Looking Back at Grade 9 Chemistry Agenda 1

Looking Back at Grade 9 Chemistry

Agenda 1. Atomic Theory 2. Periodic Table 3. Counting Atoms 4. Classifying Matter

Copper has. . . A. 29 protons, 29 electrons and 29 neutrons B. 29 protons, 29 electrons and 35 neutrons C. 29 protons, 29 electrons and 64 neutrons D. 64 protons, 64 electrons and 29 neutrons
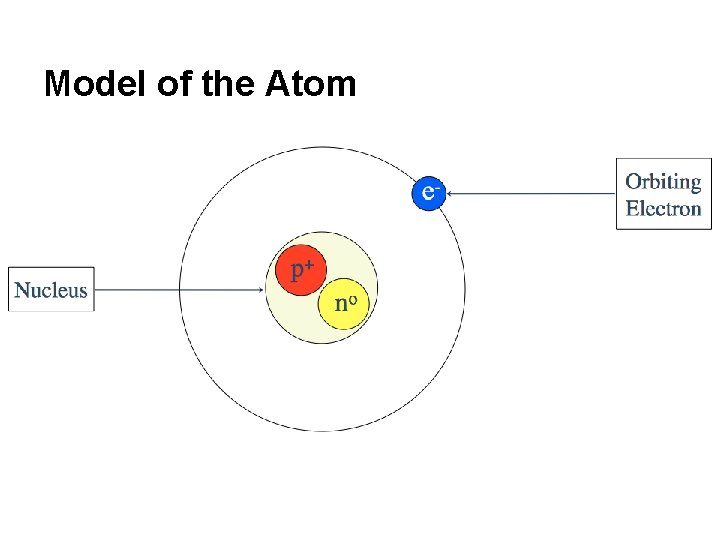
Model of the Atom
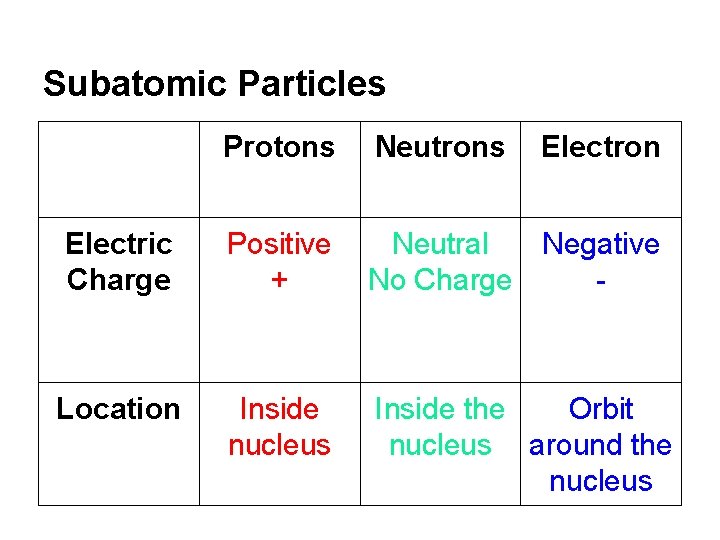
Subatomic Particles Protons Neutrons Electron Electric Charge Positive + Neutral Negative No Charge - Location Inside nucleus Inside the Orbit nucleus around the nucleus
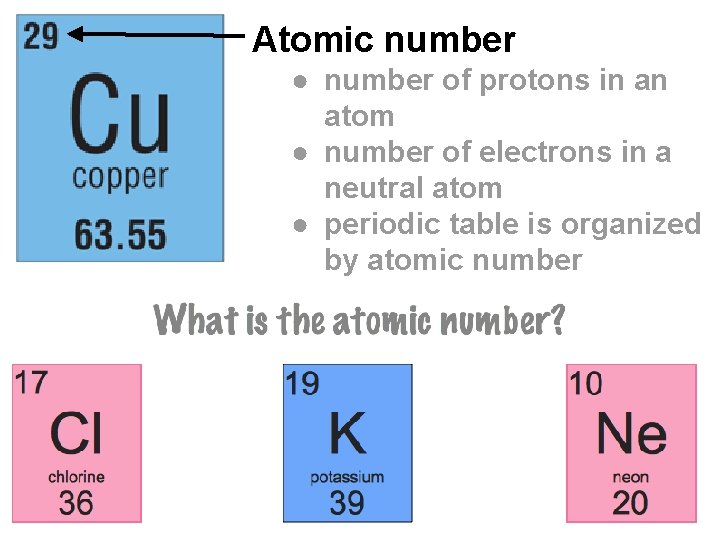
