Lung Cancer Screening Promise and Pitfalls Christine D











![Summary of Selected Cohort Trials Trial Criteria N [+] Screens ELCAP 60+ Yr 2001 Summary of Selected Cohort Trials Trial Criteria N [+] Screens ELCAP 60+ Yr 2001](https://slidetodoc.com/presentation_image_h/f4c4d4b404f34b45d76e68b1fcd4bb07/image-18.jpg)




![Results Classifications n [-] Screen No significant findings –or – minimal findings not significant Results Classifications n [-] Screen No significant findings –or – minimal findings not significant](https://slidetodoc.com/presentation_image_h/f4c4d4b404f34b45d76e68b1fcd4bb07/image-32.jpg)


- Slides: 39

Lung Cancer Screening: Promise and Pitfalls Christine D. Berg, M. D. Chief, Early Detection Research Group Division of Cancer Prevention

The opinions expressed in this presentation represent the views of the author and do not necessarily represent those of the United States Department of Health and Human Services or the United States Federal Government.

Lung Cancer Only 7% cured in 1971: only 15% cured today.

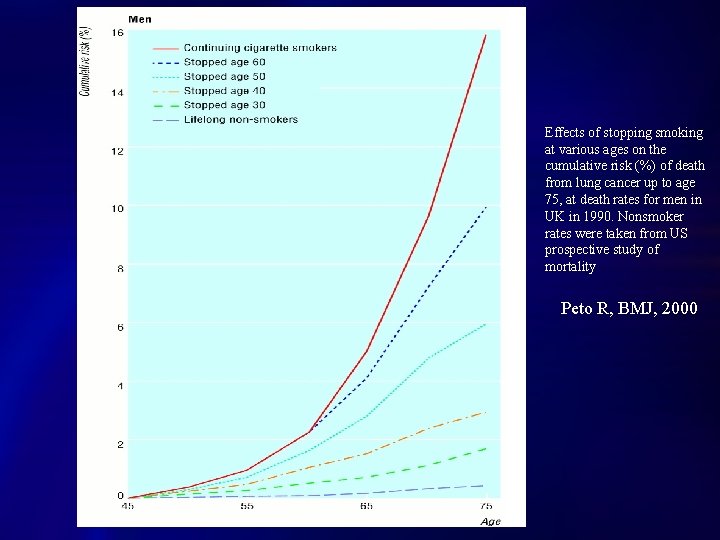
What would help most for lung cancer? SMOKING CESSATION U. S. population with direct smoking exposure: l 46. 5 million former smokers l 45. 1 million current smokers CDC MMWR 10/27/06

Effects of stopping smoking at various ages on the cumulative risk (%) of death from lung cancer up to age 75, at death rates for men in UK in 1990. Nonsmoker rates were taken from US prospective study of mortality Peto R, BMJ, 2000

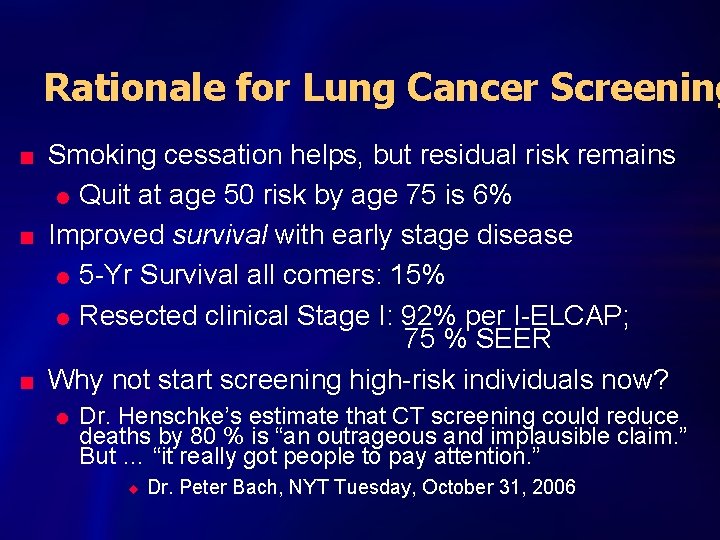
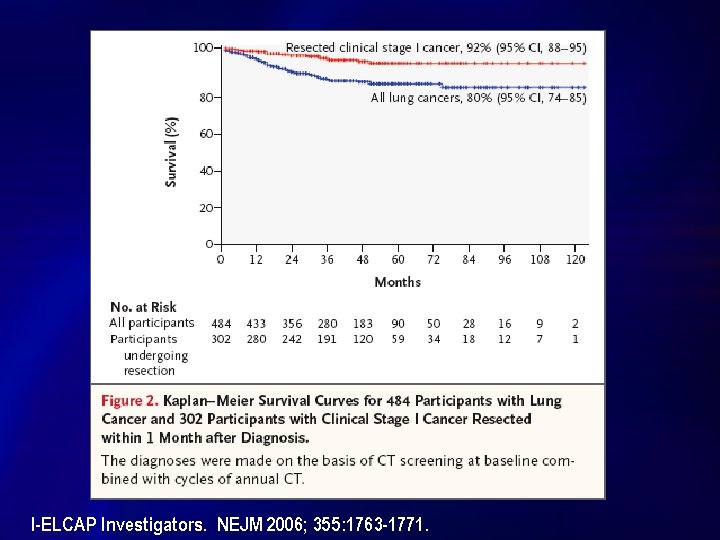
Rationale for Lung Cancer Screening n n n Smoking cessation helps, but residual risk remains l Quit at age 50 risk by age 75 is 6% Improved survival with early stage disease l 5 -Yr Survival all comers: 15% l Resected clinical Stage I: 92% per I-ELCAP; 75 % SEER Why not start screening high-risk individuals now? l Dr. Henschke’s estimate that CT screening could reduce deaths by 80 % is “an outrageous and implausible claim. ” But … “it really got people to pay attention. ” u Dr. Peter Bach, NYT Tuesday, October 31, 2006

