LO Recognize some important hazard symbols Hazard Symbols

LO: Recognize some important hazard symbols. Hazard Symbols Match the word, symbol, and explanation you were given as you came in. Sit with the people you match with.


DILUTION AND SAFETY

LO: Describe some safety precautions to follow when using acids and alkalis. Safety with Acids and Alkalis � Concentrated acids and alkalis are corrosive. �They can destroy substances including metals and living tissue. � Dilute acids and alkalis are a moderate hazard. �They make your skin red or blistered and damage your eyes.

LO: Describe some safety precautions to follow when using acids and alkalis. Safety with Acids and Alkalis � ALWAYS wear eye protection � ALWAYS wash your hands after using acids and alkalis � WASH any areas splashed with acids or alkalis with plenty of water
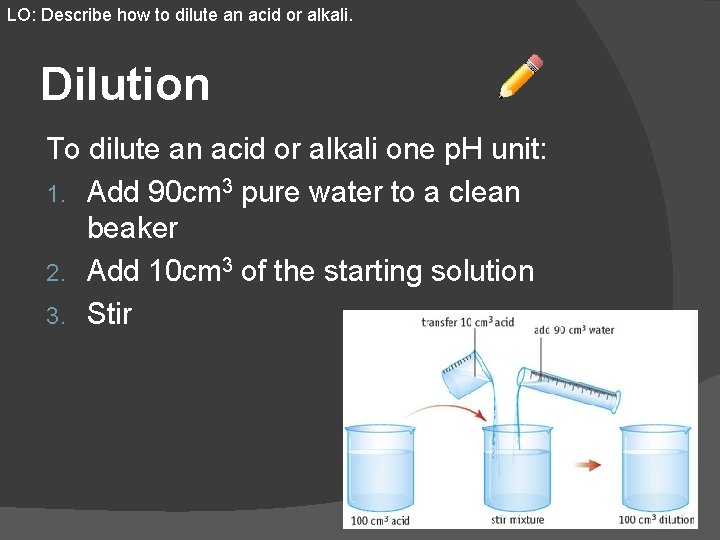
LO: Describe how to dilute an acid or alkali. Dilution To dilute an acid or alkali one p. H unit: 1. Add 90 cm 3 pure water to a clean beaker 2. Add 10 cm 3 of the starting solution 3. Stir

Acid Bath Murderer Complete the worksheet.
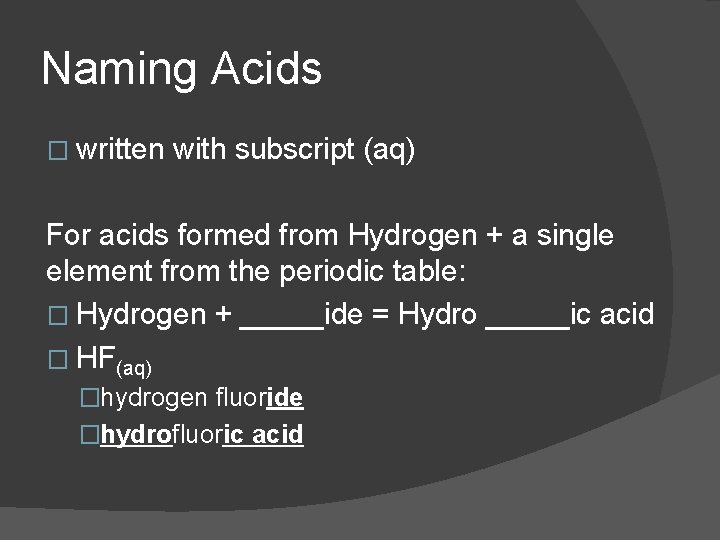
Naming Acids � written with subscript (aq) For acids formed from Hydrogen + a single element from the periodic table: � Hydrogen + _____ide = Hydro _____ic acid � HF(aq) �hydrogen fluoride �hydrofluoric acid
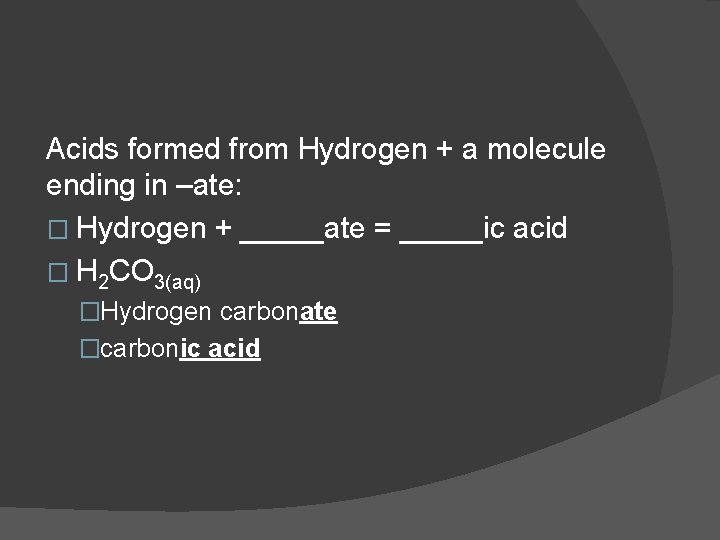
Acids formed from Hydrogen + a molecule ending in –ate: � Hydrogen + _____ate = _____ic acid � H 2 CO 3(aq) �Hydrogen carbonate �carbonic acid
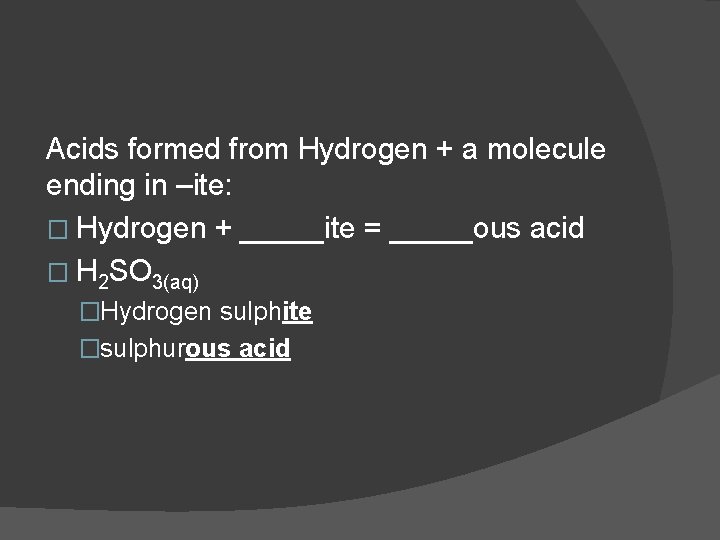
Acids formed from Hydrogen + a molecule ending in –ite: � Hydrogen + _____ite = _____ous acid � H 2 SO 3(aq) �Hydrogen sulphite �sulphurous acid


Naming Bases � Name the cation (metal) and add hydroxide � Na. OH(aq) = Sodium hydroxide � Mg(OH)2(aq) = Magnesium hydroxide

1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Hydrofluoric Acid Hydrochloric Acid Nitric Acid Sulfuric Acid Phosphoric Acid Carbonic Acid Boric Acid Silicic Acid Chloric Acid Acetic Acid 1. HF(aq) 2. HCl(aq) 3. HNO 3(aq) 4. H 2 SO 4(aq) 5. H 3 PO 4(aq) 6. H 2 CO 3(aq) 7. H 3 BO 3(aq) 8. H 2 Si. O 3(aq) 9. HCl. O 3(aq) 10. CH 2 COOH(aq)
- Slides: 13