Lowtemperature oxidation of volatile organic compounds VOCs Modeling

![Vertical distribution [Ehhalt, 1999] Vertical distribution [Ehhalt, 1999]](https://slidetodoc.com/presentation_image_h2/4bcc4f23dc07a32f334856d3523a1713/image-7.jpg)

![VOC chemistry and global ozone O 3 P(O 3) Wang et al. [1998] Ozone VOC chemistry and global ozone O 3 P(O 3) Wang et al. [1998] Ozone](https://slidetodoc.com/presentation_image_h2/4bcc4f23dc07a32f334856d3523a1713/image-11.jpg)
- Slides: 25

Low-temperature oxidation of volatile organic compounds (VOCs) Modeling perspective Lyatt Jaeglé Department of Atmospheric Sciences, University of Washington, Seattle UT/LS workshop – July 25 2001 Breckenridge, Colorado

OUTLINE § Volatile organic compounds: sources, chemistry, and observed distributions § Impact of VOCs on atmospheric chemistry (O 3, NOx, HOx) § Role of short-lived VOC compounds in UT chemistry § Chemical reactions in need of better data – field measurements/modeling perspective

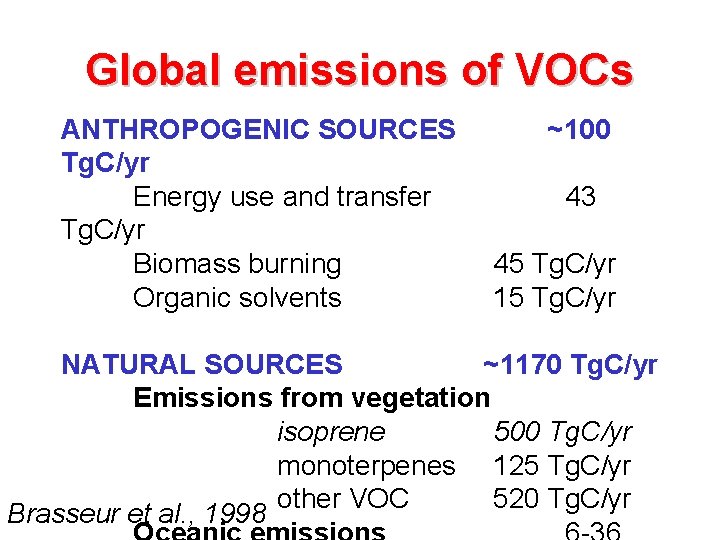
Global emissions of VOCs ANTHROPOGENIC SOURCES Tg. C/yr Energy use and transfer Tg. C/yr Biomass burning Organic solvents ~100 43 45 Tg. C/yr 15 Tg. C/yr NATURAL SOURCES ~1170 Tg. C/yr Emissions from vegetation isoprene 500 Tg. C/yr monoterpenes 125 Tg. C/yr other VOC 520 Tg. C/yr Brasseur et al. , 1998