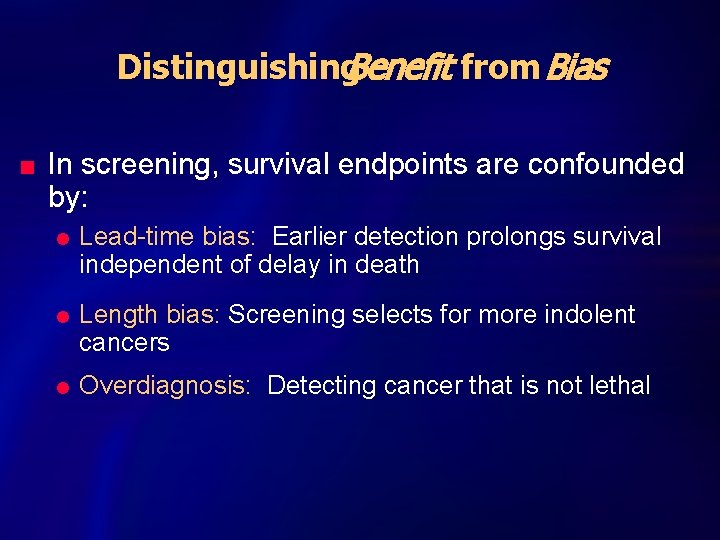
Distinguishing. Benefit from Bias n In screening, survival endpoints are confounded by: l l l Lead-time bias: Earlier detection prolongs survival independent of delay in death Length bias: Screening selects for more indolent cancers Overdiagnosis: Detecting cancer that is not lethal

Quebec Neuroblastoma Screening Projec – Neuroblastoma deaths u SIR 1. 11 compared to control group in Ontario – 22 deaths, 17 missed on screening, I false-negative, 3 diagnosed prior to screening starting and 1 not screened – 43 diagnosed by screening; all alive u u One received doxorubicin/cylcophosphamide and developed a secondary leukemia One in persistent vegetative state as a result of complications from surgery to remove the neuroblastoma v Woods WG NEJM 2002; 346: 1041 -6

Current Data from CXR & CT Screening Studies

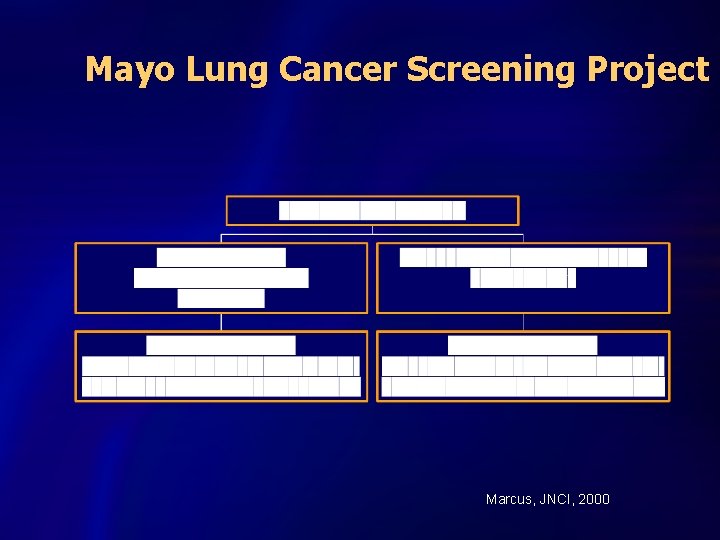
Mayo Lung Cancer Screening Project Marcus, JNCI, 2000

Mayo Lung Project Lung Cancer Survival S u r v i v a l Screened (n=206) P r o b. Usual care (n=160) Years Since Diagnosis Marcus, JNCI 2000

Mayo Lung Project Cumulative Lung Cancer Deaths # D e a t h s Screened (n=337) Usual care (n=303) Follow-up time (years) Marcus, JNCI 2000

INTERPRETATION n Overdiagnosis exists n CXR not effective in reducing mortality n Problems: – Study underpowered for a realistic result, 10% mortality decrease could have been missed – Contamination and compliance n PLCO launched

Prostate, Lung, Colorectal and Ovarian (PLCO Cancer Screening Trial: Screening vs. No Screening n Multicenter RCT involving 154, 942 men and women aged 55 -74 l 1: 1 randomization to CXR screening vs. no screening l Smokers: CXR at baseline and then annually for 3 screens l Non-smokers: CXR annually for 3 screens n Primary endpoint: lung cancer-specific mortality n PLCO Prevalence Screen Results Oken, et al, JNCI 2005

Low-Dose Helical CT n Allows entire chest to be surveyed in a single breathhold l Time: approximately 7 - 15 seconds l Reduces motion artifact l Eliminates respiratory misregistration n Narrower slice thickness n Hourly throughput - 4 patients per hour n Radiation dose one tenth of diagnostic CT

