Live Cell Imaging Applications in Confocal Microscopy BMS
Live Cell Imaging Applications in Confocal Microscopy BMS 524 - “Introduction to Confocal Microscopy and Image Analysis” 1 Credit course offered by Purdue University Department of Basic Medical Sciences, School of Veterinary Medicine J. Paul Robinson, Ph. D. Professor of Immunopharmacology Director, Purdue University Cytometry Laboratories These slides are intended for use in a lecture series. Copies of the graphics are distributed and students encouraged to take their notes on these graphics. The intent is to have the student NOT try to reproduce the figures, but to LISTEN and UNDERSTAND the material. All material copyright J. Paul Robinson unless otherwise stated, however, the material may be freely used for lectures, tutorials and workshops. It may not be used for any commercial purpose. The text for this course is Pawley “Introduction to Confocal Microscopy”, Plenum Press, 2 nd Ed. A number of the ideas and figures in these lecture notes are taken from this text. © 1993 -2004 J. Paul Robinson, Purdue University Cytometry UPDATED Laboratories April 2003 Slide 1 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
Applications • Organelle Structure & Function – Mitochondria (Rhodamine 123) – Golgi (C 6 -NBD-Ceramide) – Actin (NBD-Phaloidin) – Lipid (DPH) © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 3 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
