Luminal Metastatic Breast Cancer role of CDK 46

Luminal Metastatic Breast Cancer: role of CDK 4/6 inhibitors from preclinical data to clinical practice Vincenzo Adamo Oncologia Medica AO Papardo & Università degli Studi di Messina vadamo@unime. it
OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø Final Remarkers
OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø Final Remarkers

“Sequential hormone therapy is the preferential treatment for most women with HRpositive MBC” “Treatment recommendations should be based on type of adjuvant treatment, diseasefree interval and organ function” “Assessment of menopausal status is critical; ovarian suppression or ablation should be included in premenopausal women” Rugo H, et al. JCO 2016; 34: 3069 -3103

Top Line recomendations from ESO-ESMO ABC 4 panel (Lisbona 2017) “Endocrine therapy plus a CDK 4 -6 inhibitor, is the preferred option for hormone receptor positive disease, even in the presence of visceral disease, unless there is visceral crisis or concern/proof of endocrine resistance” courtesy by A. Prat
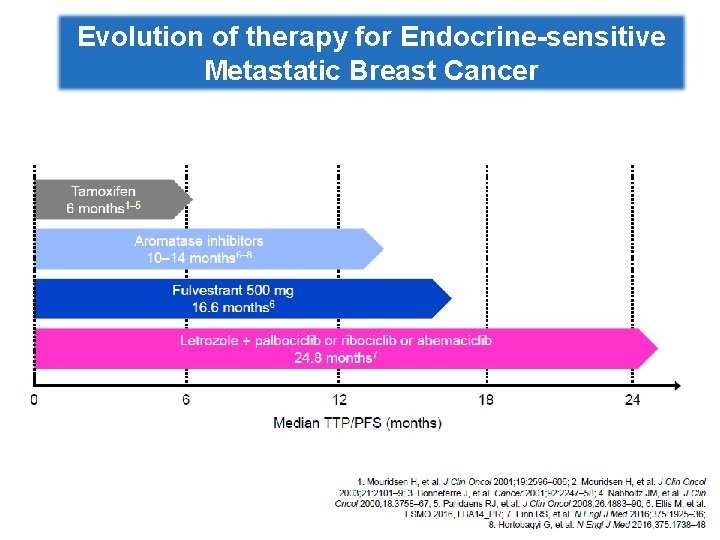
Evolution of therapy for Endocrine-sensitive Metastatic Breast Cancer
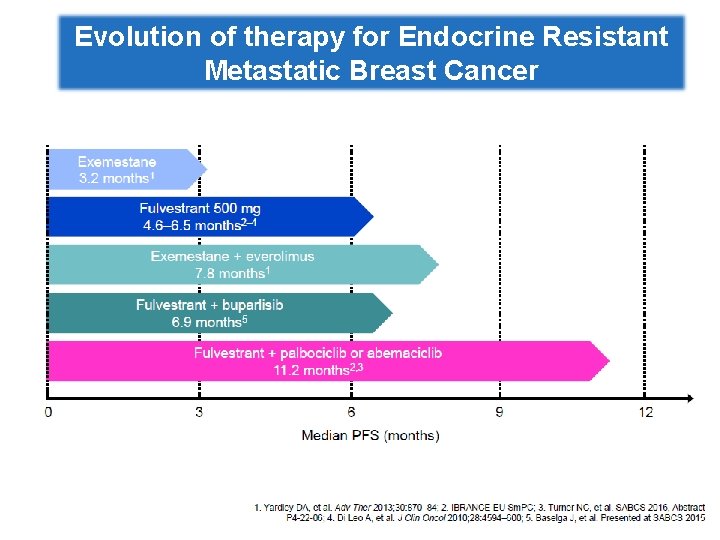
Evolution of therapy for Endocrine Resistant Metastatic Breast Cancer

OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø Final Remarkers
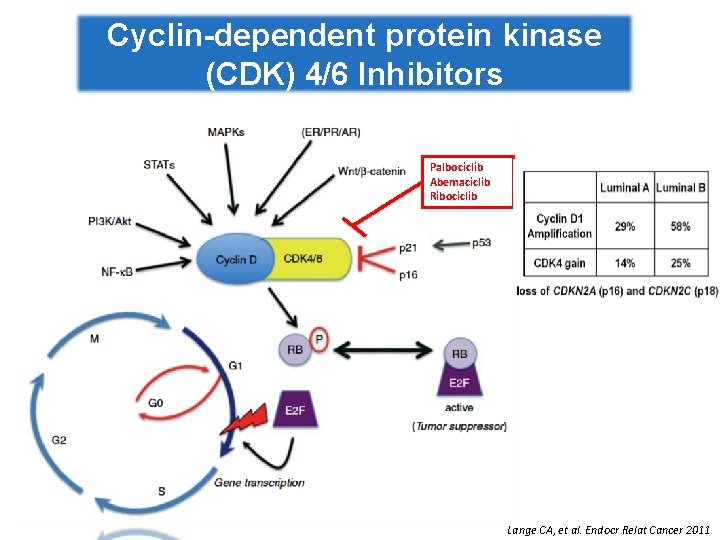