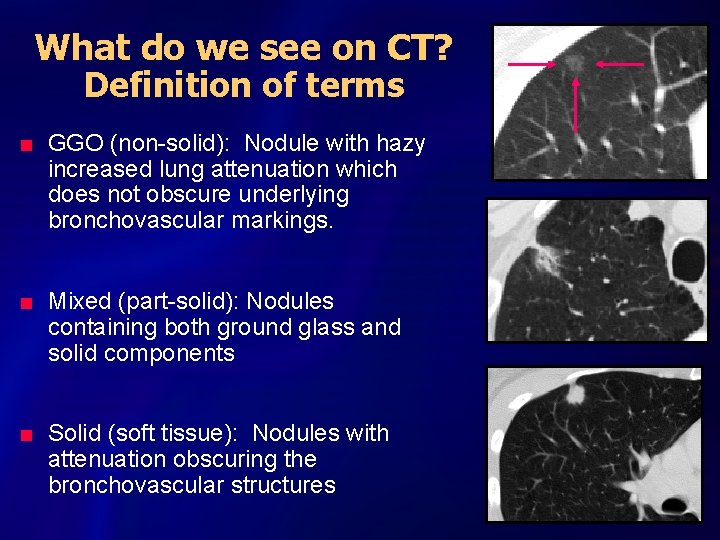
What do we see on CT? Definition of terms n n n GGO (non-solid): Nodule with hazy increased lung attenuation which does not obscure underlying bronchovascular markings. Mixed (part-solid): Nodules containing both ground glass and solid components Solid (soft tissue): Nodules with attenuation obscuring the bronchovascular structures

Downstream Effects of CT Screening n n Radiation carcinogenesis l screening & consequent diagnostic tests: CT, PET Additional minimally invasive procedures l Percutaneous Lung FNA l Bronchoscopy l VATS Thoracotomy for benign disease l Is there an acceptable percentage? l Potential post-operative morbidity & mortality l Treatment for disease without biopsy? Evaluation for other observations: cardiac, renal, liver, adrenal disease
![Summary of Selected Cohort Trials Trial Criteria N Screens ELCAP 60 Yr 2001 Summary of Selected Cohort Trials Trial Criteria N [+] Screens ELCAP 60+ Yr 2001](https://slidetodoc.com/presentation_image_h/f4c4d4b404f34b45d76e68b1fcd4bb07/image-18.jpg)
Summary of Selected Cohort Trials Trial Criteria N [+] Screens ELCAP 60+ Yr 2001 10 Pk Yr CT + CXR Yr 0: 1000 Yr 1: 841 Yr 2: 343 Baseline 233 (23. 3%) Incidence 40 (3. 4%) Yr 0 1520 Yr 1: 1478 Yr 2: 1438 Overall >95% Baseline: 782 (51%) Incidence: 9. 313. 5% Yr 0: 31, 567 Incid: 27, 456 Baseline 4186 (13%) Incidence: 1460 (5%) Swensen CT annual x 5 yrs 50+ Yr 20 Pk. Yr Quit <10 Yr I-ELCAP Site Specific Total Cancers Baseline: 31 (3. 1%) Incidence: 07 Interval: 2 Baseline: 31 (2%) Incidence: 32 Interval: 3 Baseline: 405 Incidence: 74 Interval: 5 Stage I NSCLC Survival Baseline: 23 (74%) Incidence: 5 (55%) All with cancer alive at 2. 5 Yrs; 5 deaths other causes No mortality data Baseline: 20 (65%) Incid: 17 (61%) 42 deaths overall: 09 lung ca (1. 6) 33 all cause (6. 0) [per 1000 person-Yr] Baseline: Incidence: Total: 347 (72%) F/U = median 3. 3 Yrs Estimates: -Overall 80% 10 Yr -Resected c. Stage 1 92%

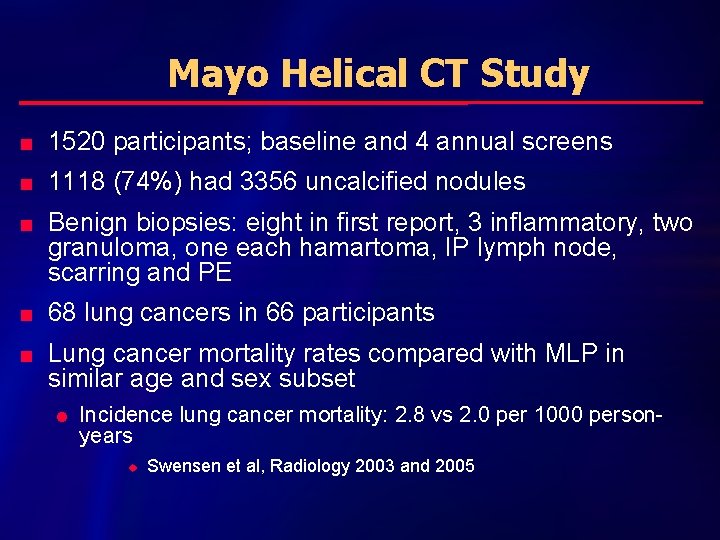
Mayo Helical CT Study n 1520 participants; baseline and 4 annual screens n 1118 (74%) had 3356 uncalcified nodules n n n Benign biopsies: eight in first report, 3 inflammatory, two granuloma, one each hamartoma, IP lymph node, scarring and PE 68 lung cancers in 66 participants Lung cancer mortality rates compared with MLP in similar age and sex subset l Incidence lung cancer mortality: 2. 8 vs 2. 0 per 1000 personyears u Swensen et al, Radiology 2003 and 2005

