LongTerm Safety of Certolizumab Pegol in Plaque Psoriasis

Long-Term Safety of Certolizumab Pegol in Plaque Psoriasis: Pooled Analysis over 3 Years from Three Phase III, Randomized, Placebo-Controlled Studies A. Blauvelt, 1 C. Paul, 2 P. van de Kerkhof, 3 R. B. Warren, 4 A. B. Gottlieb, 5 R. G. Langley, 6 F. Brock, 7 C. Arendt, 8 M. Boehnlein, 9 M. Lebwohl, 5 K. Reich 10, 11 1 Oregon Medical Research Center, Portland, OR, USA; 2 Paul Sabatier University, Toulouse, France; 3 Radboud University, Nijmegen, The Netherlands; 4 Dermatology Centre, Salford Royal NHS Foundation Trust, Manchester NIHR Biomedical Research Centre, The University of Manchester, UK; 5 Icahn School of Medicine at Mount Sinai, New York, NY, USA; 6 Dalhousie University, Nova Scotia, Canada; 7 UCB Pharma, Slough, UK; 8 UCB Pharma, Brussels, Belgium; 9 UCB Pharma, Monheim, Germany; 10 Translational Research in Inflammatory Skin Diseases, Institute for Health Services Research in Dermatology and Nursing, University Medical Center Hamburg-Eppendorf, Germany; 11 Skinflammation® Center, Hamburg, Germany British Journal of Dermatology. DOI: 10. 111/bjd. 19314

Lead Author Andrew Blauvelt, M. D. , M. B. A. Board-certified dermatologist who specializes in caring for patients with psoriasis and those with complex skin diseases President of Oregon Medical Research Center, Portland, OR, USA British Journal of Dermatology. DOI: 10. 111/bjd. 19314

Introduction What’s already known? • Certolizumab pegol (CZP) is an Fc-free, PEGylated, anti-tumor necrosis factor (anti-TNF) biologic approved for adults with moderate to severe plaque psoriasis (PSO)1, 2 • Safety data from phase 3 trials in PSO have found the incidence of adverse events to be generally similar over 16 weeks’ treatment between evaluated CZP doses 200 mg and 400 mg every two weeks and placebo 3 • CZP has a safety profile in line with others in the anti-TNF class over 48 weeks’ treatment 4, 5 British Journal of Dermatology. DOI: 10. 111/bjd. 19314