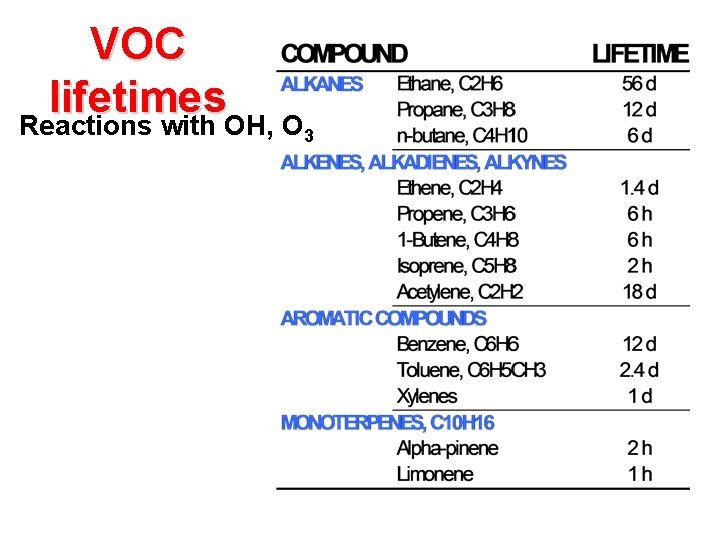
VOC lifetimes Reactions with OH, O 3

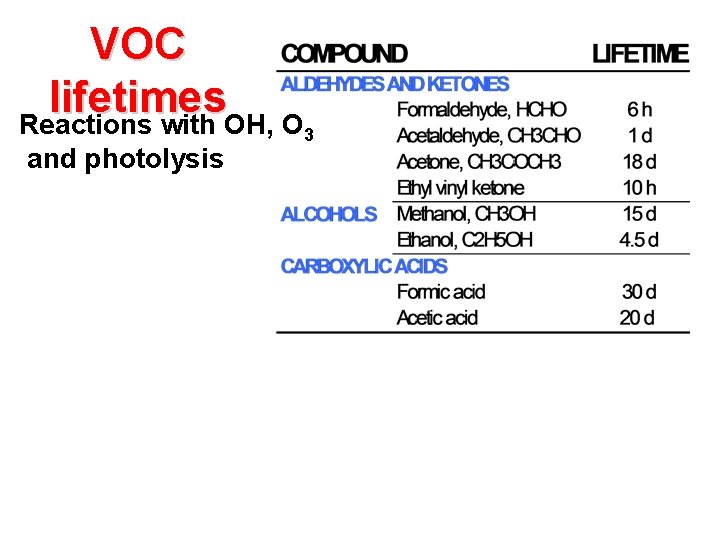
VOC lifetimes Reactions with OH, O and photolysis 3

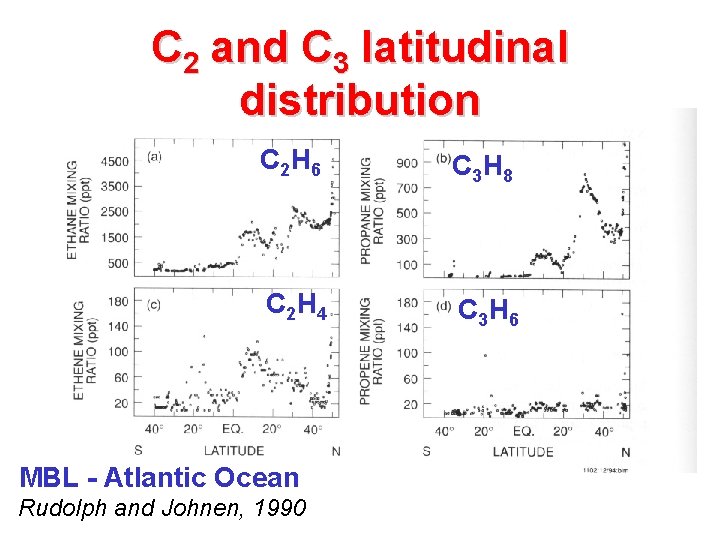
C 2 and C 3 latitudinal distribution C 2 H 6 C 3 H 8 C 2 H 4 C 3 H 6 MBL - Atlantic Ocean Rudolph and Johnen, 1990
![Vertical distribution Ehhalt 1999 Vertical distribution [Ehhalt, 1999]](https://slidetodoc.com/presentation_image_h2/4bcc4f23dc07a32f334856d3523a1713/image-7.jpg)
Vertical distribution [Ehhalt, 1999]

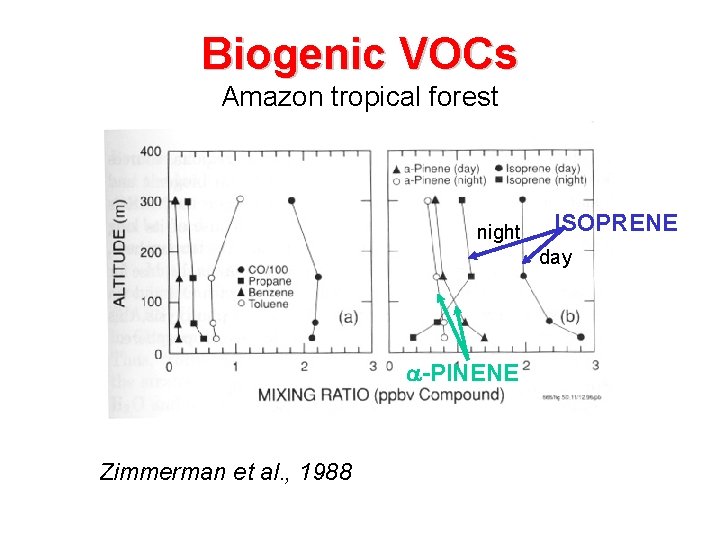
Biogenic VOCs Amazon tropical forest night ISOPRENE day -PINENE Zimmerman et al. , 1988