Cyclin-dependent protein kinase (CDK) 4/6 Inhibitors Palbociclib Abemaciclib Ribociclib Lange CA, et al. Endocr Relat Cancer 2011
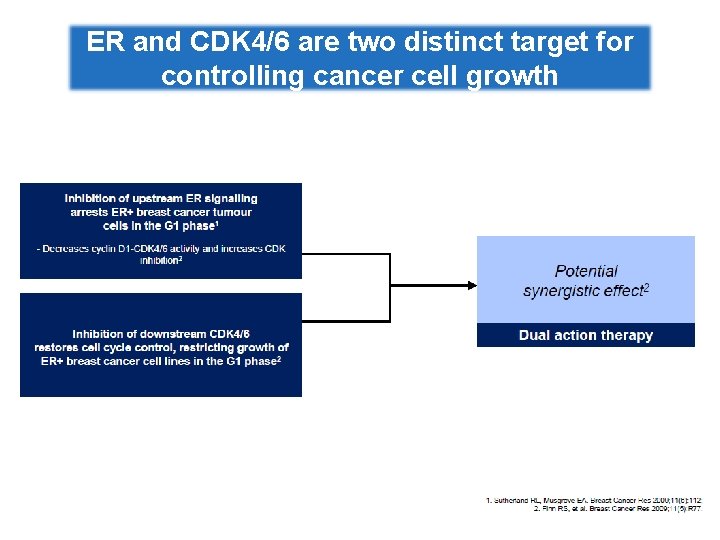
ER and CDK 4/6 are two distinct target for controlling cancer cell growth
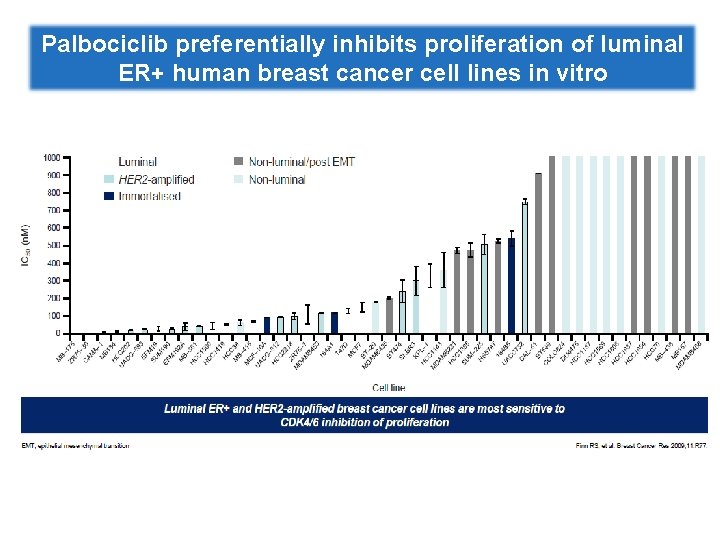
Palbociclib preferentially inhibits proliferation of luminal ER+ human breast cancer cell lines in vitro
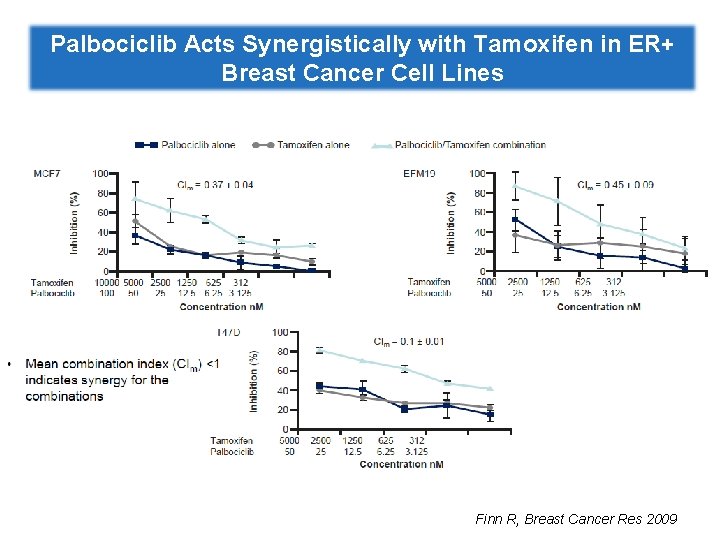
Palbociclib Acts Synergistically with Tamoxifen in ER+ Breast Cancer Cell Lines Finn R, Breast Cancer Res 2009
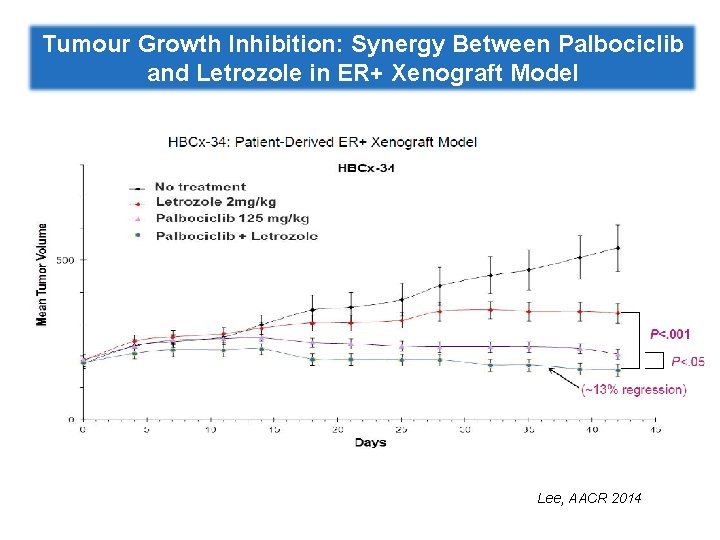
Tumour Growth Inhibition: Synergy Between Palbociclib and Letrozole in ER+ Xenograft Model Lee, AACR 2014
OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø Final Remarkers
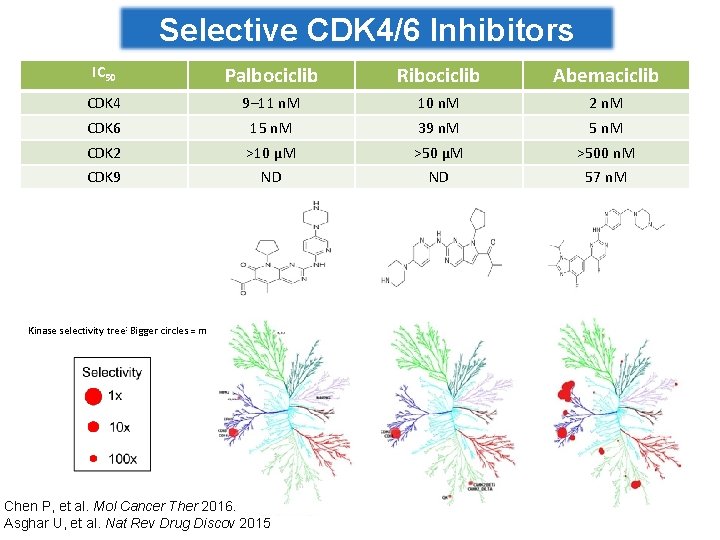
Selective CDK 4/6 Inhibitors IC 50 Palbociclib Ribociclib Abemaciclib CDK 4 9– 11 n. M 10 n. M 2 n. M CDK 6 15 n. M 39 n. M 5 n. M CDK 2 >10 µM >500 n. M CDK 9 ND ND 57 n. M Kinase selectivity tree: Bigger circles = more inhibition Chen P, et al. Mol Cancer Ther 2016. Asghar U, et al. Nat Rev Drug Discov 2015
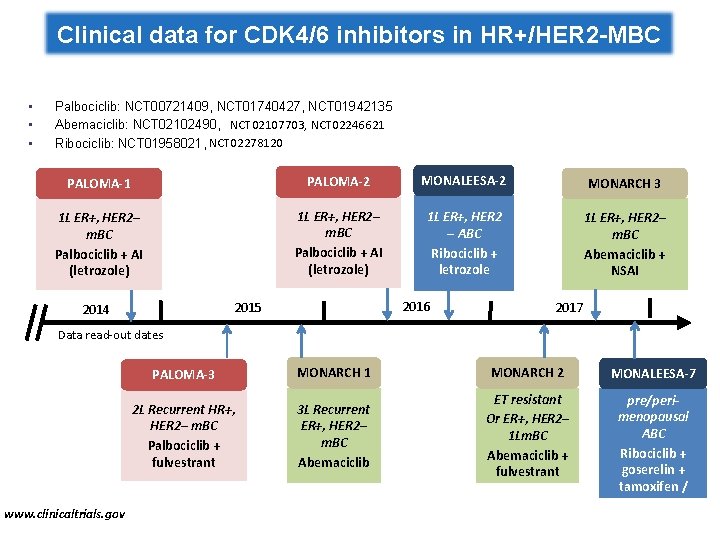
Clinical data for CDK 4/6 inhibitors in HR+/HER 2 -MBC • • • Palbociclib: NCT 00721409, NCT 01740427, NCT 01942135 Abemaciclib: NCT 02102490, NCT 02107703, NCT 02246621 Ribociclib: NCT 01958021, NCT 02278120 PALOMA-1 PALOMA-2 MONALEESA-2 MONARCH 3 1 L ER+, HER 2– m. BC Palbociclib + AI (letrozole) 1 L ER+, HER 2 – ABC Ribociclib + letrozole 1 L ER+, HER 2– m. BC Abemaciclib + NSAI 2016 2015 2014 2017 Data read-out dates www. clinicaltrials. gov PALOMA-3 MONARCH 1 MONARCH 2 2 L Recurrent HR+, HER 2– m. BC Palbociclib + fulvestrant 3 L Recurrent ER+, HER 2– m. BC Abemaciclib ET resistant Or ER+, HER 2– 1 Lm. BC Abemaciclib + fulvestrant MONALEESA-7 1 L ER+ HER 2– pre/perimenopausal ABC Ribociclib + goserelin + tamoxifen / NSAI
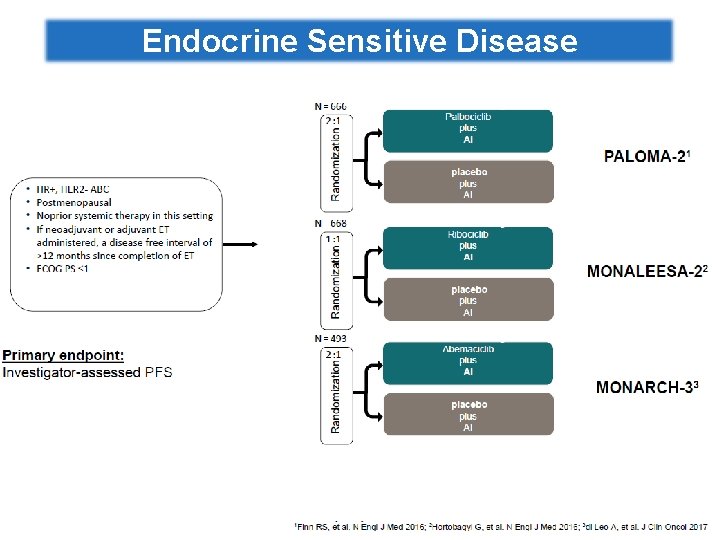
Endocrine Sensitive Disease
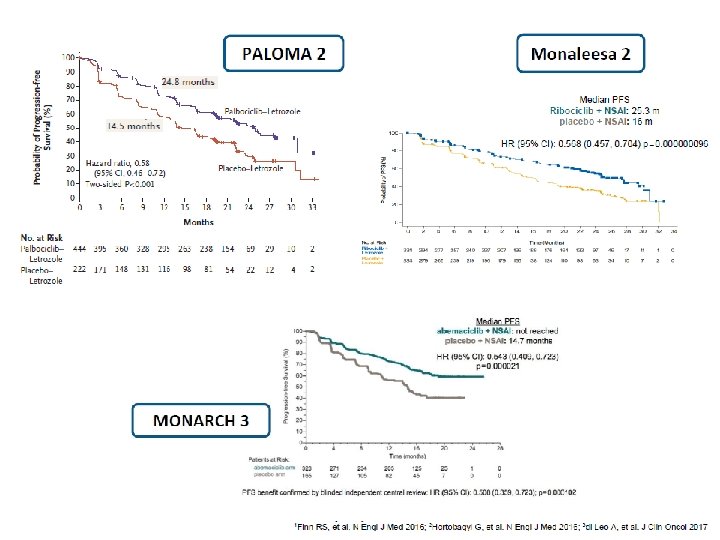
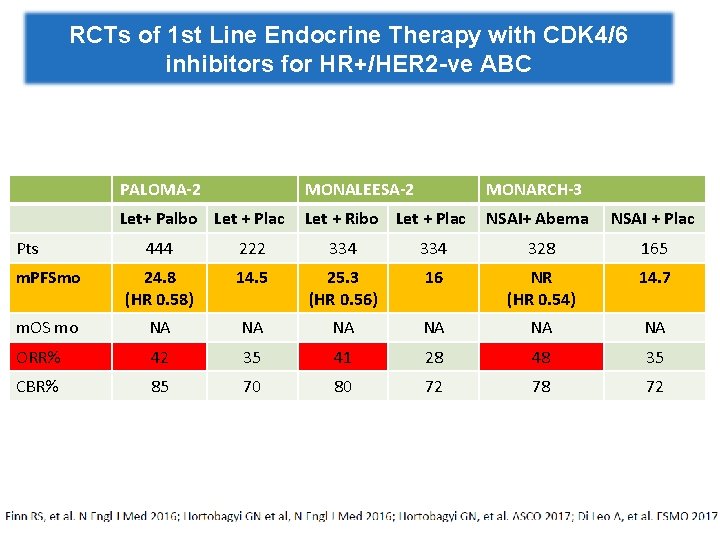
RCTs of 1 st Line Endocrine Therapy with CDK 4/6 inhibitors for HR+/HER 2 -ve ABC Pts PALOMA-2 MONALEESA-2 MONARCH-3 Let+ Palbo Let + Plac Let + Ribo Let + Plac NSAI+ Abema NSAI + Plac 444 222 334 328 165 m. PFSmo 24. 8 (HR 0. 58) 14. 5 25. 3 (HR 0. 56) 16 NR (HR 0. 54) 14. 7 m. OS mo NA NA NA ORR% 42 35 41 28 48 35 CBR% 85 70 80 72 78 72
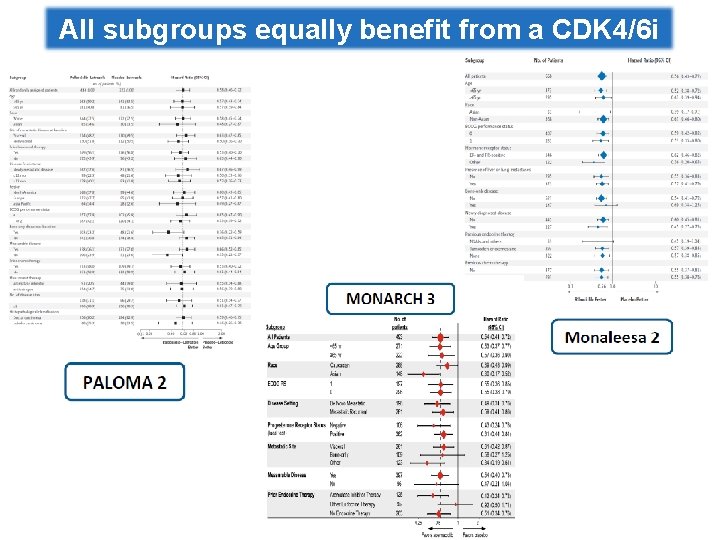
All subgroups equally benefit from a CDK 4/6 i
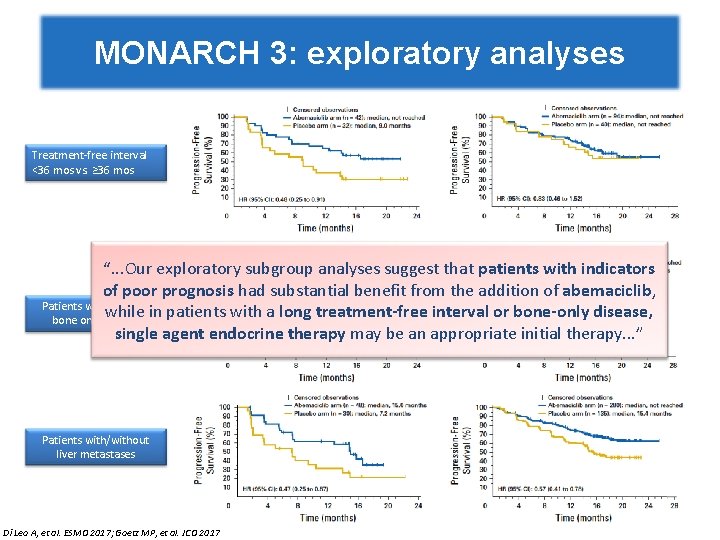
MONARCH 3: exploratory analyses Treatment-free interval <36 mos vs. ≥ 36 mos “. . . Our exploratory subgroup analyses suggest that patients with indicators of poor prognosis had substantial benefit from the addition of abemaciclib, Patients with/without while in patients with a long treatment-free interval or bone-only disease, bone only disease single agent endocrine therapy may be an appropriate initial therapy. . . ” Patients with/without liver metastases Di Leo A, et al. ESMO 2017; Goetz MP, et al. JCO 2017