Atomic number ● number of protons in an atom ● number of electrons in a neutral atom ● periodic table is organized by atomic number
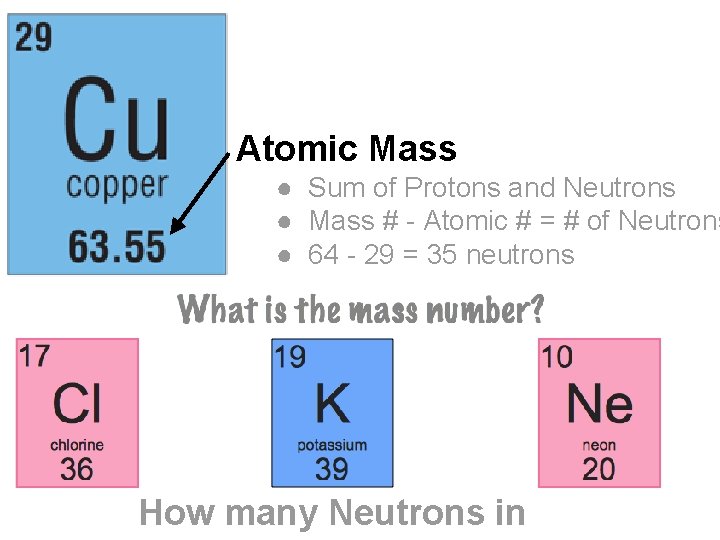
Atomic Mass ● Sum of Protons and Neutrons ● Mass # - Atomic # = # of Neutrons ● 64 - 29 = 35 neutrons How many Neutrons in
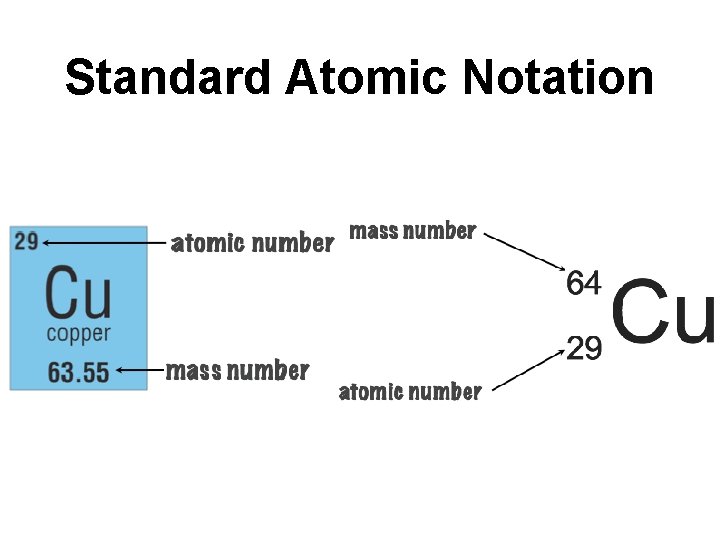
Standard Atomic Notation
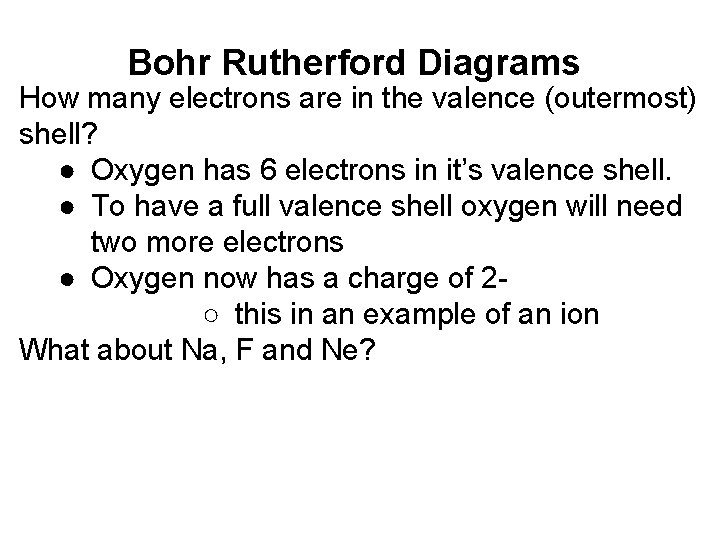
Bohr Rutherford Diagrams How many electrons are in the valence (outermost) shell? ● Oxygen has 6 electrons in it’s valence shell. ● To have a full valence shell oxygen will need two more electrons ● Oxygen now has a charge of 2○ this in an example of an ion What about Na, F and Ne?