Objective To report cumulative three-year safety data from three phase 3 trials of certolizumab pegol (CZP) in plaque psoriasis (PSO): CIMPASI-1, CIMPASI-2, and CIMPACT British Journal of Dermatology. DOI: 10. 111/bjd. 19314
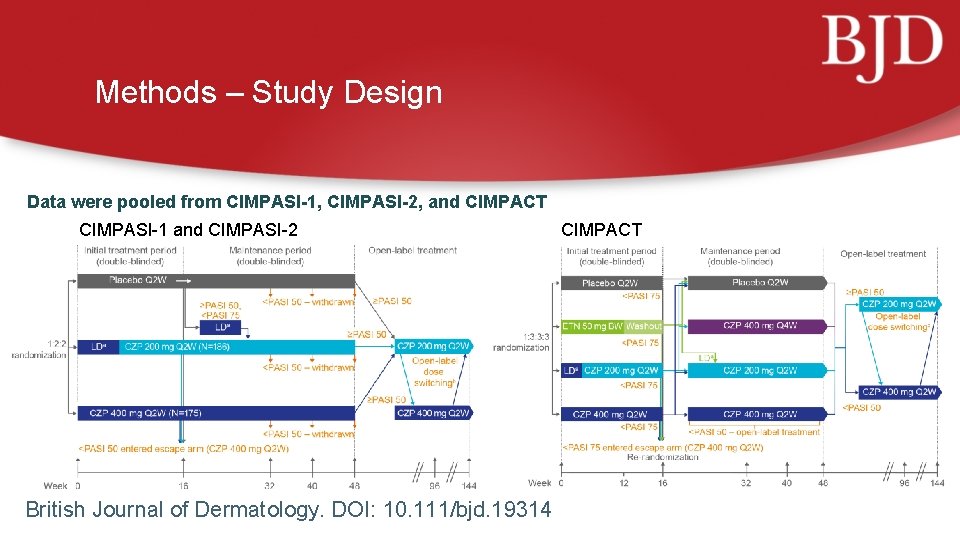
Methods – Study Design Data were pooled from CIMPASI-1, CIMPASI-2, and CIMPACT CIMPASI-1 and CIMPASI-2 British Journal of Dermatology. DOI: 10. 111/bjd. 19314 CIMPACT

Methods – Study Participants Inclusion criteria: • Individuals ≥ 18 years of age • Moderate to severe PSO for ≥ 6 months • PASI ≥ 12 • ≥ 10% body surface area (BSA) affected • PGA ≥ 3 on a 5 -point scale • Candidates for systemic PSO therapy, phototherapy and/or photochemotherapy Full exclusion criteria have been published previously 1, 2 In this analysis, safety data were pooled for patients who received ≥ 1 dose of CZP with up to 144 weeks exposure prior to study completion British Journal of Dermatology. DOI: 10. 111/bjd. 19314
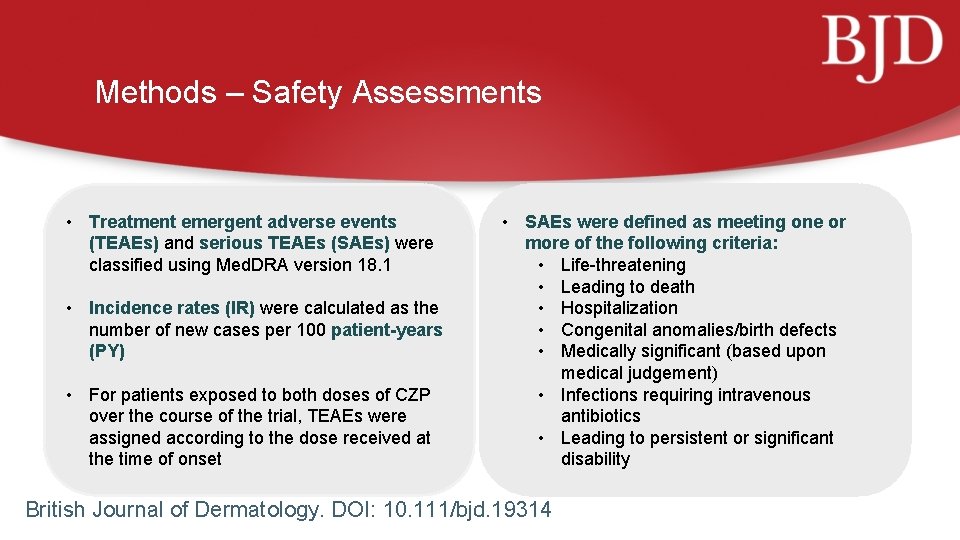
Methods – Safety Assessments • Treatment emergent adverse events (TEAEs) and serious TEAEs (SAEs) were classified using Med. DRA version 18. 1 • Incidence rates (IR) were calculated as the number of new cases per 100 patient-years (PY) • For patients exposed to both doses of CZP over the course of the trial, TEAEs were assigned according to the dose received at the time of onset • SAEs were defined as meeting one or more of the following criteria: • Life-threatening • Leading to death • Hospitalization • Congenital anomalies/birth defects • Medically significant (based upon medical judgement) • Infections requiring intravenous antibiotics • Leading to persistent or significant disability British Journal of Dermatology. DOI: 10. 111/bjd. 19314