Endocrine Resistant Disease
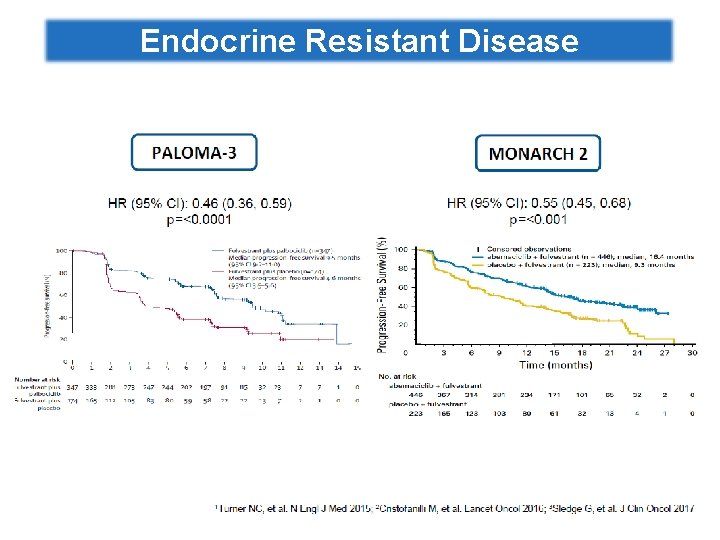
Endocrine Resistant Disease
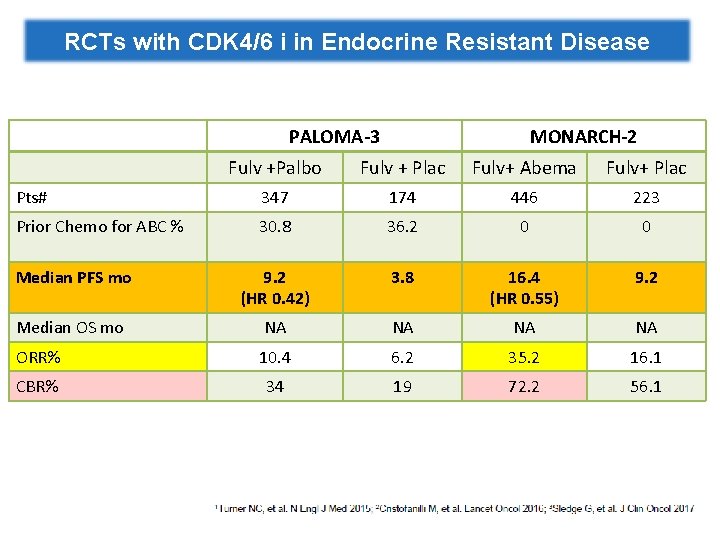
RCTs with CDK 4/6 i in Endocrine Resistant Disease PALOMA-3 MONARCH-2 Fulv +Palbo Fulv + Plac Fulv+ Abema Fulv+ Plac Pts# 347 174 446 223 Prior Chemo for ABC % 30. 8 36. 2 0 0 Median PFS mo 9. 2 (HR 0. 42) 3. 8 16. 4 (HR 0. 55) 9. 2 Median OS mo NA NA ORR% 10. 4 6. 2 35. 2 16. 1 CBR% 34 19 72. 2 56. 1
OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø Final Remarkers
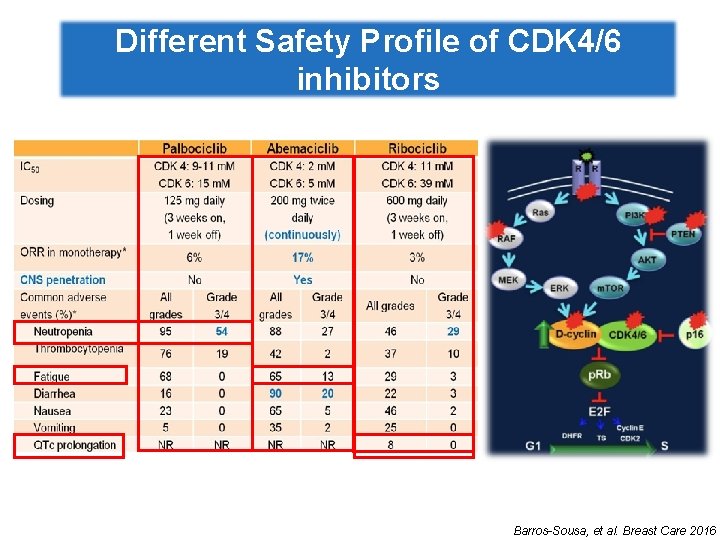
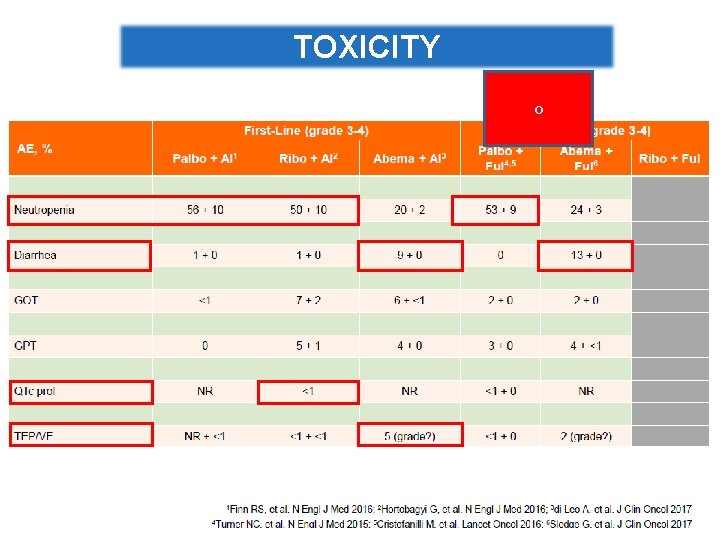
Different Safety Profile of CDK 4/6 inhibitors Barros-Sousa, et al. Breast Care 2016
OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø Final Remarkers
Premenopausal women and clinical trials Ø Premenopausal women generally excluded from large registrational trials HR+ ABC. Ø Clinical data limited to only a few small phase 2 trials. Ø PALOMA-3 included 108 premenopausal women ad is the only phase 3 study to date to report outcomes data for fulvestrant 500 mg with ovarian suppression in premenopausal patients. Ø MONALEESA-7 is the only phase 3 trial conducted in premenopausal women and is the only phase 3 trial to date report outcomes data in this populations.