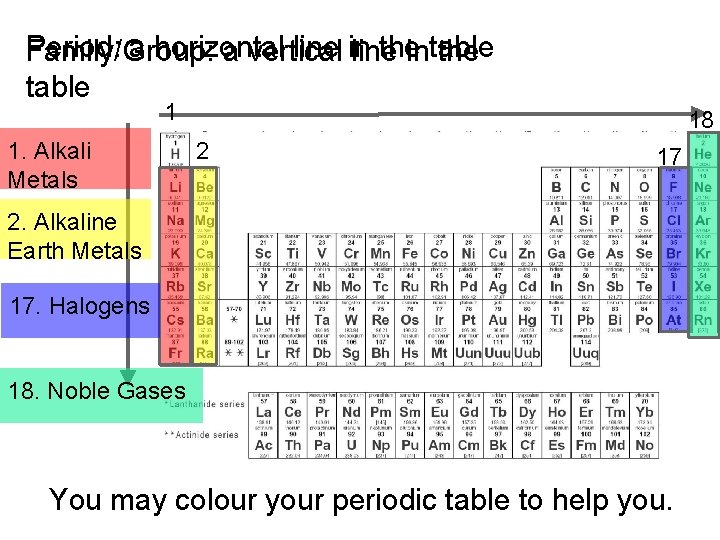
Period: a horizontal line in the Family/Group: a vertical line intable the table 1 1. Alkali Metals 18 2 17 2. Alkaline Earth Metals 17. Halogens 18. Noble Gases You may colour your periodic table to help you.
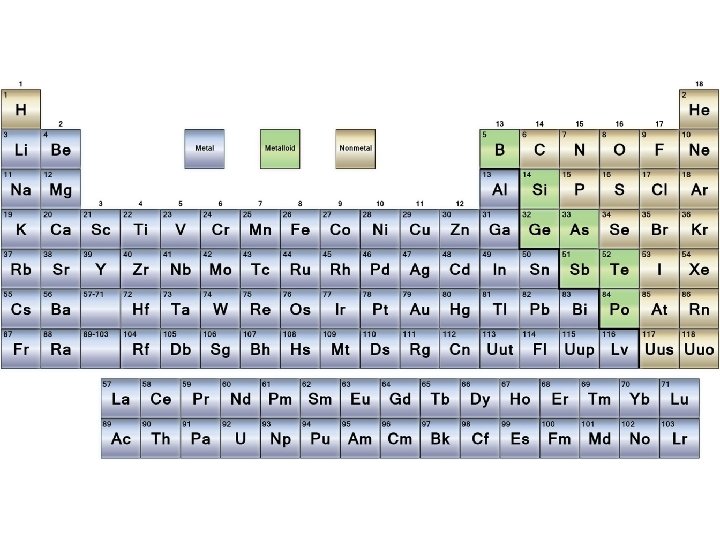

Periodic Table Scavenger Hunt For the next 15 -20 minutes you will be working on a worksheet to familiarize yourself with the periodic table. It is important that you understand the periodic table as it will greatly help you to predict how elements will react together for future labs in class.
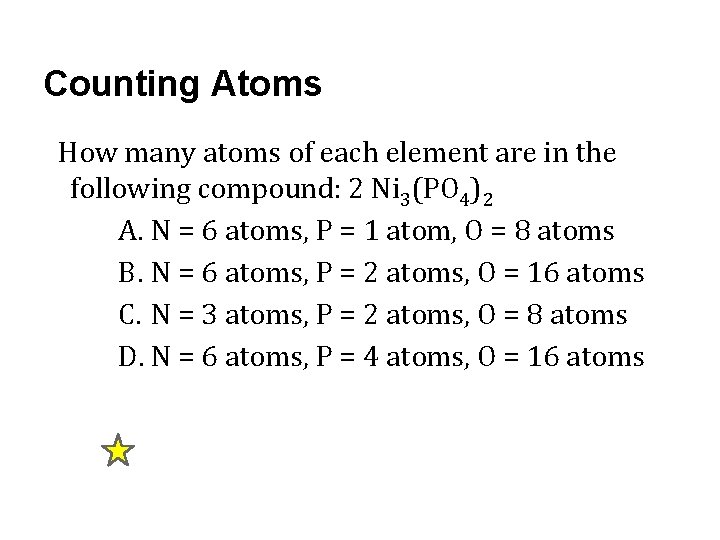
Counting Atoms How many atoms of each element are in the following compound: 2 Ni 3(PO 4)2 A. N = 6 atoms, P = 1 atom, O = 8 atoms B. N = 6 atoms, P = 2 atoms, O = 16 atoms C. N = 3 atoms, P = 2 atoms, O = 8 atoms D. N = 6 atoms, P = 4 atoms, O = 16 atoms
