LLE LiquidLiquid Extractions Primary Forensic Applications Analysis of



- Slides: 16

LLE: Liquid-Liquid Extractions Primary Forensic Applications: Analysis of Toxicology Samples and Controlled Substances Parts of slideshow courtesy of Adam Hall, Instructor of Forensic Sciences, Boston University

LLE: Analytical Separations Separation of analytes from a liquid matrix by the use of another liquid (solvent) based upon selective partitioning aka. Solvent Extraction Suitable solvents are selected based on their abilities to partition target analytes within a liquid matrix Consider: Miscibility of solvents: Et. OH and Water vs. Pentane Immiscible solvents are employed for liquid-liquid extractions

LLE: Analyte Considerations Remember: like-dissolves-like Consider: Ionic, non-ionic, polar, and non-polar analytes Acid / Base / Amphoteric Nature of Analytes Solubility of Salts … i. e. Cocaine Hydrochloride (HCl) General Comments: Neutral molecules are soluble in organic solvents Charged species are water soluble

LLE: p. H, p. Ka and ionization Manipulation and control of p. H maximizes separation efficiency with LLE’s and SPE’s Ionization of a compound alters its physical behavior and properties such as solubility and lipophilicity ▪ Ionization will increase a compounds solubility in water ▪ However, ionization will decrease a compounds lipophilicity

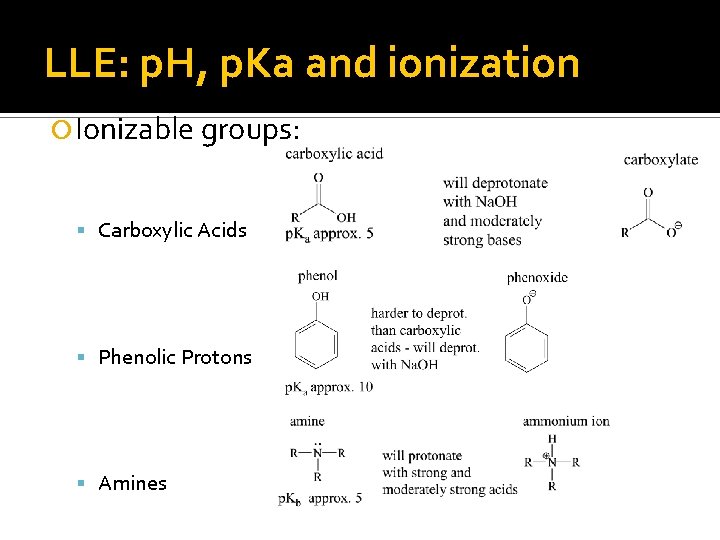
LLE: p. H, p. Ka and ionization Ionizable groups: Carboxylic Acids Phenolic Protons Amines