VOC oxidation mechanism

VOCs and tropospheric chemistry § Most early global models of tropospheric chemistry ignored NMHC chemistry until late 1990 s: complex, costly, and unclear global effects § Over last 3 years number of specific studies on global role of NMHCs: Houweling et al. [1998], Wang et al. [1998], Horowitz and Jacob [1999], Roelofs and Lelieveld [2000], Poisson et al. [2001]
![VOC chemistry and global ozone O 3 PO 3 Wang et al 1998 Ozone VOC chemistry and global ozone O 3 P(O 3) Wang et al. [1998] Ozone](https://slidetodoc.com/presentation_image_h2/4bcc4f23dc07a32f334856d3523a1713/image-11.jpg)
VOC chemistry and global ozone O 3 P(O 3) Wang et al. [1998] Ozone increases by 10 -80% in summer NH boundary layer, 8 -16% global increase P(O 3) increases by 10 -50% in UT Direct effect of NMHC (increase in organic peroxy radicals) is small Changes in O 3 are due to indirect effects: changes in NOx (PAN) changes in OH

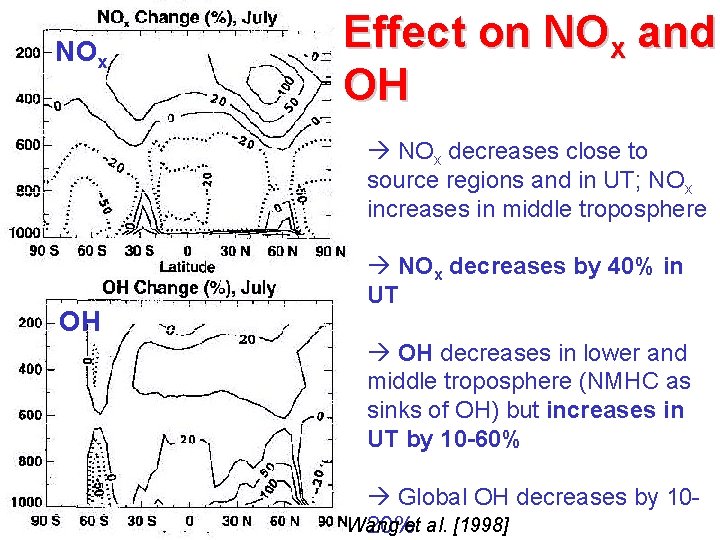
NOx Effect on NOx and OH NOx decreases close to source regions and in UT; NOx increases in middle troposphere OH NOx decreases by 40% in UT OH decreases in lower and middle troposphere (NMHC as sinks of OH) but increases in UT by 10 -60% Global OH decreases by 10 Wang 20%et al. [1998]

Lumping vs. detailed schemes for computations § Example of isoprene oxidation: ~1000 reactions and ~300 species § Computer limitations use of simplified mechanisms in global 3 -D studies (10 -40 species and 30 -100 reactions) § Intercomparison of various schemes [Pöschl et al. , 2000]: Ø Large differences in calculations of PAN, NOx, H 2 O 2, HOx, CO, O 3 (± 100%) Ø Differences associated with treatment of isoprene-related nitrates Ø Good news: some condensed mechanisms can reproduce the results of detailed schemes § BUT: detailed mechanisms have not been validated under globally relevant conditions [chamber studies at high

VOC oxidation and HOx in the UT Higher observed levels of HOx than anticipated based on H 2 O + O(1 D): tropical Pacific (STRAT, PEMTropics B), continental U. S. (SUCCESS) § Role of acetone photolysis as a source of HOx [Singh et al. , 1995; Arnold et al. , 1997; Mc. Keen et al. , 1997; Wennberg et al. , 1998]