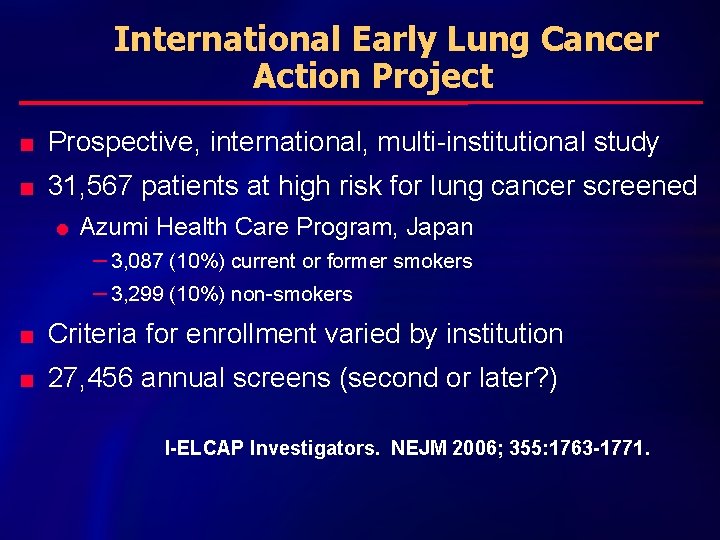
International Early Lung Cancer Action Project n Prospective, international, multi-institutional study n 31, 567 patients at high risk for lung cancer screened l Azumi Health Care Program, Japan – 3, 087 (10%) current or former smokers – 3, 299 (10%) non-smokers n Criteria for enrollment varied by institution n 27, 456 annual screens (second or later? ) I-ELCAP Investigators. NEJM 2006; 355: 1763 -1771.

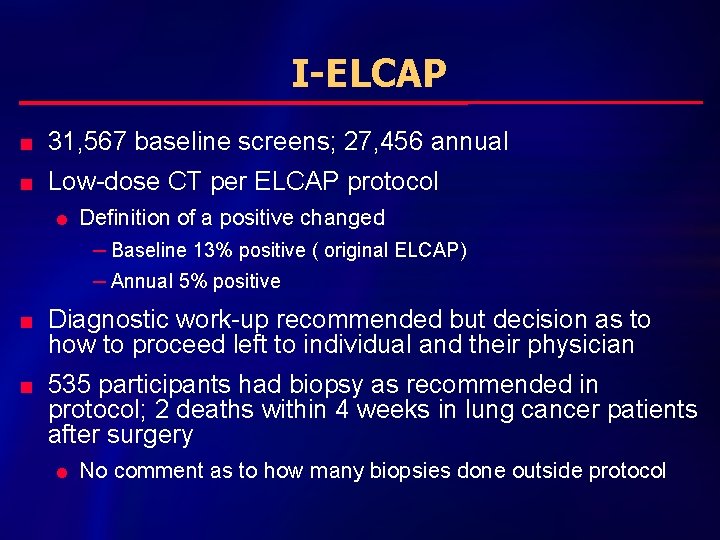
I-ELCAP n 31, 567 baseline screens; 27, 456 annual n Low-dose CT per ELCAP protocol l Definition of a positive changed – Baseline 13% positive ( original ELCAP) – Annual 5% positive n n Diagnostic work-up recommended but decision as to how to proceed left to individual and their physician 535 participants had biopsy as recommended in protocol; 2 deaths within 4 weeks in lung cancer patients after surgery l No comment as to how many biopsies done outside protocol

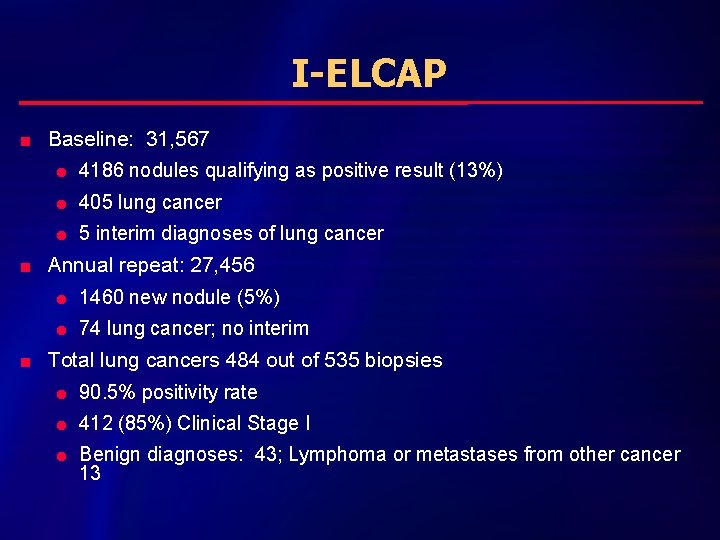
I-ELCAP n n n Baseline: 31, 567 l 4186 nodules qualifying as positive result (13%) l 405 lung cancer l 5 interim diagnoses of lung cancer Annual repeat: 27, 456 l 1460 new nodule (5%) l 74 lung cancer; no interim Total lung cancers 484 out of 535 biopsies l 90. 5% positivity rate l 412 (85%) Clinical Stage I l Benign diagnoses: 43; Lymphoma or metastases from other cancer 13

I-ELCAP Investigators. NEJM 2006; 355: 1763 -1771.