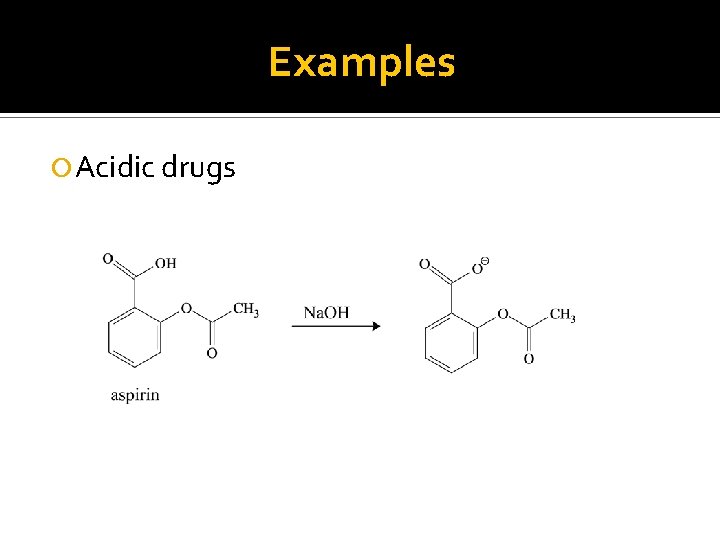
Examples Acidic drugs

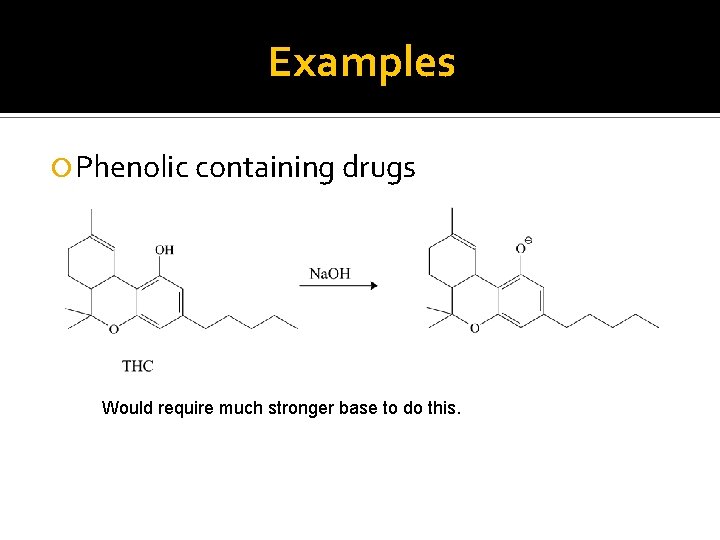
Examples Phenolic containing drugs Would require much stronger base to do this.

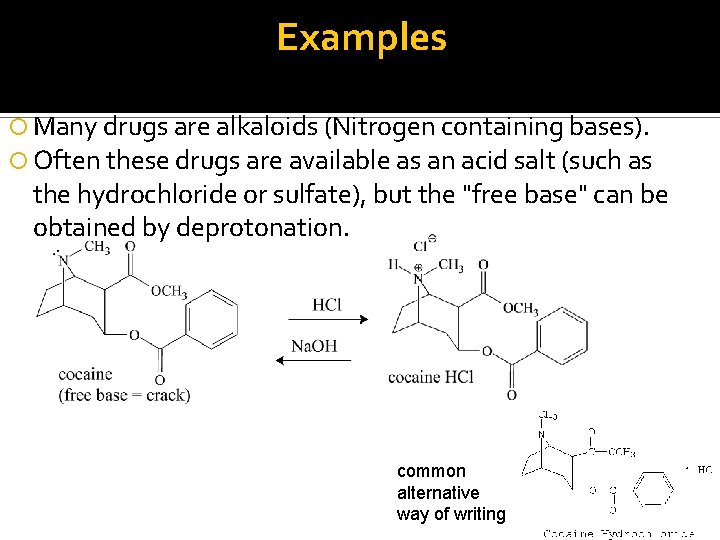
Examples Many drugs are alkaloids (Nitrogen containing bases). Often these drugs are available as an acid salt (such as the hydrochloride or sulfate), but the "free base" can be obtained by deprotonation. common alternative way of writing

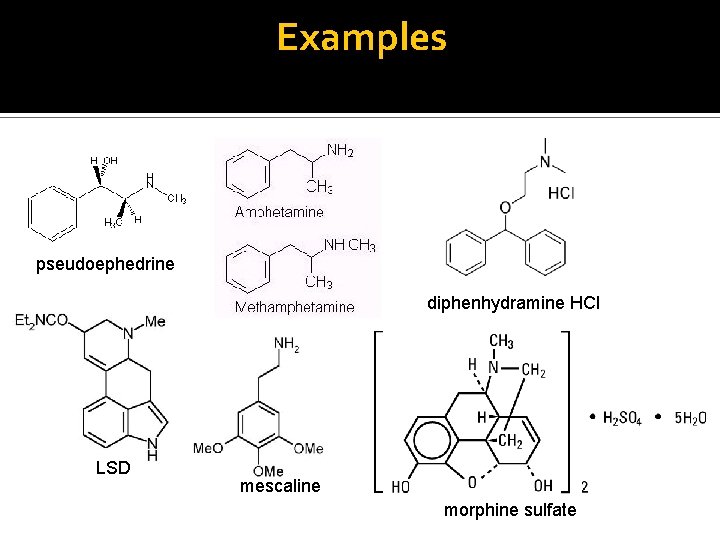
Examples pseudoephedrine diphenhydramine HCl LSD mescaline morphine sulfate