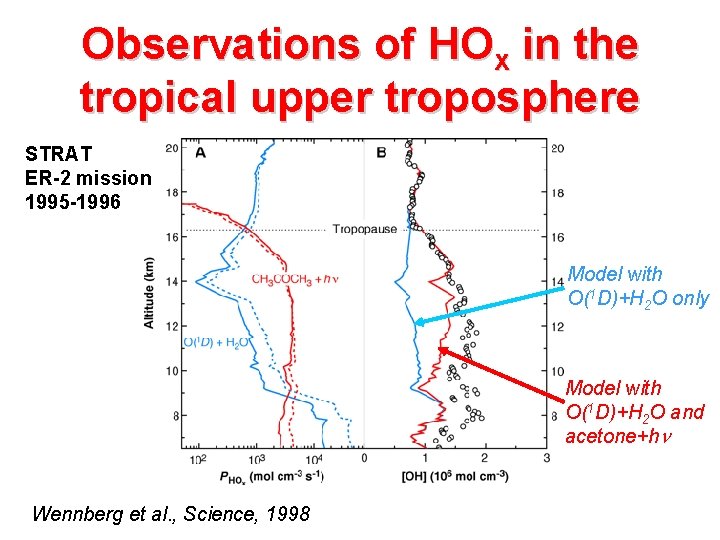
Observations of HOx in the tropical upper troposphere STRAT ER-2 mission 1995 -1996 Model with O(1 D)+H 2 O only Model with O(1 D)+H 2 O and acetone+h Wennberg et al. , Science, 1998

VOC oxidation and HOx in the UT Higher observed levels of HOx than anticipated based on H 2 O + O(1 D): tropical Pacific (STRAT, PEMTropics B), continental U. S. (SUCCESS) § Role of acetone photolysis as a source of HOx [Singh et al. , 1995; Arnold et al. , 1997; Mc. Keen et al. , 1997; Wennberg et al. , 1998] § Role of deep convection as a source of HOx precursors to the and UT: Crutzen, 1984] too soluble • H 2 O 2 [Chatfield • CH 3 OOH [Prather and Jacob, 1997; Jaeglé et al. , 1997; Cohan et al. , 1999; Mari et al. , 2000; Ravetta et al. , 2001] • CH 2 O [Müller and Brasseur, 1999; Jaeglé et al. , 1998] • Higher aldehydes [Müller and Brasseur, 1999] • Isoprene oxidation products (methylvinylketone, methyl glyoxal) [Collins et al. , 1999]

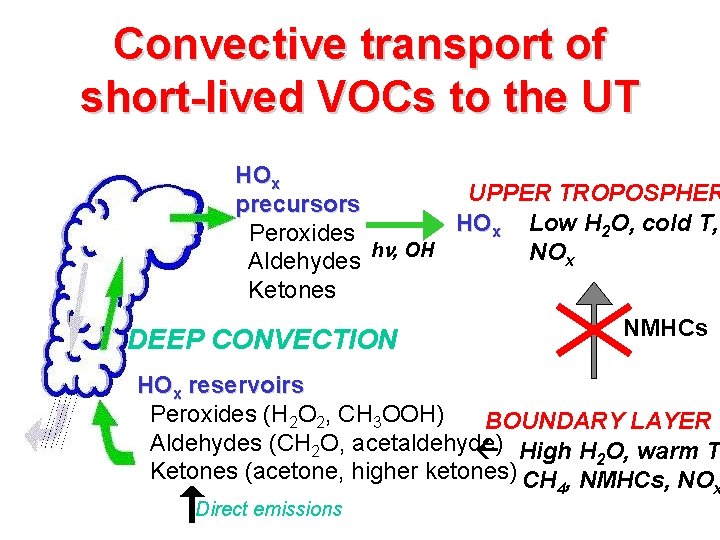
Convective transport of short-lived VOCs to the UT HOx UPPER TROPOSPHER precursors HOx Low H 2 O, cold T, Peroxides h , OH NOx Aldehydes Ketones NMHCs DEEP CONVECTION HOx reservoirs Peroxides (H 2 O 2, CH 3 OOH) BOUNDARY LAYER Aldehydes (CH 2 O, acetaldehyde) High H 2 O, warm T Ketones (acetone, higher ketones) CH , NMHCs, NO 4 Direct emissions x