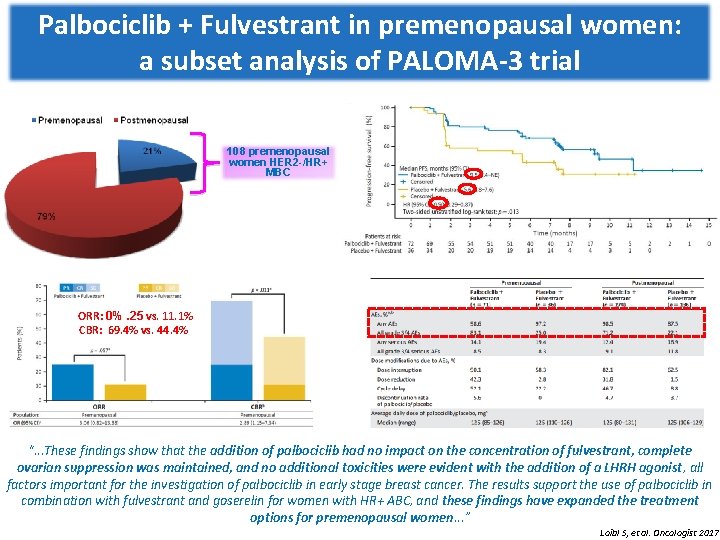
Palbociclib + Fulvestrant in premenopausal women: a subset analysis of PALOMA-3 trial 108 premenopausal women HER 2 -/HR+ MBC ORR: 0%. 25 vs. 11. 1% CBR: 69. 4% vs. 44. 4% “. . . These findings show that the addition of palbociclib had no impact on the concentration of fulvestrant, complete ovarian suppression was maintained, and no additional toxicities were evident with the addition of a LHRH agonist, all factors important for the investigation of palbociclib in early stage breast cancer. The results support the use of palbociclib in combination with fulvestrant and goserelin for women with HR+ ABC, and these findings have expanded the treatment options for premenopausal women. . . ” Loibl S, et al. Oncologist 2017
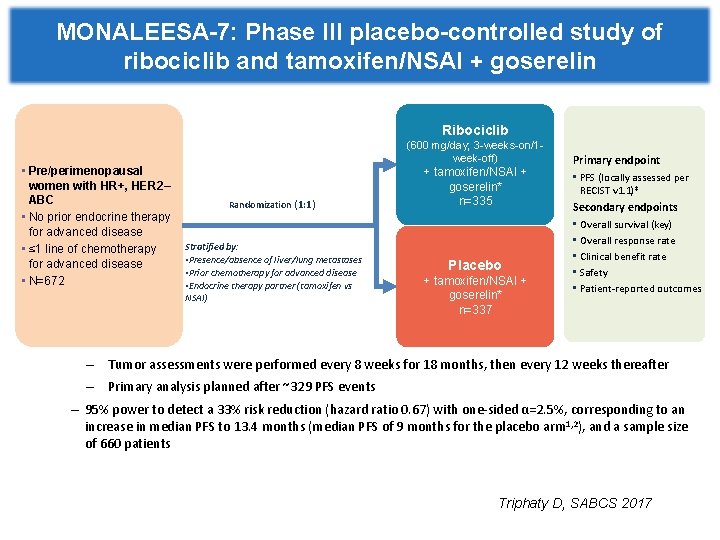
MONALEESA-7: Phase III placebo-controlled study of ribociclib and tamoxifen/NSAI + goserelin Ribociclib • Pre/perimenopausal women with HR+, HER 2– ABC • No prior endocrine therapy for advanced disease • ≤ 1 line of chemotherapy for advanced disease • N=672 (600 mg/day; 3 -weeks-on/1 week-off) Randomization (1: 1) Stratified by: • Presence/absence of liver/lung metastases • Prior chemotherapy for advanced disease • Endocrine therapy partner (tamoxifen vs NSAI) + tamoxifen/NSAI + goserelin* n=335 Placebo + tamoxifen/NSAI + goserelin* n=337 Primary endpoint • PFS (locally assessed per RECIST v 1. 1)‡ Secondary endpoints • Overall survival (key) • Overall response rate • Clinical benefit rate • Safety • Patient-reported outcomes – Tumor assessments were performed every 8 weeks for 18 months, then every 12 weeks thereafter – Primary analysis planned after ~329 PFS events – 95% power to detect a 33% risk reduction (hazard ratio 0. 67) with one-sided α=2. 5%, corresponding to an increase in median PFS to 13. 4 months (median PFS of 9 months for the placebo arm 1, 2), and a sample size of 660 patients Triphaty D, SABCS 2017
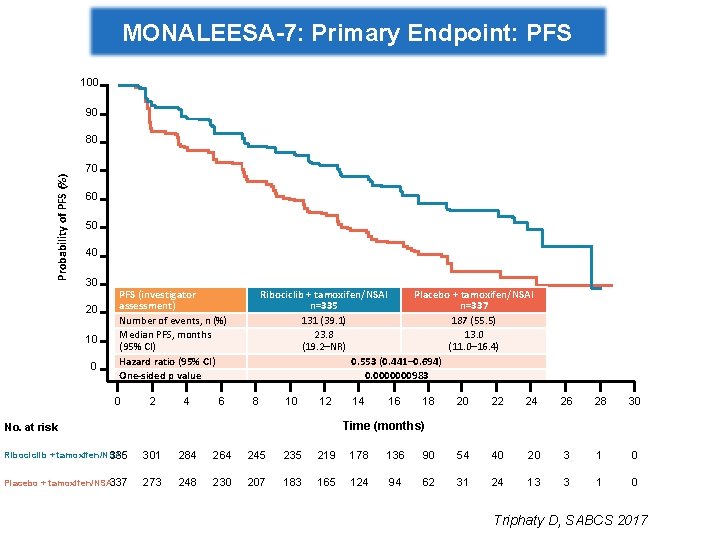
MONALEESA-7: Primary Endpoint: PFS 100 90 Probability of PFS (%) 80 70 60 50 40 30 20 10 0 PFS (investigator assessment) Number of events, n (%) Median PFS, months (95% CI) Hazard ratio (95% CI) One-sided p value 0 2 4 6 Ribociclib + tamoxifen/NSAI Placebo + tamoxifen/NSAI n=335 n=337 131 (39. 1) 187 (55. 5) 23. 8 13. 0 (19. 2–NR) (11. 0– 16. 4) 0. 553 (0. 441– 0. 694) 0. 0000000983 8 10 12 14 16 18 20 22 24 26 28 30 Time (months) No. at risk Ribociclib + tamoxifen/NSAI 335 301 284 264 245 235 219 178 136 90 54 40 20 3 1 0 Placebo + tamoxifen/NSAI 337 273 248 230 207 183 165 124 94 62 31 24 13 3 1 0 Triphaty D, SABCS 2017