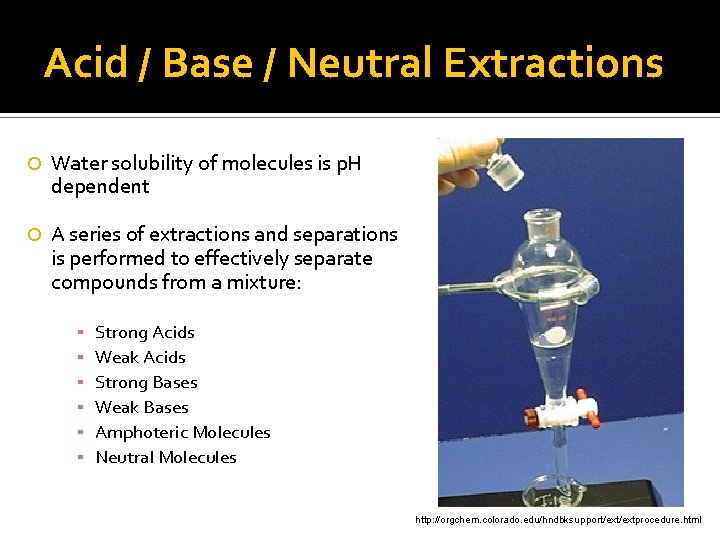
Acid / Base / Neutral Extractions Water solubility of molecules is p. H dependent A series of extractions and separations is performed to effectively separate compounds from a mixture: ▪ ▪ ▪ Strong Acids Weak Acids Strong Bases Weak Bases Amphoteric Molecules Neutral Molecules http: //orgchem. colorado. edu/hndbksupport/extprocedure. html


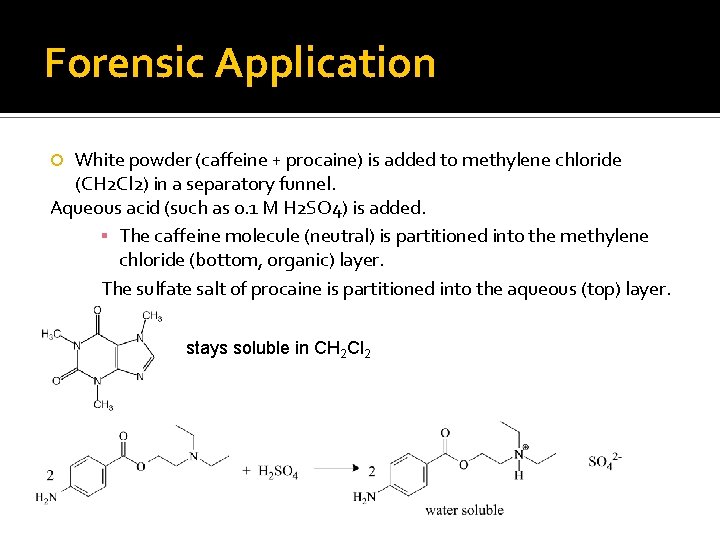
Forensic Application White powder (caffeine + procaine) is added to methylene chloride (CH 2 Cl 2) in a separatory funnel. Aqueous acid (such as 0. 1 M H 2 SO 4) is added. ▪ The caffeine molecule (neutral) is partitioned into the methylene chloride (bottom, organic) layer. The sulfate salt of procaine is partitioned into the aqueous (top) layer. stays soluble in CH 2 Cl 2