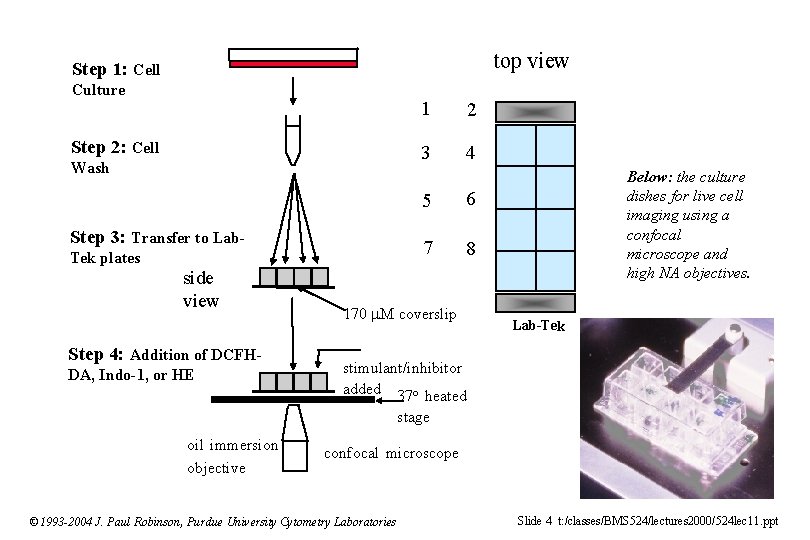
top view Step 1: Cell Culture Step 2: Cell Wash Step 3: Transfer to Lab. Tek plates side view Step 4: Addition of DCFHDA, Indo-1, or HE 1 2 3 4 5 6 7 8 170 M coverslip Below: the culture dishes for live cell imaging using a confocal microscope and high NA objectives. Lab-Tek stimulant/inhibitor added 37 o heated stage oil immersion objective confocal microscope © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 4 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
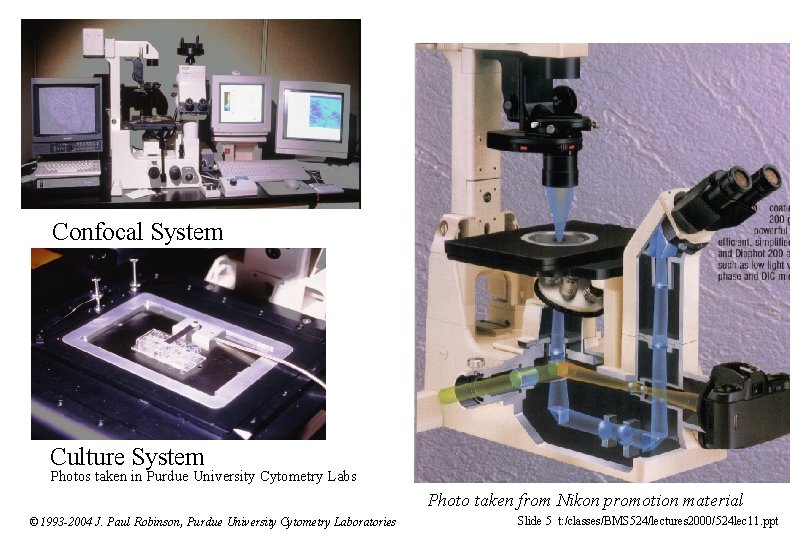
Confocal System Culture System Photos taken in Purdue University Cytometry Labs Photo taken from Nikon promotion material © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 5 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
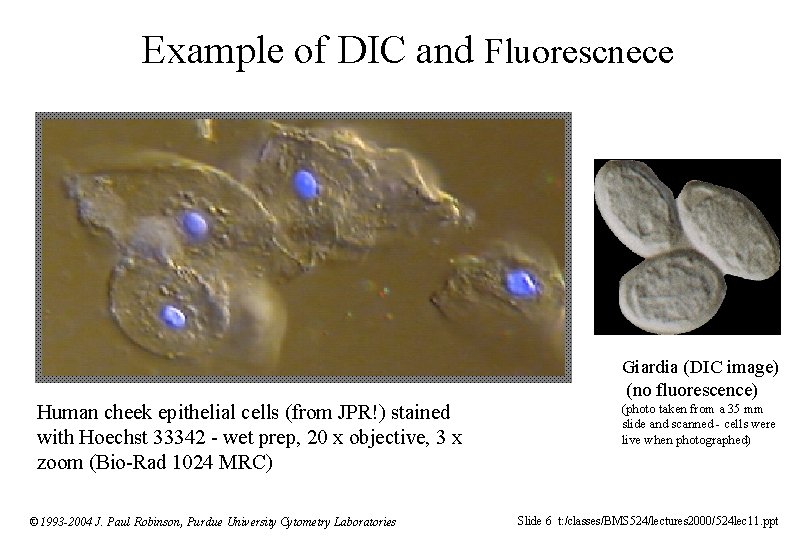
Example of DIC and Fluorescnece Giardia (DIC image) (no fluorescence) Human cheek epithelial cells (from JPR!) stained with Hoechst 33342 - wet prep, 20 x objective, 3 x zoom (Bio-Rad 1024 MRC) © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories (photo taken from a 35 mm slide and scanned - cells were live when photographed) Slide 6 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
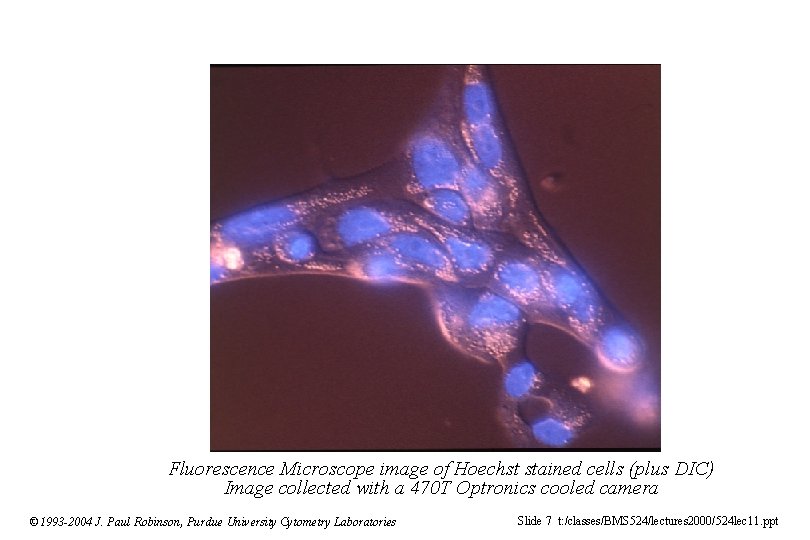
Fluorescence Microscope image of Hoechst stained cells (plus DIC) Image collected with a 470 T Optronics cooled camera © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 7 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