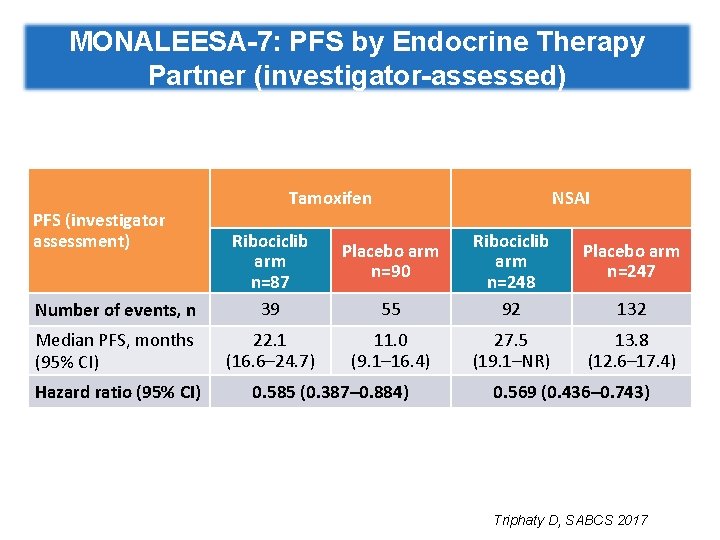
MONALEESA-7: PFS by Endocrine Therapy Partner (investigator-assessed) PFS (investigator assessment) Tamoxifen Number of events, n Ribociclib arm n=87 39 Median PFS, months (95% CI) 22. 1 (16. 6– 24. 7) Hazard ratio (95% CI) NSAI 55 Ribociclib arm n=248 92 11. 0 (9. 1– 16. 4) 27. 5 (19. 1–NR) Placebo arm n=90 0. 585 (0. 387– 0. 884) Placebo arm n=247 132 13. 8 (12. 6– 17. 4) 0. 569 (0. 436– 0. 743) Triphaty D, SABCS 2017
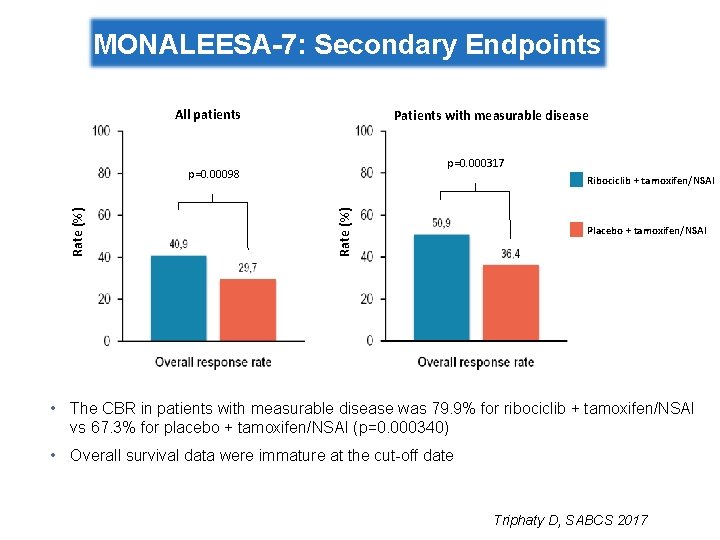
MONALEESA-7: Secondary Endpoints All patients Patients with measurable disease p=0. 000317 Ribociclib + tamoxifen/NSAI Rate (%) p=0. 00098 Placebo + tamoxifen/NSAI • The CBR in patients with measurable disease was 79. 9% for ribociclib + tamoxifen/NSAI vs 67. 3% for placebo + tamoxifen/NSAI (p=0. 000340) • Overall survival data were immature at the cut-off date Triphaty D, SABCS 2017

OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø The Future Ø Final Remarkers
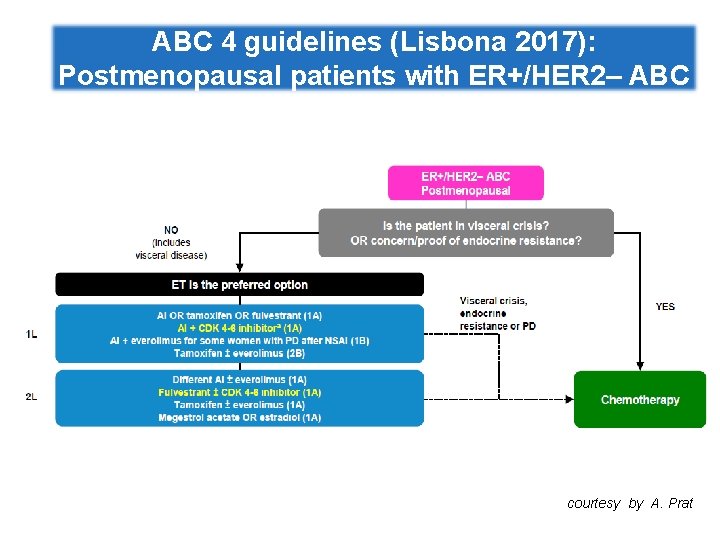
ABC 4 guidelines (Lisbona 2017): Postmenopausal patients with ER+/HER 2‒ ABC courtesy by A. Prat
OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø The Future Ø Final Remarkers
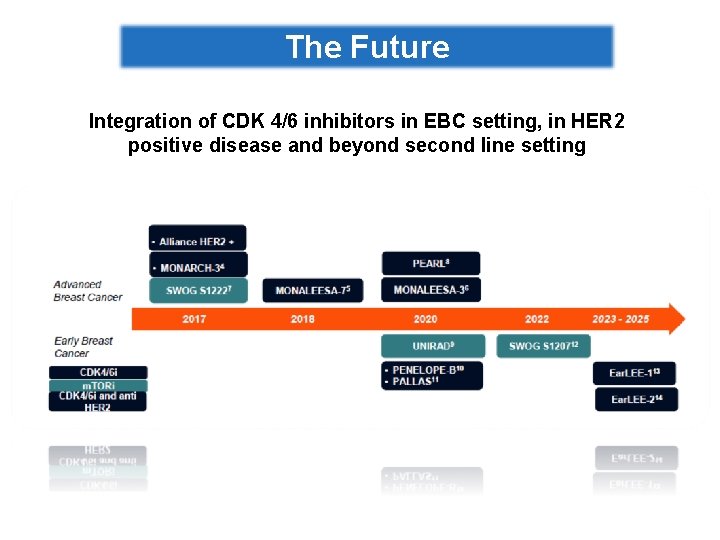
The Future Integration of CDK 4/6 inhibitors in EBC setting, in HER 2 positive disease and beyond second line setting
OUTLINE Ø Therapeutic landscape of HR+HER 2 -MBC Ø Endocrine therapy plus CDK 4/6 inhibitors as a new standard of care on HR+ advanced breast cancer-preclinical data Ø The CDK 4/6 inhibitors program Ø Toxicity Ø Premenopausal women Ø Positioning of CDK 4/6 inhibitors in standard clinical practice Ø The Future Ø Final Remarkers
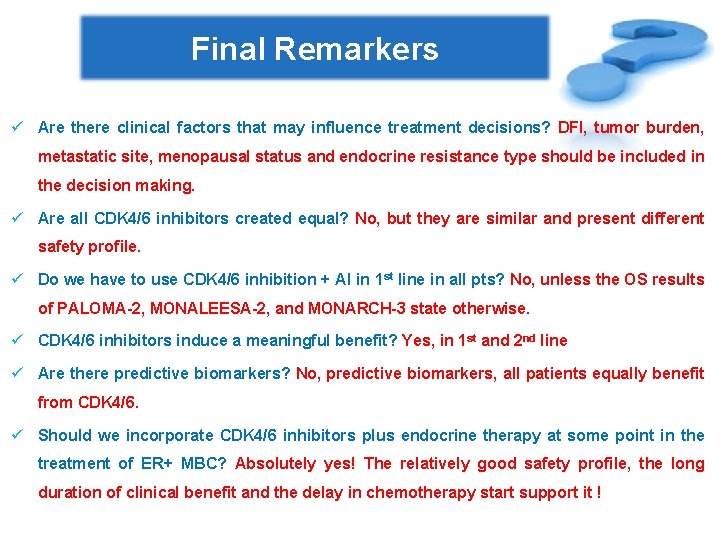
Final Remarkers ü Are there clinical factors that may influence treatment decisions? DFI, tumor burden, metastatic site, menopausal status and endocrine resistance type should be included in the decision making. ü Are all CDK 4/6 inhibitors created equal? No, but they are similar and present different safety profile. ü Do we have to use CDK 4/6 inhibition + AI in 1 st line in all pts? No, unless the OS results of PALOMA-2, MONALEESA-2, and MONARCH-3 state otherwise. ü CDK 4/6 inhibitors induce a meaningful benefit? Yes, in 1 st and 2 nd line ü Are there predictive biomarkers? No, predictive biomarkers, all patients equally benefit from CDK 4/6. ü Should we incorporate CDK 4/6 inhibitors plus endocrine therapy at some point in the treatment of ER+ MBC? Absolutely yes! The relatively good safety profile, the long duration of clinical benefit and the delay in chemotherapy start support it !