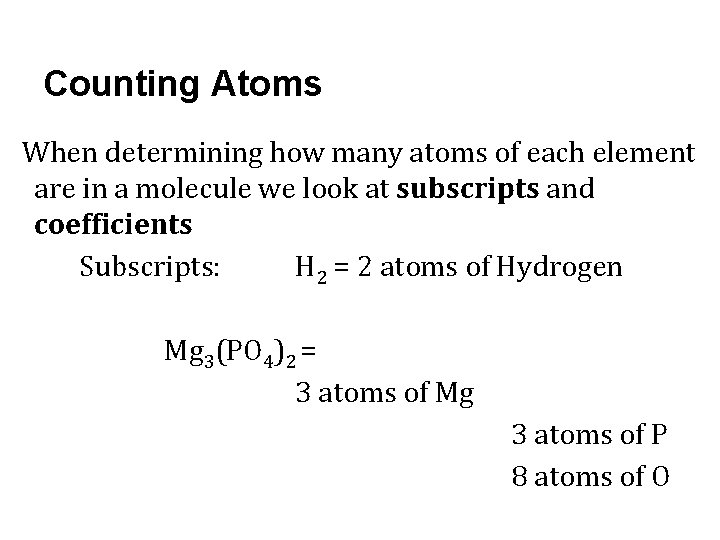
Counting Atoms When determining how many atoms of each element are in a molecule we look at subscripts and coefficients Subscripts: H 2 = 2 atoms of Hydrogen Mg 3(PO 4)2 = 3 atoms of Mg 3 atoms of P 8 atoms of O
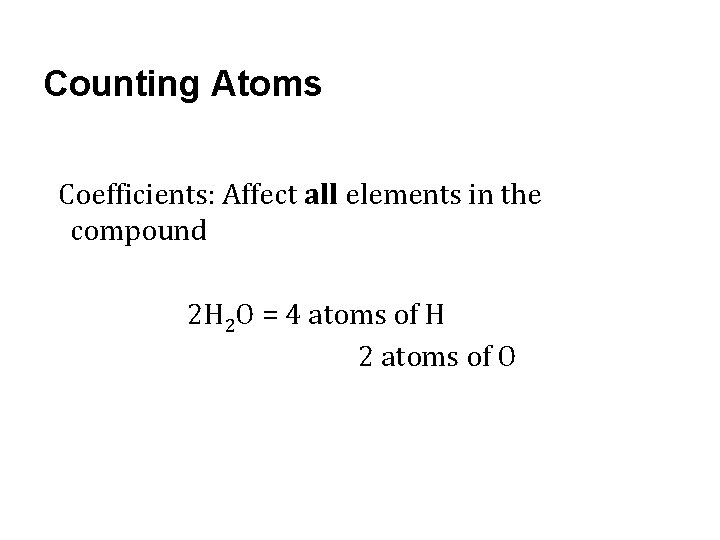
Counting Atoms Coefficients: Affect all elements in the compound 2 H 2 O = 4 atoms of H 2 atoms of O
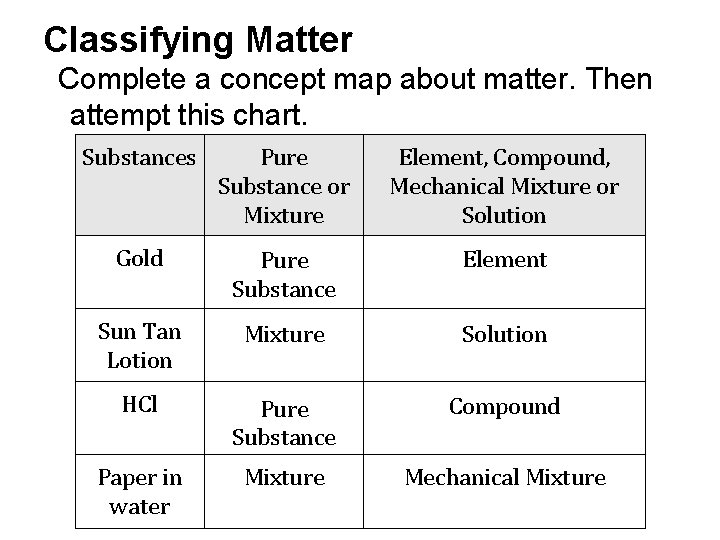
Classifying Matter Complete a concept map about matter. Then attempt this chart. Substances Pure Substance or Mixture Element, Compound, Mechanical Mixture or Solution Gold Pure Substance Element Sun Tan Lotion Mixture Solution HCl Pure Substance Compound Paper in water Mixture Mechanical Mixture
- Slides: 17