LOINC Codes and CDISC Mapping Progress and Issues


















- Slides: 31


LOINC Codes and CDISC Mapping Progress and Issues CDISC Intra. Change July 26, 2017 © CDISC 2016 2

Overview • Progress to Date • Mapping Issues • Next Steps (Beyond CDISC) • CDISC New Term Creation © CDISC 2016 3

Common LOINC Code Sources • CDISC – LOINC Mapping Project in 2005 § 611 LOINC Code § 597 Added (14 Fully Deprecated Codes not Added) • LOINC Top 2000 SI § 2194 LOINC Codes • 317 Duplicate 2005 Codes • 1426 Added to Matching List • 439 Not Added • 12 Deprecated Codes (Added) • LOINC Top 2000 US § 2180 LOINC Codes • 59 New Codes Added © CDISC 2016 4

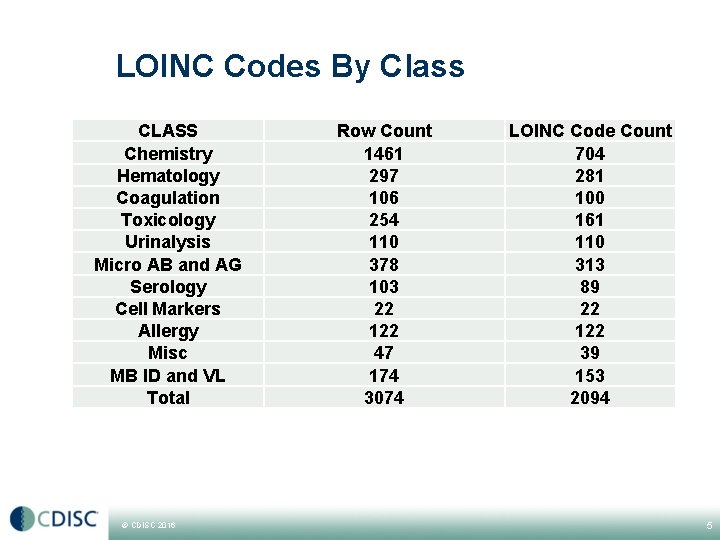
LOINC Codes By Class CLASS Chemistry Hematology Coagulation Toxicology Urinalysis Micro AB and AG Serology Cell Markers Allergy Misc MB ID and VL Total © CDISC 2016 Row Count 1461 297 106 254 110 378 103 22 122 47 174 3074 LOINC Code Count 704 281 100 161 110 313 89 22 122 39 153 2094 5

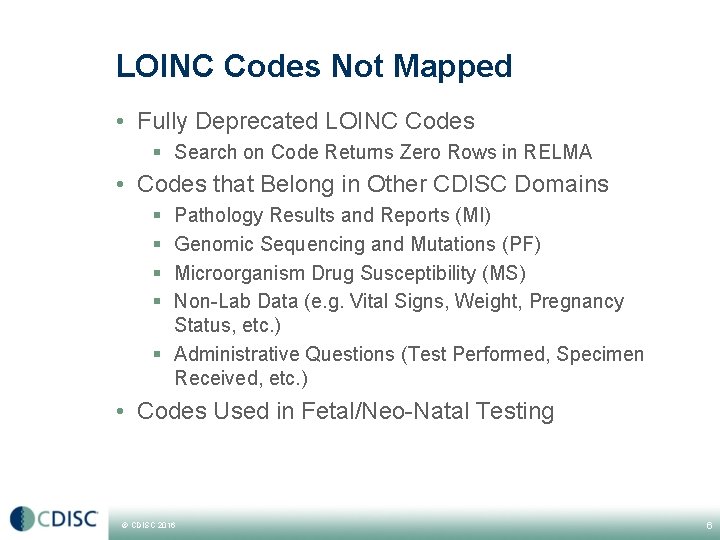
LOINC Codes Not Mapped • Fully Deprecated LOINC Codes § Search on Code Returns Zero Rows in RELMA • Codes that Belong in Other CDISC Domains § § Pathology Results and Reports (MI) Genomic Sequencing and Mutations (PF) Microorganism Drug Susceptibility (MS) Non-Lab Data (e. g. Vital Signs, Weight, Pregnancy Status, etc. ) § Administrative Questions (Test Performed, Specimen Received, etc. ) • Codes Used in Fetal/Neo-Natal Testing © CDISC 2016 6

Mapping Status • Initial Mapping Completed § Provisional Mapping, Subject to Issues Resolution. • Over 2, 000 LOINC Codes Mapped § Should Provide a High Level (90% plus? ) of Normal Lab (LB) and Microbiology (MB) Submission Data • No Obvious Large Gaps Uncovered. • Both CDISC (2005) and the Last LOINC Common Use Statistics are Several Years old, so Recent Trends in Testing are not Well Reflected. § Rise in DNA/RNA Based Microorganism Detection/Identification) § Cell Marker/Flow Cytometry use in Cancer and Auto. Immune Diseases © CDISC 2016 7