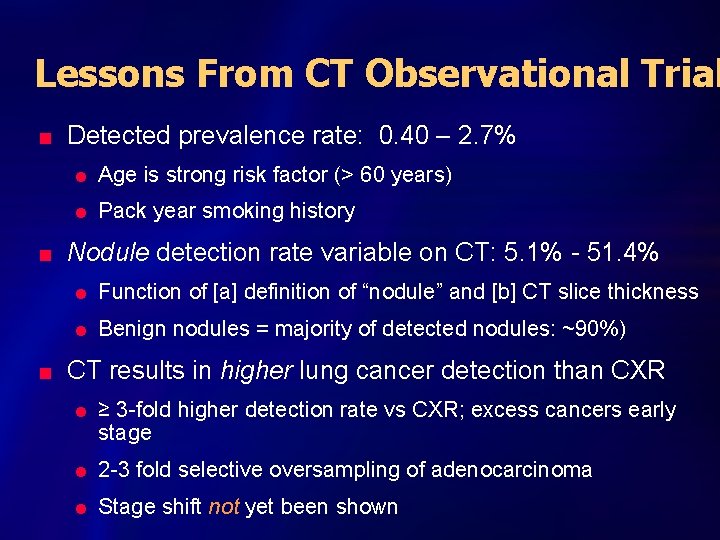
Lessons From CT Observational Trial n n n Detected prevalence rate: 0. 40 – 2. 7% l Age is strong risk factor (> 60 years) l Pack year smoking history Nodule detection rate variable on CT: 5. 1% - 51. 4% l Function of [a] definition of “nodule” and [b] CT slice thickness l Benign nodules = majority of detected nodules: ~90%) CT results in higher lung cancer detection than CXR l ≥ 3 -fold higher detection rate vs CXR; excess cancers early stage l 2 -3 fold selective oversampling of adenocarcinoma l Stage shift not yet been shown

National Lung Screening Trial n Determine effect on lung cancer mortality l 90% power, α of 5%, to detect a 20% difference n Determine magnitude if any of stage shift n Delineate adverse events n Determine the ratio between risks and benefits l l Thoracotomies for benign disease Diagnostic radiation exposure in individuals without cancer; estimate radiation carcinogenesis

Definition of High Risk Participants n Males and females n 55 -74 Yrs n Asymptomatic current or former smokers ≥ 30 pack yrs n Former smokers must have quit within ≤ 15 yrs n No prior Hx lung cancer n No Hx any cancer within past 5 years n No chest CT w/in prior 18 months

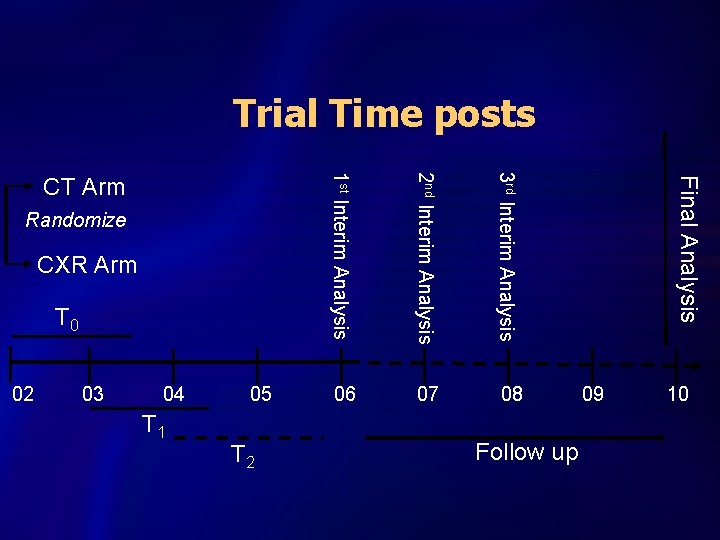
NLST Trial Design 53, 464 High-Risk Subjects CT Arm Randomize CXR Arm 3 annual screens: T 0, T 1, T 2

Trial Time posts 3 rd Interim Analysis Final Analysis 2 nd Interim Analysis Follow up T 2 T 1 10 09 02 03 04 05 1 st Interim Analysis T 0 08 CXR Arm 07 Randomize 06 CT Arm