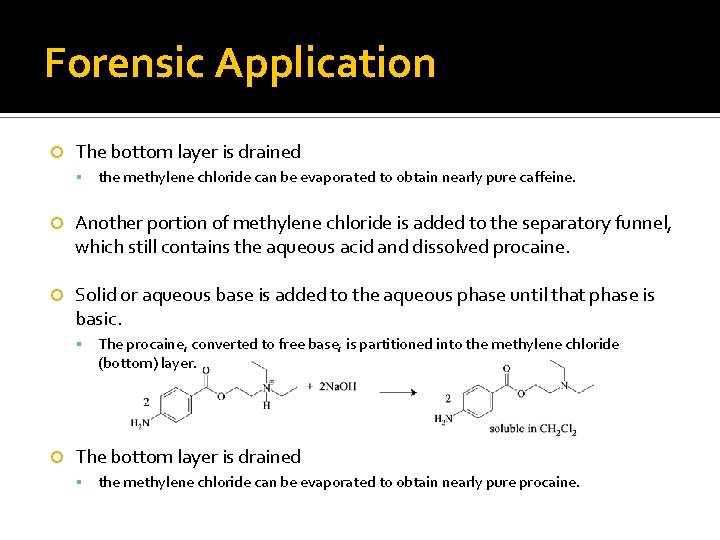
Forensic Application The bottom layer is drained the methylene chloride can be evaporated to obtain nearly pure caffeine. Another portion of methylene chloride is added to the separatory funnel, which still contains the aqueous acid and dissolved procaine. Solid or aqueous base is added to the aqueous phase until that phase is basic. The procaine, converted to free base, is partitioned into the methylene chloride (bottom) layer. The bottom layer is drained the methylene chloride can be evaporated to obtain nearly pure procaine.

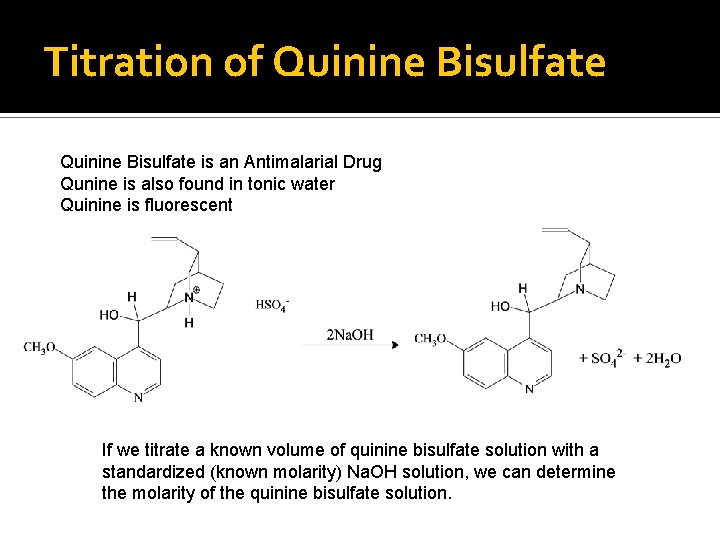
Titration of Quinine Bisulfate is an Antimalarial Drug Qunine is also found in tonic water Quinine is fluorescent If we titrate a known volume of quinine bisulfate solution with a standardized (known molarity) Na. OH solution, we can determine the molarity of the quinine bisulfate solution.

Objectives Learn how to standardize a solution by titration Learn how to perform multiple titrations with good precision Review stoichiometry Determine the concentration of a solution of quinine bisulfate of unknown molarity Observe the differences in solubility between an acidic and basic form of a drug

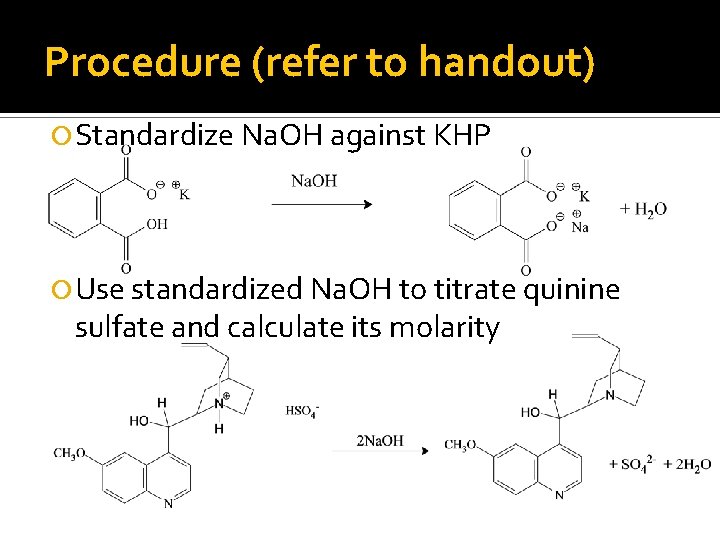
Procedure (refer to handout) Standardize Na. OH against KHP Use standardized Na. OH to titrate quinine sulfate and calculate its molarity