Grazie per l’attenzione
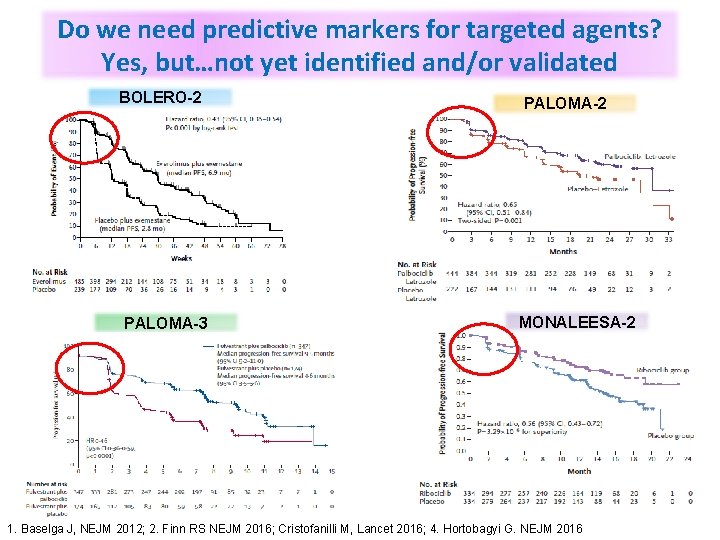
Do we need predictive markers for targeted agents? Yes, but…not yet identified and/or validated BOLERO-2 PALOMA-3 PALOMA-2 MONALEESA-2 1. Baselga J, NEJM 2012; 2. Finn RS NEJM 2016; Cristofanilli M, Lancet 2016; 4. Hortobagyi G. NEJM 2016
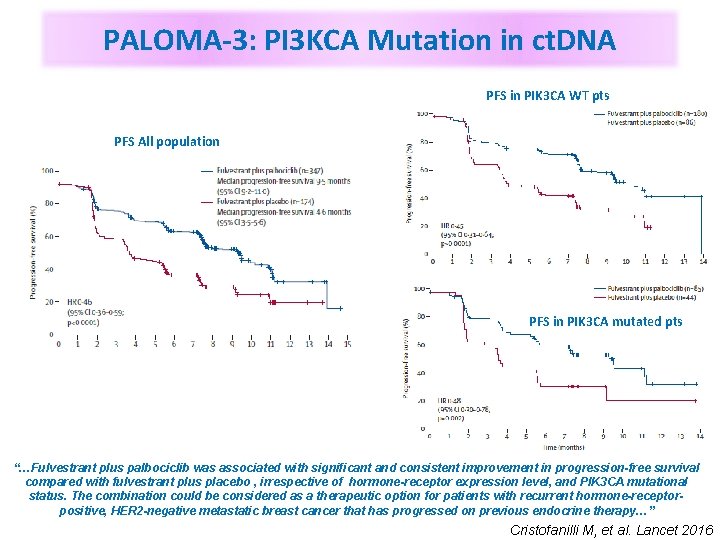
PALOMA-3: PI 3 KCA Mutation in ct. DNA PFS in PIK 3 CA WT pts PFS All population PFS in PIK 3 CA mutated pts “…Fulvestrant plus palbociclib was associated with significant and consistent improvement in progression-free survival compared with fulvestrant plus placebo , irrespective of hormone-receptor expression level, and PIK 3 CA mutational status. The combination could be considered as a therapeutic option for patients with recurrent hormone-receptorpositive, HER 2 -negative metastatic breast cancer that has progressed on previous endocrine therapy…” Cristofanilli M, et al. Lancet 2016
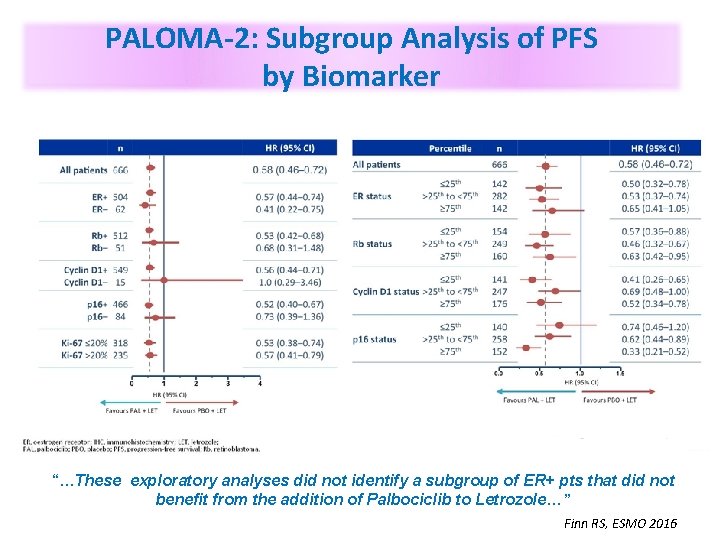
PALOMA-2: Subgroup Analysis of PFS by Biomarker “…These exploratory analyses did not identify a subgroup of ER+ pts that did not benefit from the addition of Palbociclib to Letrozole…” Finn RS, ESMO 2016
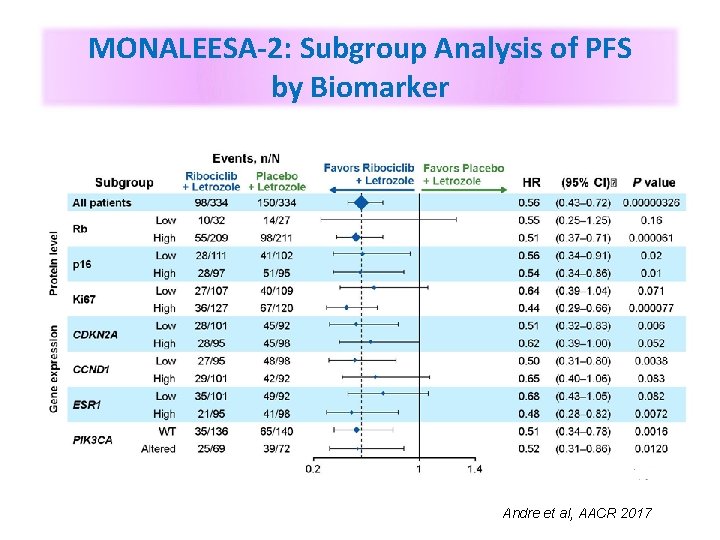
MONALEESA-2: Subgroup Analysis of PFS by Biomarker Andre et al, AACR 2017

CDK 4/6 in Breast Cancer o • The growth of HR+ breast cancer is dependent on Cyclin D 1, a direct transcriptional target of ER • Cyclin D 1 activates CDK 4/6 resulting in G 1–S phase transition and entry into the cell cycle 1 Adapted from Asghar U, Nat Rev Drug Discov 2015

Luminal MBC: Treatment Guidelines NCCN/AS CO ESMO/ABC 3 AIOM • Pts whose tumors express any level of ER and/or Pg. R. Treatment recommendations should be offered on the basis of type of adjuvant treatment, disease-free interval, and extent ofodisease at the time of recurrence. • Endocrine therapy should be recommended as initial treatment for patients with HR-positive MBC, except for patients with immediately life-threatening disease or for those experiencing rapid visceral recurrence during adjuvant endocrine therapy • Sequential hormone therapy should be offered to patients with endocrine-responsive