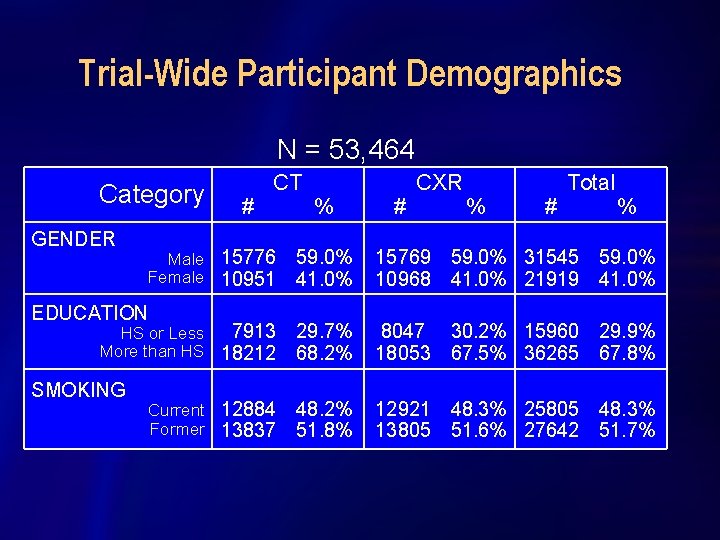
Trial-Wide Participant Demographics N = 53, 464 Category GENDER # CT Male 15776 Female 10951 EDUCATION 7913 HS or Less More than HS 18212 SMOKING Current 12884 Former 13837 % # CXR % # Total % 59. 0% 41. 0% 15769 59. 0% 31545 59. 0% 10968 41. 0% 21919 41. 0% 29. 7% 68. 2% 8047 30. 2% 15960 29. 9% 18053 67. 5% 36265 67. 8% 48. 2% 51. 8% 12921 48. 3% 25805 48. 3% 13805 51. 6% 27642 51. 7%

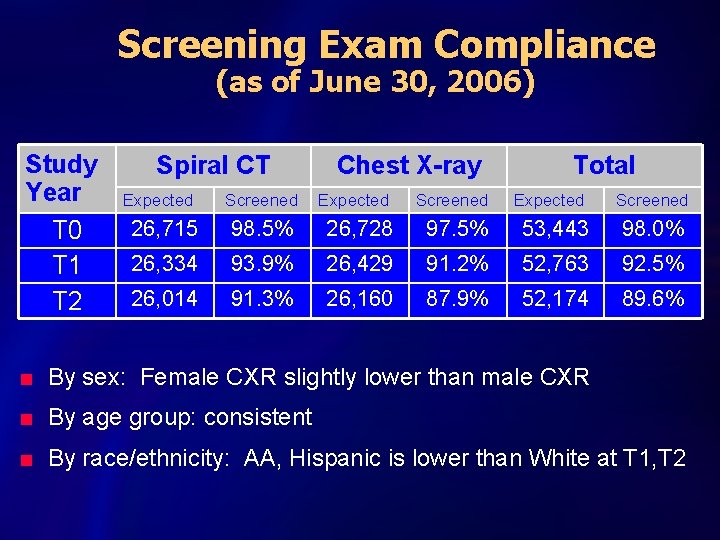
Screening Exam Compliance (as of June 30, 2006) Study Year T 0 T 1 T 2 Spiral CT Chest X-ray Total Expected Screened 26, 715 98. 5% 26, 728 97. 5% 53, 443 98. 0% 26, 334 93. 9% 26, 429 91. 2% 52, 763 92. 5% 26, 014 91. 3% 26, 160 87. 9% 52, 174 89. 6% n By sex: Female CXR slightly lower than male CXR n By age group: consistent n By race/ethnicity: AA, Hispanic is lower than White at T 1, T 2

ACRIN/NLST CT Technique NLST-ACRIN Physics Committee n CT Technique Chart l l l n n n Standardized 18 parameters 14 different CT scanners 4 manufacturers: 4 -64 channel Equipment certification annually Bi-monthly CT phantom calibration CXR techniques from CRFs reviewed
![Results Classifications n Screen No significant findings or minimal findings not significant Results Classifications n [-] Screen No significant findings –or – minimal findings not significant](https://slidetodoc.com/presentation_image_h/f4c4d4b404f34b45d76e68b1fcd4bb07/image-32.jpg)
Results Classifications n [-] Screen No significant findings –or – minimal findings not significant for lung cancer n [-] Screen Significant findings unrelated to lung cancer [Some form of diagnostic recommendation required; e. g. , echocardiogram for suspected pulmonary hypertension) n [+] Screen Findings potentially related to lung cancer [diagnostic recommendation of some form required]

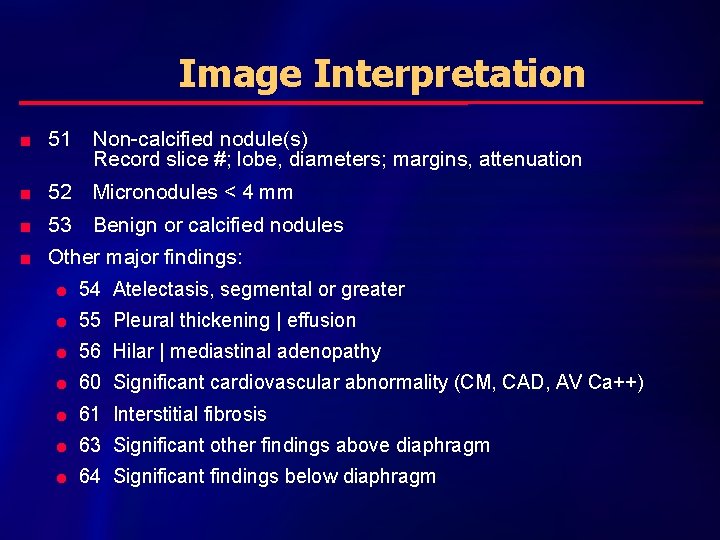
Image Interpretation n 51 Non-calcified nodule(s) Record slice #; lobe, diameters; margins, attenuation n 52 Micronodules < 4 mm n 53 Benign or calcified nodules n Other major findings: l 54 Atelectasis, segmental or greater l 55 Pleural thickening | effusion l 56 Hilar | mediastinal adenopathy l 60 Significant cardiovascular abnormality (CM, CAD, AV Ca++) l 61 Interstitial fibrosis l 63 Significant other findings above diaphragm l 64 Significant findings below diaphragm