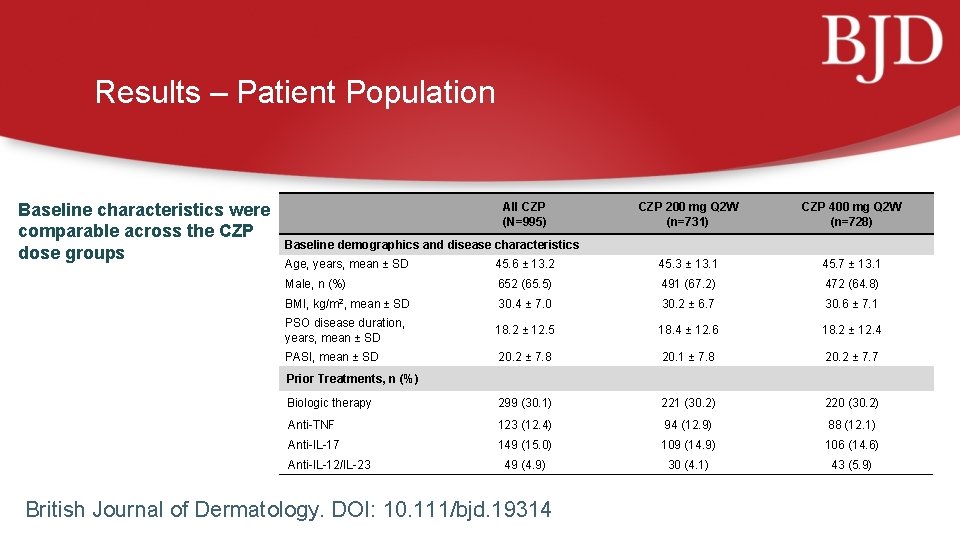
Results – Patient Population Baseline characteristics were comparable across the CZP dose groups All CZP (N=995) CZP 200 mg Q 2 W (n=731) CZP 400 mg Q 2 W (n=728) Baseline demographics and disease characteristics Age, years, mean ± SD 45. 6 ± 13. 2 45. 3 ± 13. 1 45. 7 ± 13. 1 Male, n (%) 652 (65. 5) 491 (67. 2) 472 (64. 8) BMI, kg/m 2, mean ± SD 30. 4 ± 7. 0 30. 2 ± 6. 7 30. 6 ± 7. 1 PSO disease duration, years, mean ± SD 18. 2 ± 12. 5 18. 4 ± 12. 6 18. 2 ± 12. 4 PASI, mean ± SD 20. 2 ± 7. 8 20. 1 ± 7. 8 20. 2 ± 7. 7 Biologic therapy 299 (30. 1) 221 (30. 2) 220 (30. 2) Anti-TNF 123 (12. 4) 94 (12. 9) 88 (12. 1) Anti-IL-17 149 (15. 0) 109 (14. 9) 106 (14. 6) 49 (4. 9) 30 (4. 1) 43 (5. 9) Prior Treatments, n (%) Anti-IL-12/IL-23 British Journal of Dermatology. DOI: 10. 111/bjd. 19314
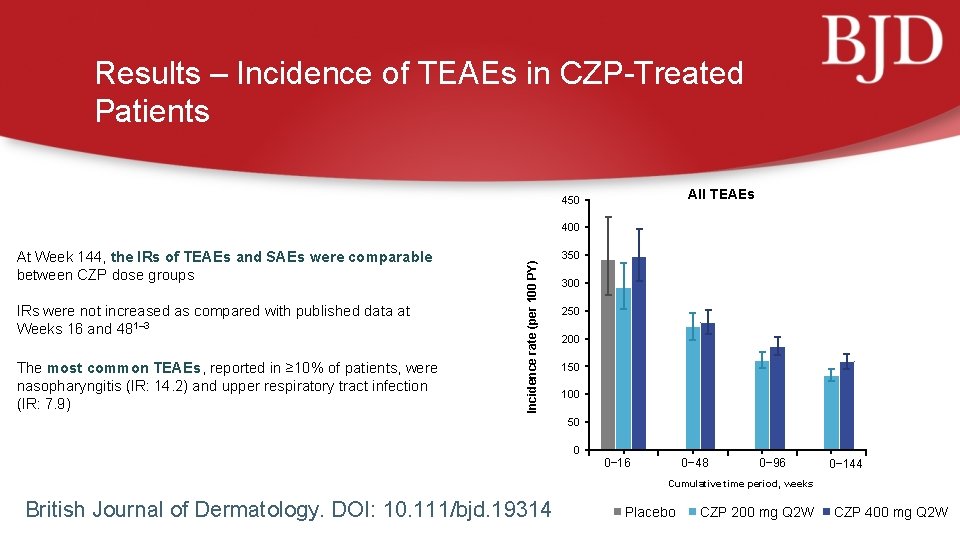
Results – Incidence of TEAEs in CZP-Treated Patients All TEAEs 450 At Week 144, the IRs of TEAEs and SAEs were comparable between CZP dose groups IRs were not increased as compared with published data at Weeks 16 and 481– 3 The most common TEAEs, reported in ≥ 10% of patients, were nasopharyngitis (IR: 14. 2) and upper respiratory tract infection (IR: 7. 9) Incidence rate (per 100 PY) 400 350 300 250 200 150 100 50 0 0− 16 0− 48 0− 96 0− 144 Cumulative time period, weeks British Journal of Dermatology. DOI: 10. 111/bjd. 19314 Placebo CZP 200 mg Q 2 W CZP 400 mg Q 2 W
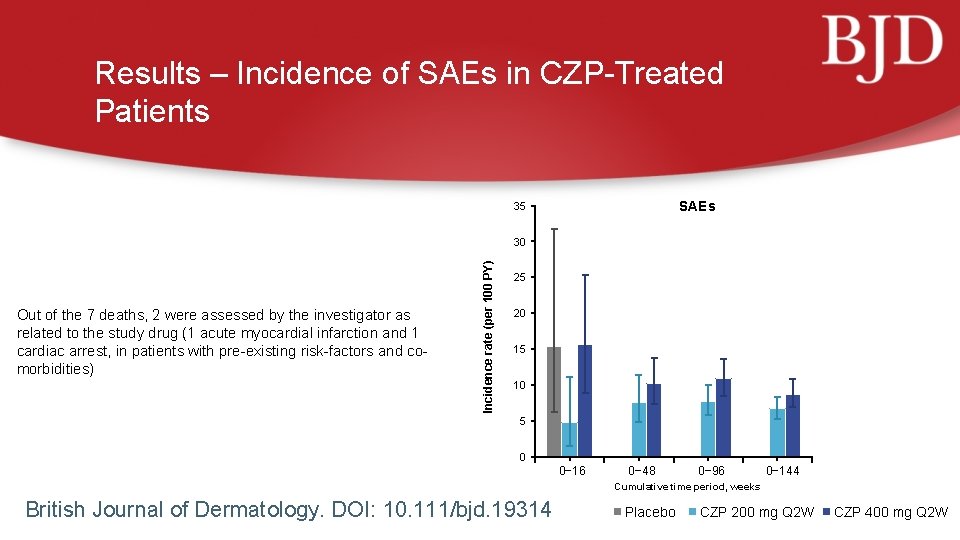