Uniqueness of Mapping • General Rules § Any Unique Combination of CDISC Terms Must Map to One and Only One LOINC Code § Multiple CDISC Combination of Terms May Map to a Single LOINC Code, Especially when a LOINC Concept is Generic (e. g. the XXX System Type) or NULL (e. g. Methodless LOINC Codes) • Implications § If the LOINC Terms Used for a Concept have Finer Granularity than CDISC Terms, CDISC Must Develop New Terminology that Matches the LOINC Level of Detail. § If the CDISC Terms Used for a Concept have Finer Granularity than that LOINC Uses, a Many to 1 Mapping Shall be Accepted © CDISC 2016 8

General Issues Deprecated Codes • Issue § Two Levels of LOINC Deprecation Exist • No Entry in LOINC • Flagged But Still In LOINC § Small Number of Such Codes, (26) • Proposed Resolution § Determine if Newer Code with Same Characteristics has Been Mapped • If So, Remove as Replacement Already in Place • If Not, Find the Best Fit Matching, Active Code in LOINC and Use It as the LOINC Mapping © CDISC 2016 9

General Issues CDISC New Terminology • Issue § During Mapping a Number of LOINC Terms had no Equivalent in CDISC § CDISC Terminology Sets Affected Include • • LBTEST/MBTEST Specimen Material Method Units of Measure • Proposed Resolution § Create New Terms as Needed § Other Issue Resolution May Impact this Issue © CDISC 2016 10

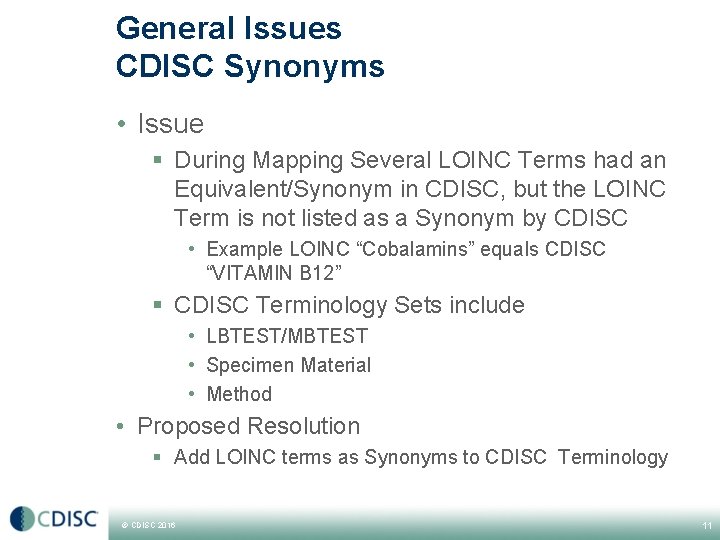
General Issues CDISC Synonyms • Issue § During Mapping Several LOINC Terms had an Equivalent/Synonym in CDISC, but the LOINC Term is not listed as a Synonym by CDISC • Example LOINC “Cobalamins” equals CDISC “VITAMIN B 12” § CDISC Terminology Sets include • LBTEST/MBTEST • Specimen Material • Method • Proposed Resolution § Add LOINC terms as Synonyms to CDISC Terminology © CDISC 2016 11

General Issues Property and UOM • Issue § The LOINC Property Concept Does Not Have an Equivalent Concept in CDISC • Proposed Resolution § As a Short-term Solution, the Mapping Sheets Indicate an Example UOM in the CDISC Mapping § As a Long-term Solution, CDISC should consider Adding the Property • As a field in the Findings Domains, or as • A Metadata Field that Classifies UOM Values © CDISC 2016 12

General Issue Cell Markers • Issue § LOINC Common 2000 Lists Contain a Small Number (22) of Cell Marker/Flow Cytometry/Phenotyping Tests. § These have been Included in the Mapping, § CDISC Terminology for this Class of Test is Still in “For Public Comment Only” State • Proposed Resolution § CDISC Should Move at Least this Set of Terms to Production Use § The Test/Property Should be the targeted Cell Type/Sub -type § The Marker String Field Should Carry the Receptor Pattern © CDISC 2016 13

Analyte/Component Issue Impression Values • Issue § In Several Classes, but Especially the Microbiology Class, LOINC includes a number of “Impression” results, which are data type as “Nominal” (e. g. Free Text). § CDISC has Values for the Primary Test (e. g. Organism Antigen/Antibody Codes), which can Take a Present/Absent, or Numeric Result. • Proposed resolution § CDISC should Use a Supporting field such as Test Detail to indicate the Result is an Impression/Interpretation § Alternately, new CDISC LBTEST/MBTEST terms need be created for the Impression Results. © CDISC 2016 14