disease • Treatment should be administered until there is unequivocal evidence of disease progression. • the preferential use of endocrine therapy, even in the presence of visceral metastases, until clear evidence of endocrine resistance. • Chemotherapy should be reserved for cases of rapidly progressive disease or proven endocrine resistance. • Endocrine treatment after CT (maintenance ET) to maintain benefit is a reasonable option, although this approach has not been assessed in randomized trials. • Tumori ormonosensibili HER 2 negativi, in assenza di malattia aggressiva e crisi viscerale. • Il trattamento ormonale dovrebbe essere proseguito (anche con linee di terapia successive) fino a quando è possibile considerare la malattia ormonosensibile. • la scelta della terapia, sia per la prima linea che per quelle successive, si basa soprattutto sullo stato menopausale della paziente e sulle terapie precedentemente eseguite in fase adiuvante o metastatica. Rugo HS, et al. JCO 2017 Cardoso F, et al. Ann Oncol 2016 Linee Guida AIOM 2016
Treatment for HR+HER 2 neg MBC CDK 4/6 inhibitors + AI o 7% CDK 4/6 + fulvestrant Breast Cancer Everolimus + exemestane Toremifene Exemestane Letrozolo Buparlisib + fulvestrant Fulvestrant Tamoxifene 30% Anastrozolo 70% 1975 1980 1985 1990 1995 2000 2005 2010 2015 2016
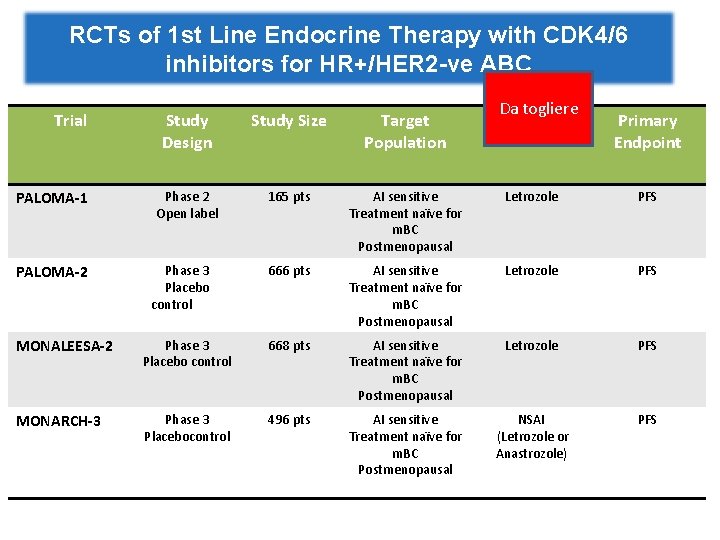
RCTs of 1 st Line Endocrine Therapy with CDK 4/6 inhibitors for HR+/HER 2 -ve ABC Trial PALOMA-1 PALOMA-2 Da togliere Partner ET Study Design Study Size Target Population Phase 2 Open label 165 pts AI sensitive Treatment naïve for m. BC Postmenopausal Letrozole PFS 666 pts AI sensitive Treatment naïve for m. BC Postmenopausal Letrozole PFS Phase 3 Placebo control Primary Endpoint MONALEESA-2 Phase 3 Placebo control 668 pts AI sensitive Treatment naïve for m. BC Postmenopausal Letrozole PFS MONARCH-3 Phase 3 Placebocontrol 496 pts AI sensitive Treatment naïve for m. BC Postmenopausal NSAI (Letrozole or Anastrozole) PFS
TOXICITY o
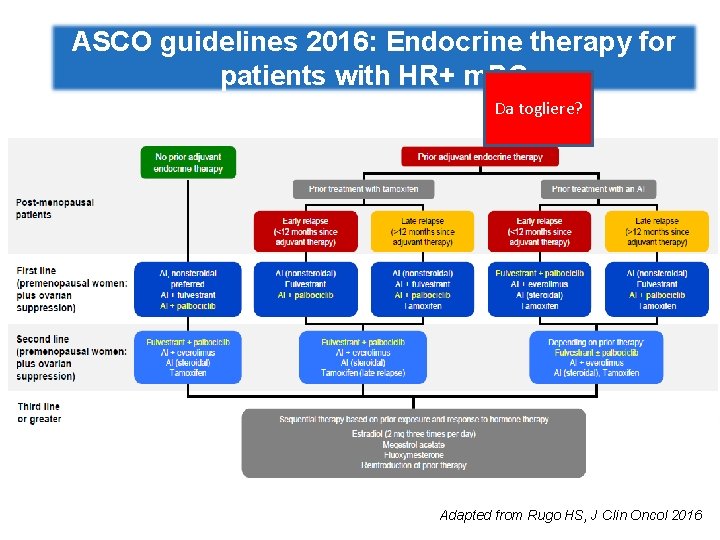
ASCO guidelines 2016: Endocrine therapy for patients with HR+ m. BC Da togliere? Adapted from Rugo HS, J Clin Oncol 2016
- Slides: 50