Results – Incidence of SAEs in CZP-Treated Patients SAEs 35 Out of the 7 deaths, 2 were assessed by the investigator as related to the study drug (1 acute myocardial infarction and 1 cardiac arrest, in patients with pre-existing risk-factors and comorbidities) Incidence rate (per 100 PY) 30 25 20 15 10 5 0 0− 16 0− 48 0− 96 0− 144 Cumulative time period, weeks British Journal of Dermatology. DOI: 10. 111/bjd. 19314 Placebo CZP 200 mg Q 2 W CZP 400 mg Q 2 W
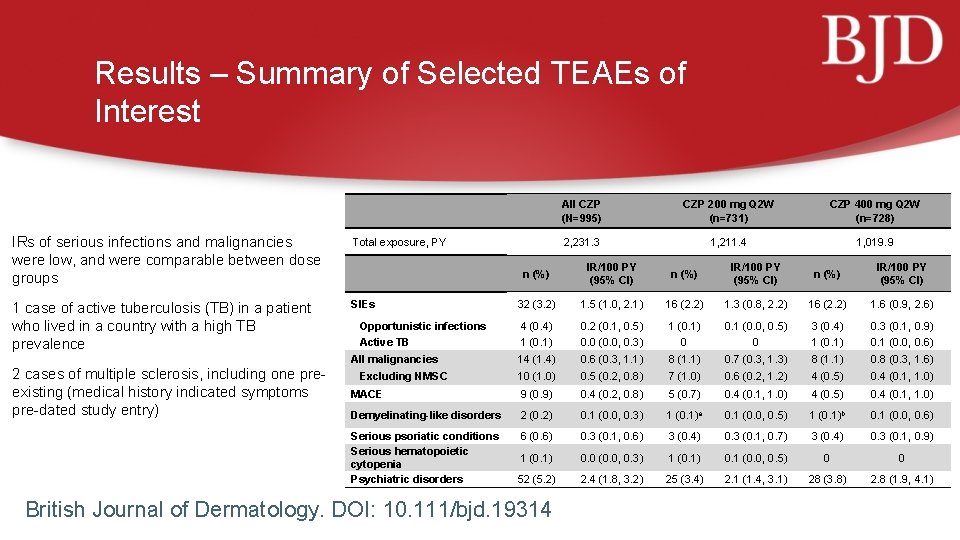
Results – Summary of Selected TEAEs of Interest IRs of serious infections and malignancies were low, and were comparable between dose groups Total exposure, PY 1 case of active tuberculosis (TB) in a patient who lived in a country with a high TB prevalence SIEs 2 cases of multiple sclerosis, including one preexisting (medical history indicated symptoms pre-dated study entry) All CZP (N=995) CZP 200 mg Q 2 W (n=731) CZP 400 mg Q 2 W (n=728) 2, 231. 3 1, 211. 4 1, 019. 9 n (%) IR/100 PY (95% CI) 32 (3. 2) 1. 5 (1. 0, 2. 1) 16 (2. 2) 1. 3 (0. 8, 2. 2) 16 (2. 2) 1. 6 (0. 9, 2. 6) Opportunistic infections 4 (0. 4) 0. 2 (0. 1, 0. 5) 1 (0. 1) 0. 1 (0. 0, 0. 5) 3 (0. 4) 0. 3 (0. 1, 0. 9) Active TB 1 (0. 1) 0. 0 (0. 0, 0. 3) 0 0 1 (0. 1) 0. 1 (0. 0, 0. 6) 14 (1. 4) 0. 6 (0. 3, 1. 1) 8 (1. 1) 0. 7 (0. 3, 1. 3) 8 (1. 1) 0. 8 (0. 3, 1. 6) 10 (1. 0) 0. 5 (0. 2, 0. 8) 7 (1. 0) 0. 6 (0. 2, 1. 2) 4 (0. 5) 0. 4 (0. 1, 1. 0) MACE 9 (0. 9) 0. 4 (0. 2, 0. 8) 5 (0. 7) 0. 4 (0. 1, 1. 0) 4 (0. 5) 0. 4 (0. 1, 1. 0) Demyelinating-like disorders 2 (0. 2) 0. 1 (0. 0, 0. 3) 1 (0. 1)a 0. 1 (0. 0, 0. 5) 1 (0. 1)b 0. 1 (0. 0, 0. 6) Serious psoriatic conditions Serious hematopoietic cytopenia Psychiatric disorders 6 (0. 6) 0. 3 (0. 1, 0. 6) 3 (0. 4) 0. 3 (0. 1, 0. 7) 3 (0. 4) 0. 3 (0. 1, 0. 9) 1 (0. 1) 0. 0 (0. 0, 0. 3) 1 (0. 1) 0. 1 (0. 0, 0. 5) 0 0 52 (5. 2) 2. 4 (1. 8, 3. 2) 25 (3. 4) 2. 1 (1. 4, 3. 1) 28 (3. 8) 2. 8 (1. 9, 4. 1) All malignancies Excluding NMSC British Journal of Dermatology. DOI: 10. 111/bjd. 19314