Analyte/Component Issue Microorgaism Identification Tests • Issue § For Identifying Organisms by Culture or Staining Methods, LOINC Usually Specifies the Type of Organism (e. g. Bacteria, Fungus, Mycobacterium, Virus) Being Identified § CDISC MBTEST Terminology Currently has Only “Microorganism Identified”. • Proposed Resolution § To Maintain Mapping Uniqueness, CDISC Should Expand its MBTEST List to Match LOINC § Alternately, the Organism Type Could Go in a Supporting Field (probably Test Target) © CDISC 2016 15

Analyte/Component Issue Organism Identification Granularity • Issue § In the Microbiology Class, LOINC Includes a Number of Tests that Target the Organism at a Sub-species Level (i. e. Serotype, Polyprotein, etc. ). § CDISC Antibody/Antigen and Identification Test Codes Generally Target only to the Species Level • Proposed Resolution § CDISC needs to determine what Supporting Field (e. g. Test Detail or Test Target) the Sub-Species Information should be Placed into § Alternately, new CDISC LBTEST/MBTEST terms need be created for Each Sub-Species Value © CDISC 2016 16

Analyte/Component Issue Multi-Sub-Species Testing • Issue § In the Microbiology Class, LOINC Includes a Small Number of Tests (e. g. HPV High and Low Risk) that Target Multiple Sub-species Values § CDISC Currently has Only Organism Level MBTEST codes • Proposed Resolution § Use the Marker String Field to Carry the List of Serotypes § Use a Supporting Field (Test Target) to Carry the Nominal Type (e. g. High/Low Risk) © CDISC 2016 17

Analyte/Component Issue Cell marker Relative Tests • Issue § In the LOINC Cell Marker tests from the top 2000 lists, the relative terms (Ratio/Percent) all have a denominator of “ 100 Cells”, but do not in the definition specify any particular cell type. § CDISC requires explicit definition of the denominator, and with the examples collected to date, this is often a cell type (e. g. Lymphocytes). • Proposed Resolution § Confirm with the LOINC Staff that the Denominator is “All Cells”, with no Pre-gating. § If So, Create New CDISC Tests with “All Cells” as the Denominator § If Not, Have LOINC Clearly Define the Denominator © CDISC 2016 18

Specimen Issue LOINC “XXX” Specimen Type • Issue § In Many Classes, LOINC uses “XXX” for the System (Specimen Type) § In the Mapping Sheets, we Indicate that “Any”, LBSPEC/MBSPEC Value is Allowed § In Many Cases, the Same Test Exists as Alternate LOINC Code(s) with a Specific System/Specimen Type. • In Microbiology, Cytomegalovirus DNA can be Tested for in “XXX”, Blood or Serum/Plasma. • Proposed Resolution § In an Implementation Guide, CDISC Must Stress that the Exact Match Should be Used § Only if No Match on Specimen Type is Available Should an “XXX Match be Used © CDISC 2016 19

Specimen Issue LOINC Ser/Plas Specimen Type • Issue § LOINC Often Uses a Specimen Type (System) of “Ser/Plas”. § CDISC has this Joint Term, as Well as Separate Terms for Serum and Plasma § Central Labs Report the Specimen Material Actually Tested, Local Lab Reports May Show “Ser/Plas” • Proposed Resolution § Use a 1: 3 (LOINC to CDISC) mapping showing on Separate Rows the Three CDISC Specimen Type Terms. © CDISC 2016 20

Specimen Issue Urine Sediment Specimen Type • Issue § In Urinalysis, LOINC Often Uses a Specimen Type (System) of “Urine Sediment” § CDISC Specimen Type is “URINE”, since that is what is actually collected. § In the LOINC Codes Mapped, Several Entities (e. g Leukocytes, Erythrocytes, Fungi) Can be Tested in Both Urine and Urine Sediment • Proposed Resolution § To Maintain Mapping Uniqueness, CDISC Should Expand its Specimen Type List to Include “URINE SEDIMENT”. © CDISC 2016 21

Specimen Issue LOINC Location as Specimen Type • Issue § In Several Areas, but Especially in the Microbiology Culture/DNA/RNA Tests, LOINC Uses an Anatomical location (Vaginal, Cervix, Urethra, Genital, Nose, Eye, Throat, etc. ) as the System § Thus the CDISC Mapping Includes an LBLOC/MBLOC Value. § This Leaves Open the LBSPEC/MBSPEC Field, • Proposed Solution § For Some Locations, a Generic CDISC Specimen Type of “FLUID”, or “TISSUE” is Appropriate. § For Others, CDISC Needs to Define Examples with a Clear Specimen Type to Always be Used © CDISC 2016 22