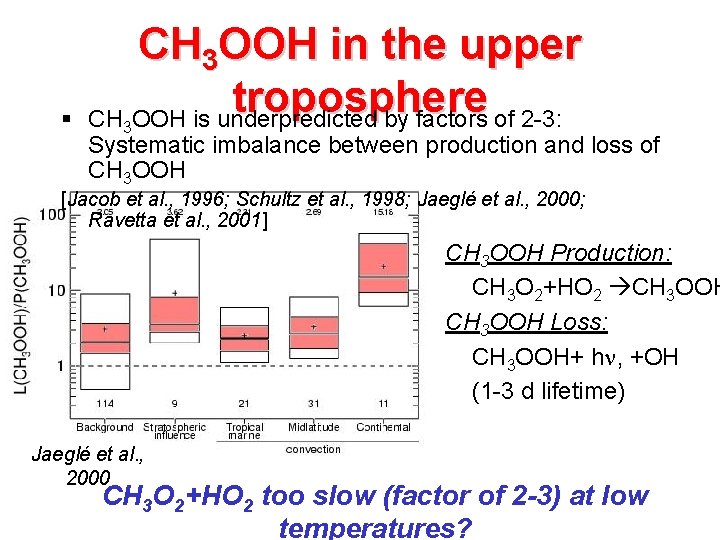
§ CH 3 OOH in the upper troposphere CH OOH is underpredicted by factors of 2 -3: 3 Systematic imbalance between production and loss of CH 3 OOH [Jacob et al. , 1996; Schultz et al. , 1998; Jaeglé et al. , 2000; Ravetta et al. , 2001] CH 3 OOH Production: CH 3 O 2+HO 2 CH 3 OOH Loss: CH 3 OOH+ h , +OH (1 -3 d lifetime) Jaeglé et al. , 2000 CH 3 O 2+HO 2 too slow (factor of 2 -3) at low temperatures?

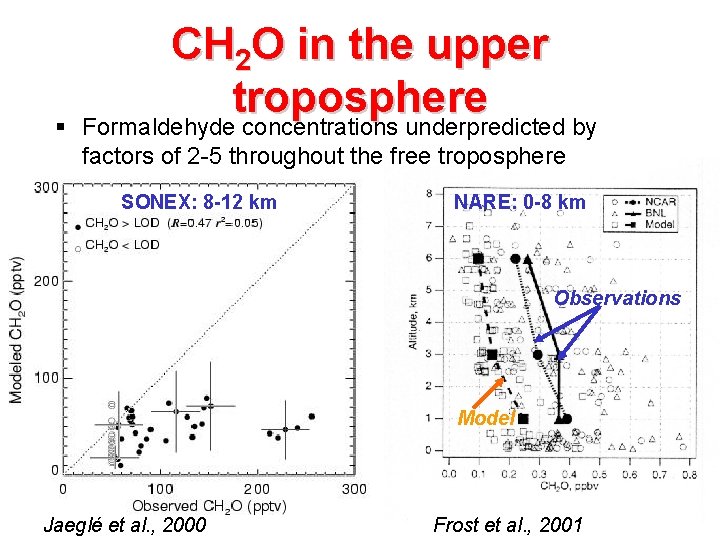
§ CH 2 O in the upper troposphere Formaldehyde concentrations underpredicted by factors of 2 -5 throughout the free troposphere SONEX: 8 -12 km NARE: 0 -8 km Observations Model Jaeglé et al. , 2000 Frost et al. , 2001