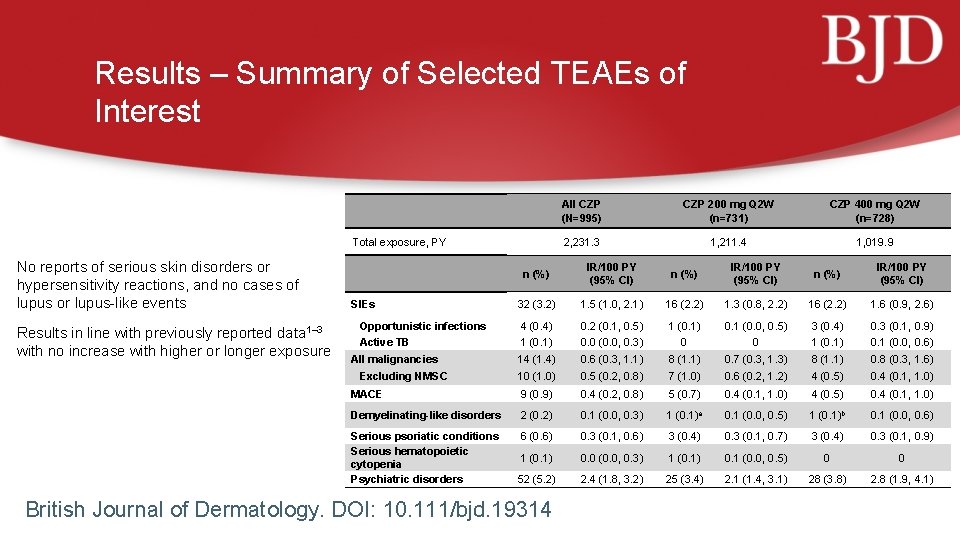
Results – Summary of Selected TEAEs of Interest Total exposure, PY No reports of serious skin disorders or hypersensitivity reactions, and no cases of lupus or lupus-like events Results in line with previously reported data 1– 3 with no increase with higher or longer exposure All CZP (N=995) CZP 200 mg Q 2 W (n=731) CZP 400 mg Q 2 W (n=728) 2, 231. 3 1, 211. 4 1, 019. 9 n (%) IR/100 PY (95% CI) 32 (3. 2) 1. 5 (1. 0, 2. 1) 16 (2. 2) 1. 3 (0. 8, 2. 2) 16 (2. 2) 1. 6 (0. 9, 2. 6) Opportunistic infections 4 (0. 4) 0. 2 (0. 1, 0. 5) 1 (0. 1) 0. 1 (0. 0, 0. 5) 3 (0. 4) 0. 3 (0. 1, 0. 9) Active TB 1 (0. 1) 0. 0 (0. 0, 0. 3) 0 0 1 (0. 1) 0. 1 (0. 0, 0. 6) 14 (1. 4) 0. 6 (0. 3, 1. 1) 8 (1. 1) 0. 7 (0. 3, 1. 3) 8 (1. 1) 0. 8 (0. 3, 1. 6) 10 (1. 0) 0. 5 (0. 2, 0. 8) 7 (1. 0) 0. 6 (0. 2, 1. 2) 4 (0. 5) 0. 4 (0. 1, 1. 0) MACE 9 (0. 9) 0. 4 (0. 2, 0. 8) 5 (0. 7) 0. 4 (0. 1, 1. 0) 4 (0. 5) 0. 4 (0. 1, 1. 0) Demyelinating-like disorders 2 (0. 2) 0. 1 (0. 0, 0. 3) 1 (0. 1)a 0. 1 (0. 0, 0. 5) 1 (0. 1)b 0. 1 (0. 0, 0. 6) Serious psoriatic conditions Serious hematopoietic cytopenia Psychiatric disorders 6 (0. 6) 0. 3 (0. 1, 0. 6) 3 (0. 4) 0. 3 (0. 1, 0. 7) 3 (0. 4) 0. 3 (0. 1, 0. 9) 1 (0. 1) 0. 0 (0. 0, 0. 3) 1 (0. 1) 0. 1 (0. 0, 0. 5) 0 0 52 (5. 2) 2. 4 (1. 8, 3. 2) 25 (3. 4) 2. 1 (1. 4, 3. 1) 28 (3. 8) 2. 8 (1. 9, 4. 1) SIEs All malignancies Excluding NMSC British Journal of Dermatology. DOI: 10. 111/bjd. 19314

