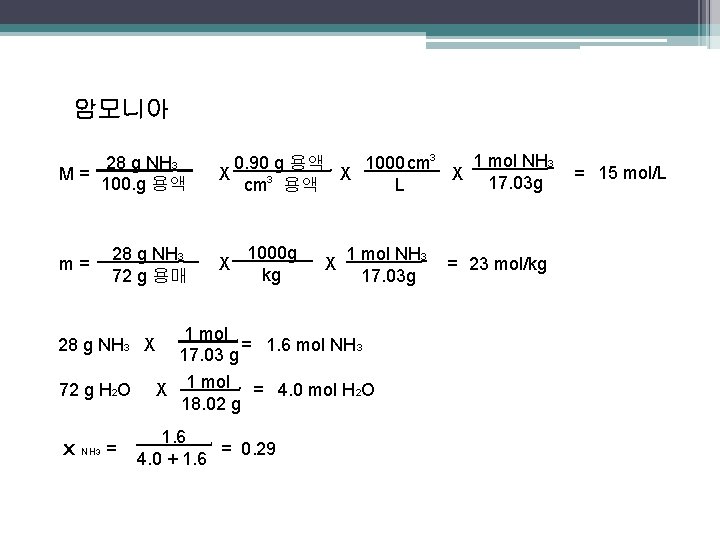
암모니아 28 g NH 3 M= 100. g 용액 m= 28 g NH 3 72 g 용매 28 g NH 3 X 72 g H 2 O x NH 3 = 1 mol NH 3 1000 cm 3 0. 90 g 용액 X X X 17. 03 g L cm 3 용액 . . . 1000 g kg X . . X 1 mol NH 3 17. 03 g 1 mol = 1. 6 mol NH 3 17. 03 g. X 1 mol = 4. 0 mol H 2 O 18. 02 g. 1. 6 = 0. 29 4. 0 + 1. 6. = 23 mol/kg = 15 mol/L