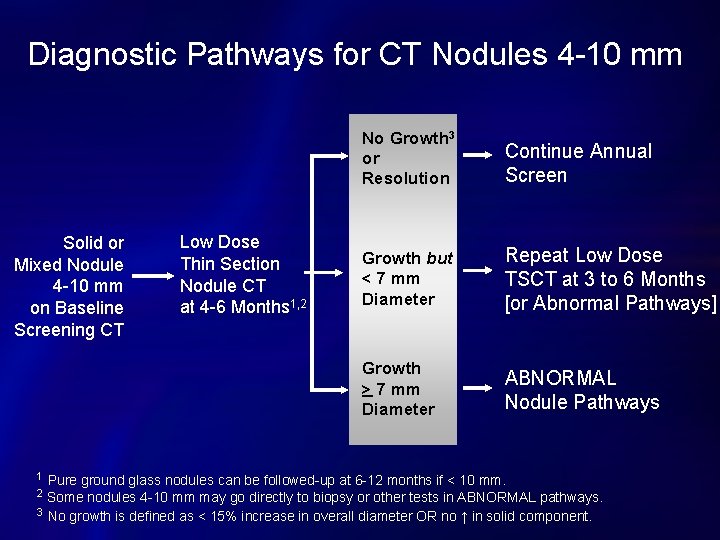
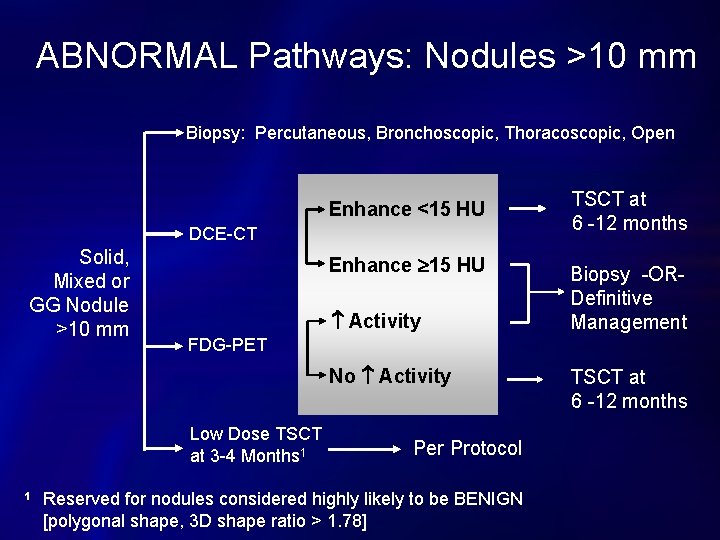
Diagnostic Pathways for CT Nodules 4 -10 mm Solid or Mixed Nodule 4 -10 mm on Baseline Screening CT 1 Low Dose Thin Section Nodule CT at 4 -6 Months 1, 2 No Growth 3 or Resolution Continue Annual Screen Growth but < 7 mm Diameter Repeat Low Dose TSCT at 3 to 6 Months [or Abnormal Pathways] Growth > 7 mm Diameter ABNORMAL Nodule Pathways Pure ground glass nodules can be followed-up at 6 -12 months if < 10 mm. Some nodules 4 -10 mm may go directly to biopsy or other tests in ABNORMAL pathways. 3 No growth is defined as < 15% increase in overall diameter OR no ↑ in solid component. 2

ABNORMAL Pathways: Nodules >10 mm Biopsy: Percutaneous, Bronchoscopic, Thoracoscopic, Open Enhance <15 HU DCE-CT Solid, Mixed or GG Nodule >10 mm Enhance 15 HU Activity Biopsy -ORDefinitive Management FDG-PET No Activity Low Dose TSCT at 3 -4 Months 1 1 TSCT at 6 -12 months Per Protocol Reserved for nodules considered highly likely to be BENIGN [polygonal shape, 3 D shape ratio > 1. 78] TSCT at 6 -12 months

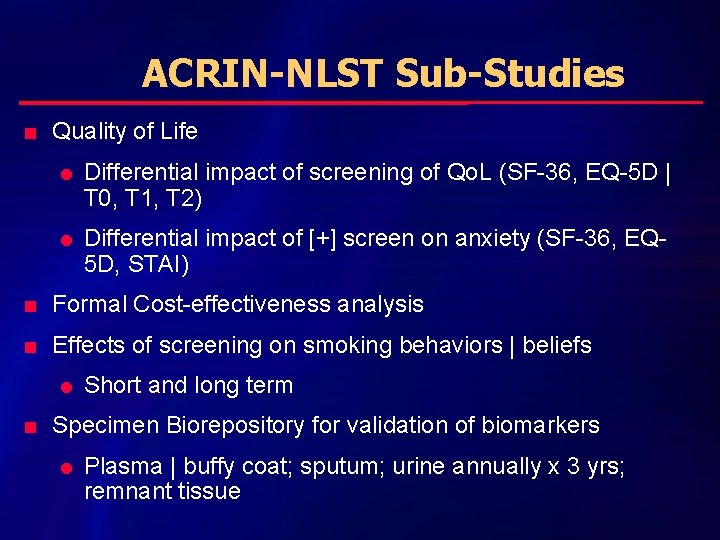
ACRIN-NLST Sub-Studies n Quality of Life l l Differential impact of screening of Qo. L (SF-36, EQ-5 D | T 0, T 1, T 2) Differential impact of [+] screen on anxiety (SF-36, EQ 5 D, STAI) n Formal Cost-effectiveness analysis n Effects of screening on smoking behaviors | beliefs l n Short and long term Specimen Biorepository for validation of biomarkers l Plasma | buffy coat; sputum; urine annually x 3 yrs; remnant tissue