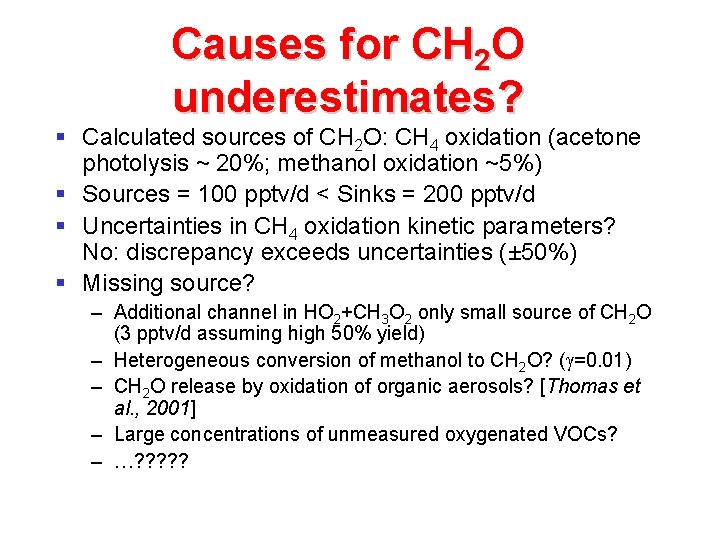
Causes for CH 2 O underestimates? § Calculated sources of CH 2 O: CH 4 oxidation (acetone photolysis ~ 20%; methanol oxidation ~5%) § Sources = 100 pptv/d < Sinks = 200 pptv/d § Uncertainties in CH 4 oxidation kinetic parameters? No: discrepancy exceeds uncertainties (± 50%) § Missing source? – Additional channel in HO 2+CH 3 O 2 only small source of CH 2 O (3 pptv/d assuming high 50% yield) – Heterogeneous conversion of methanol to CH 2 O? ( =0. 01) – CH 2 O release by oxidation of organic aerosols? [Thomas et al. , 2001] – Large concentrations of unmeasured oxygenated VOCs? – …? ? ?

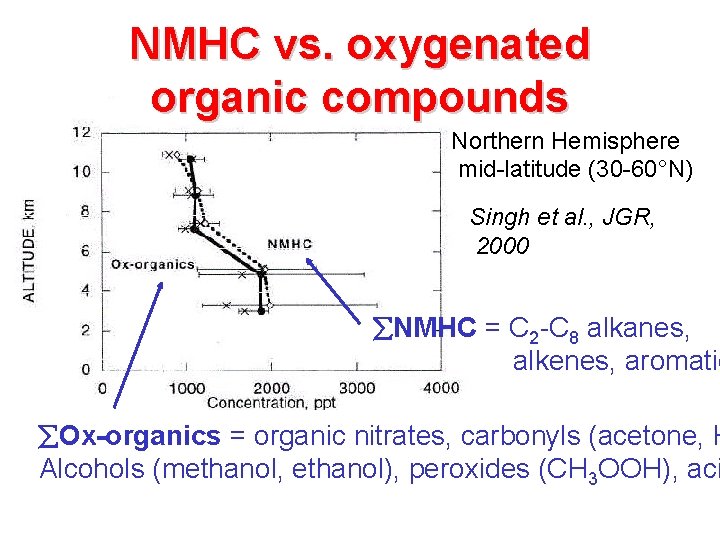
NMHC vs. oxygenated organic compounds Northern Hemisphere mid-latitude (30 -60°N) Singh et al. , JGR, 2000 NMHC = C 2 -C 8 alkanes, alkenes, aromatic Ox-organics = organic nitrates, carbonyls (acetone, H Alcohols (methanol, ethanol), peroxides (CH 3 OOH), aci

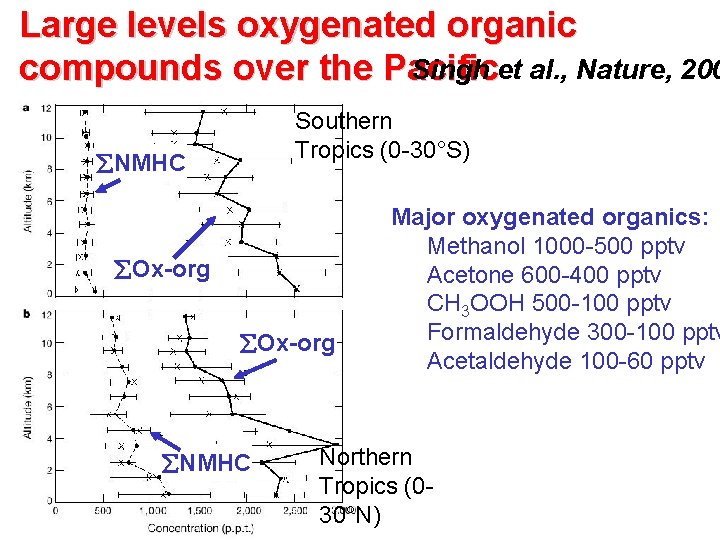
Large levels oxygenated organic Singh et al. , Nature, 200 compounds over the Pacific Southern Tropics (0 -30°S) NMHC Ox-org NMHC Major oxygenated organics: Methanol 1000 -500 pptv Acetone 600 -400 pptv CH 3 OOH 500 -100 pptv Formaldehyde 300 -100 pptv Acetaldehyde 100 -60 pptv Northern Tropics (030°N)