Discussion • The three-year safety data presented here, comprising 2, 231. 3 PY of exposure, represent the longest report to date of CZP safety in patients with moderate to severe PSO • No new safety signals were identified as compared to previously reported data for CZP in other indications, or compared to other anti-TNF medications approved for PSO 1– 3 • The risk of TEAEs did not increase with longer or higher CZP exposure; the safety profiles of the two dose groups were similar • While anti-TNF biologics remain a mainstay of treatment for moderate to severe PSO due to their wellestablished safety and efficacy profiles, further comparative studies are needed to fully investigate the risk/benefit of all biologics British Journal of Dermatology. DOI: 10. 111/bjd. 19314
Conclusions What does this study add? • PSO is a chronic disease for which patients require lifetime management; therefore, long-term safety data are important to understand the benefits and risk of prolonged treatment • This study reported three-year safety data from a pooled analysis of three phase 3 trials of CZP in the treatment of moderate to severe PSO, representing 2, 231. 3 patient-years’ exposure • No new safety signals were identified and the risk of TEAEs did not increase with longer or higher CZP exposure British Journal of Dermatology. DOI: 10. 111/bjd. 19314

The Authors Andrew Blauvelt Fiona Brock Carle Paul Peter van de Kerkhof Catherine Arendt Richard B. Warren Marion Boehnlein British Journal of Dermatology. DOI: 10. 111/bjd. 19314 Alice B. Gottlieb Mark Lebwohl Richard G. Langley Kristian Reich
Call for correspondence Why not join the debate on this article through our correspondence section? Rapid responses should not exceed 350 words, four references and one figure Further details can be found on the BJD website
- Slides: 16