Method Issue LOINC Methodless Codes • Issue § In Many Classes, LOINC has no value for the Method § In the Mapping Sheets, we Indicate that “Any”, LBMETH/MBMETH Value is Allowed § In Many Cases, the Same Test Exists as Alternate LOINC Code(s) with a Specific Method. • In Chemistry, Albumin can be Tested for in Ser/Plass with Electrophoresis or no Method • Proposed Resolution § In an Implementation Guide, CDISC Must Stress that the Exact Match Should be Used § Only if No Match on Method is Available Should a Methodless Match be Used © CDISC 2016 23

Method Issue Detection Limit as Method • Issue § In Some Chemistry Tests, LOINC Uses a Detection Limit, or a “High Sensitivity” Value as its Method § CDISC for now Would Consider Such a Limit as a Supplemental Qualifier on a True Method. § In Some Cases, the Same Test Exists as Alternate LOINC Code(s) with a Specific Methodless. • Thyrotropin can be Tested for in Ser/Plass with a Detection Limit or no Method • Proposed Solution § Remove the Detection Limit Code and make sure a Methodless Code Exists in the Mapping, § If no Methodless Code has been mapped, Add it. © CDISC 2016 24

Method Issue Multiple Count Methods • Issue § In its Hematology Counts, and Urine Sediment Counts, LOINC Often Differentiates Between “Manual Count”, “Automated Count” and “Light Microscopy”, Thus LOINC has Three Codes (and Maybe a Fourth for Methodless). § CDISC has : LIGHT MICROSCOPY” as a Method, but no Count Methods § Central Labs use Automated Counting, but May Review/Confirm with a Supervisor Manual Count. This Often Does not Surface up to the Data Delivery Engines • Proposed Solution § CDISC Needs to Add Automated Count as a Method § Local Lab Data Needs Discussion, as Does Review/Confirm © CDISC 2016 25

Method Issue Drug Screen/Confirm Methods • Issue § In its DRUG/TOX Results, LOINC Often Uses “Screen” or “Confirm” as its Method § CDISC Would Use the True Method, and Example Values (e. g. EIA, LC/MS/MS) are Shown in the Mapping § Central Labs Often Only Report one Value, all Positive Screen Values having been Confirmed. • Proposed Solution § Needs Some Thought © CDISC 2016 26

Method Issue Coagulation Method • Issue § In its Coagulation Class, LOINC Often Uses “Coag” as its Method, § CDISC has a Number of Clot Detection methods (Manual, Mechanical, Photometric) that Would Roll-up to “Coag”. • Proposed Solution § Allow the 1: Many (LOINC to CDISC) Mapping, and List Out on Separate Rows the CDISC Methods © CDISC 2016 27

Method Issues Microbial Cultures • Issue § In the LOINC Microbiology Class, LOINC has a Generic “Culture” Method, as Well as “Organism specific culture” § An Organism Can be Identified by Both Methods, Hence Two LOINC Codes Can be Mapped § CDISC has a Single Method Term for Microbial Culture. • Proposed Solution § To Maintain Mapping Uniqueness, CDISC Should Expand its Method List to Include “ORGANISM SPECIFIC CULTURE”. © CDISC 2016 28

Unit Of Measure Issues Arbitrary Units • CDISC Needs to Review and Clarify its Use of “U”, ”IU” and “Enzyme U” via Multiple Examples, • CDISC Needs to Determine an Equivalent for LOINC’s “Index_Value”, and Does it Need a Unit? • The CDISC term “Enzyme U” Needs Concentration Terms Added. • LOINC when showing example Units for Enzymes and Antibodies is Inconsistent (perhaps with reason) Need to Clarify This, and if Need be, Carefully map “U”, “IU”, and “Enzyme U” CDISC Terms • © CDISC 2016 29

Next Steps • Mapping § In the Mapping, Implement the Decisions Made Today § For Open Issues, Identify a CDISC/FDA/LOINC Subteam to Reach Resolution and Implement § Once a Beta, (All Issues Resolved) Mapping is Available, Work with LOINC and Other Interested Parties (NLM? ) to Confirm or Modify the Mapping • Implementation Guide § Identify a CDISC/FDA/LOINC Sub-team to Identify Issues to be Addressed, and Develop a First Draft © CDISC 2016 30

CDISC New Terminology • Define the New Terms to be Request • Target December Release for All Terms • Evaluate CDISC LAB Terminology Team Capacity, and if Short, Enlist Additional Support. © CDISC 2016 31