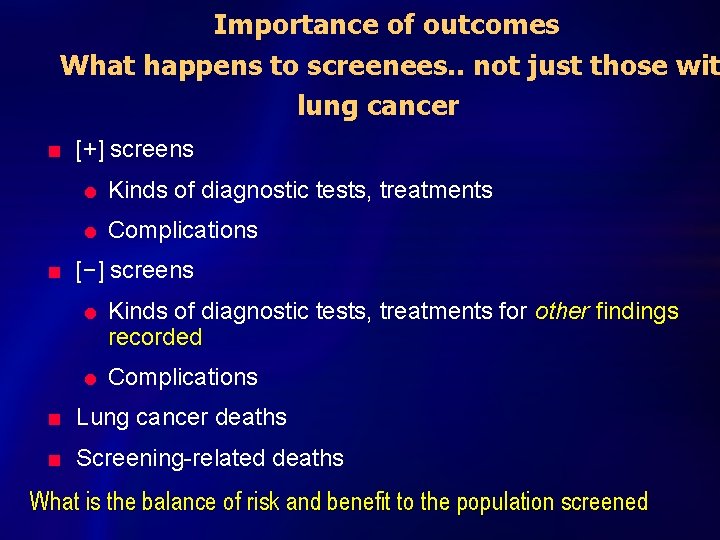
Importance of outcomes What happens to screenees. . not just those wit lung cancer n n [+] screens l Kinds of diagnostic tests, treatments l Complications [−] screens l l Kinds of diagnostic tests, treatments for other findings recorded Complications n Lung cancer deaths n Screening-related deaths What is the balance of risk and benefit to the population screened

Summary n n n The most effective way to reduce smoking-related deaths is to stop smoking. CT screening reveals many non-calcified nodules, the majority of which will be benign. Observational studies of CT screening indicate a high rate of Stage I lung cancers | unknown effects on numbers of late stage cancers. We do not know if screening reduces lung cancer mortality. Interventions resulting from screening come at economic, emotional, and medical cost.

With appreciation n LSS and ACRIN Colleagues n Site Coordinators and Staff n Trial participants without whom these studies would not have been possible
 Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Tnm staging lung cancer
Tnm staging lung cancer Di vs csw
Di vs csw Gesundheit central european lung cancer patient network
Gesundheit central european lung cancer patient network Servier medical art
Servier medical art Smart goals for ineffective airway clearance
Smart goals for ineffective airway clearance Dr. amy ford
Dr. amy ford Optimal lung cancer pathway
Optimal lung cancer pathway Lifetime risk of lung cancer
Lifetime risk of lung cancer C traps and pitfalls
C traps and pitfalls Nitcar
Nitcar Latchup in vlsi
Latchup in vlsi What are pitfalls in differentiation
What are pitfalls in differentiation The protocol ensures freedom from deadlock.
The protocol ensures freedom from deadlock. Contoh pertanyaan bipolar dalam wawancara
Contoh pertanyaan bipolar dalam wawancara Pitfalls in relational database design with example
Pitfalls in relational database design with example Graph based locking protocol
Graph based locking protocol What are two pitfalls (problems) of lock-based protocols
What are two pitfalls (problems) of lock-based protocols Pitfalls of operator overloading in c++
Pitfalls of operator overloading in c++ Pitfalls of object oriented programming
Pitfalls of object oriented programming Security handshake pitfalls
Security handshake pitfalls Pitfalls of gauss elimination method
Pitfalls of gauss elimination method Strategic management process
Strategic management process The pitfalls of a differentiation strategy include
The pitfalls of a differentiation strategy include Pitfalls of prototyping
Pitfalls of prototyping Circuit pitfalls in vlsi
Circuit pitfalls in vlsi Pitfalls in selecting new ventures
Pitfalls in selecting new ventures Pitfalls of lock based protocol
Pitfalls of lock based protocol Decide model aviation
Decide model aviation Girl scout pledge and law
Girl scout pledge and law Cpcc career and college promise
Cpcc career and college promise Chapter 24 the immune and lymphatic systems and cancer
Chapter 24 the immune and lymphatic systems and cancer Chapter 24 the immune and lymphatic systems and cancer
Chapter 24 the immune and lymphatic systems and cancer Pleura
Pleura Cural
Cural What causes tactile fremitus
What causes tactile fremitus Difference between restrictive and obstructive lung disease
Difference between restrictive and obstructive lung disease Obstructive and restrictive lung disease
Obstructive and restrictive lung disease Vocal cord
Vocal cord Short tall grande venti
Short tall grande venti