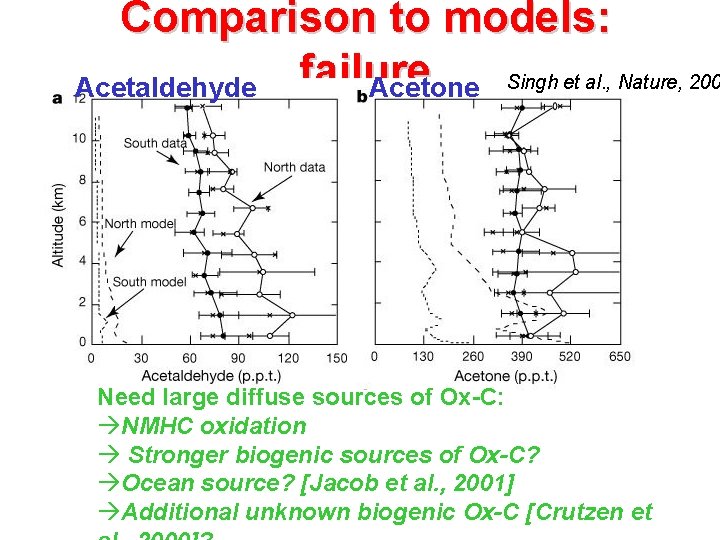
Comparison to models: failure Acetaldehyde Acetone Singh et al. , Nature, 200 Need large diffuse sources of Ox-C: NMHC oxidation Stronger biogenic sources of Ox-C? Ocean source? [Jacob et al. , 2001] Additional unknown biogenic Ox-C [Crutzen et

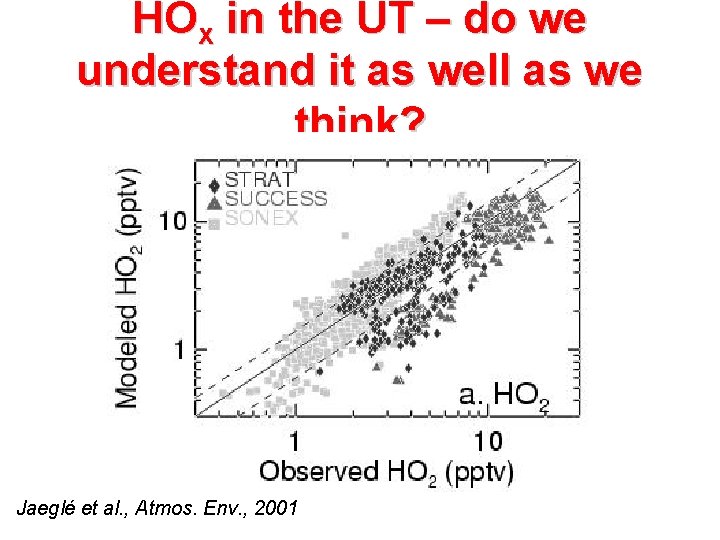
HOx in the UT – do we understand it as well as we think? Jaeglé et al. , Atmos. Env. , 2001

Summary Major uncertainties in VOC oxidation: 1) Kinetics of CH 3 OOH formation at low temp? 2) Chemistry and sources of CH 2 O 3) Chemistry and sources of acetone, acetaldehyde, methanol, and other oxygenated VOCs 4) Production and fate of isoprene oxidation products (organic nitrates and HOx producing molecules) 5) Yields and mechanisms for organic aerosol formation