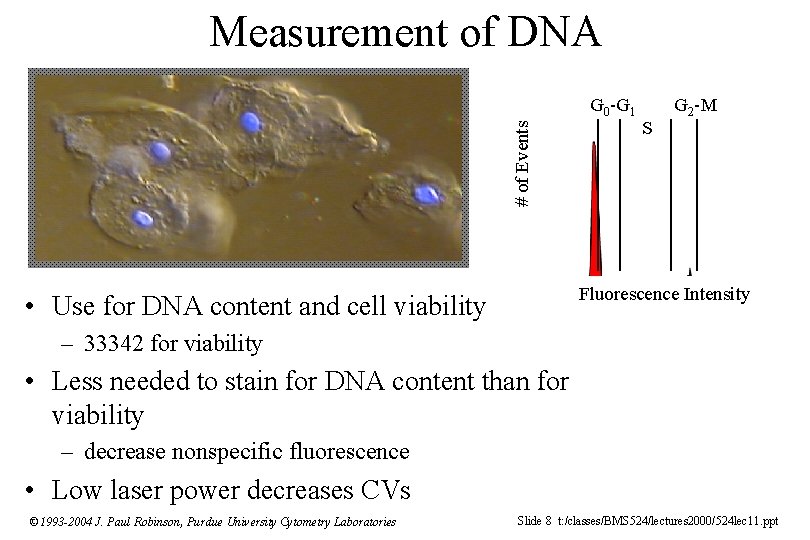
Measurement of DNA # of Events G 0 -G 1 S G 2 -M Fluorescence Intensity • Use for DNA content and cell viability – 33342 for viability • Less needed to stain for DNA content than for viability – decrease nonspecific fluorescence • Low laser power decreases CVs © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 8 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
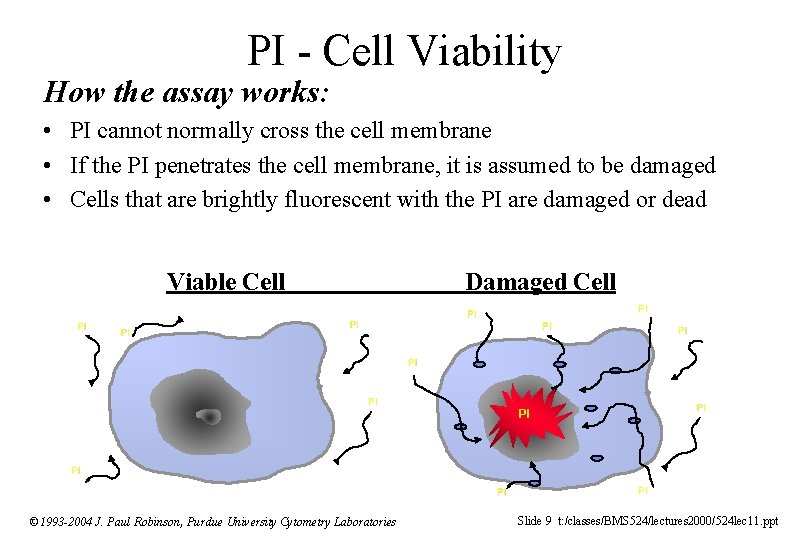
PI - Cell Viability How the assay works: • PI cannot normally cross the cell membrane • If the PI penetrates the cell membrane, it is assumed to be damaged • Cells that are brightly fluorescent with the PI are damaged or dead Viable Cell PI PI Damaged Cell PI PI PI © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories PI Slide 9 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
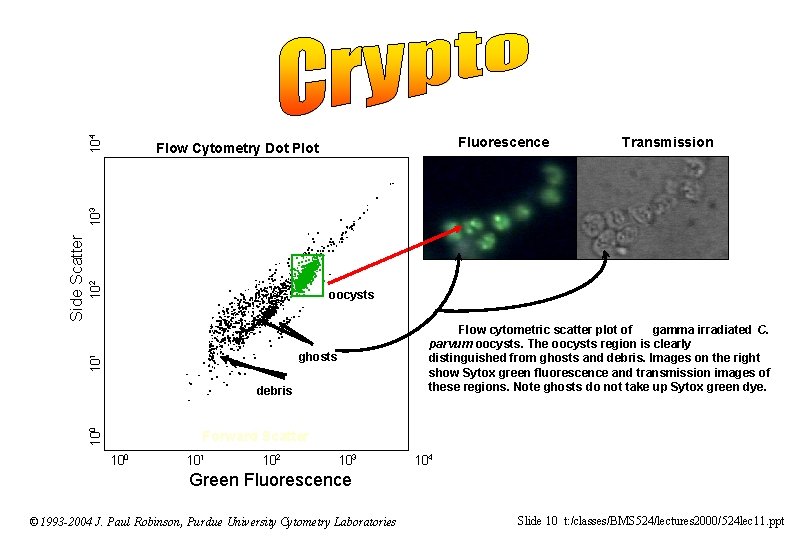
104 Fluorescence Transmission 102 oocysts Flow cytometric scatter plot of gamma irradiated C. parvum oocysts. The oocysts region is clearly distinguished from ghosts and debris. Images on the right show Sytox green fluorescence and transmission images of these regions. Note ghosts do not take up Sytox green dye. ghosts 10 1 Side Scatter 103 Flow Cytometry Dot Plot 0 debris 10 Forward Scatter 100 101 102 103 104 Green Fluorescence © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 10 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
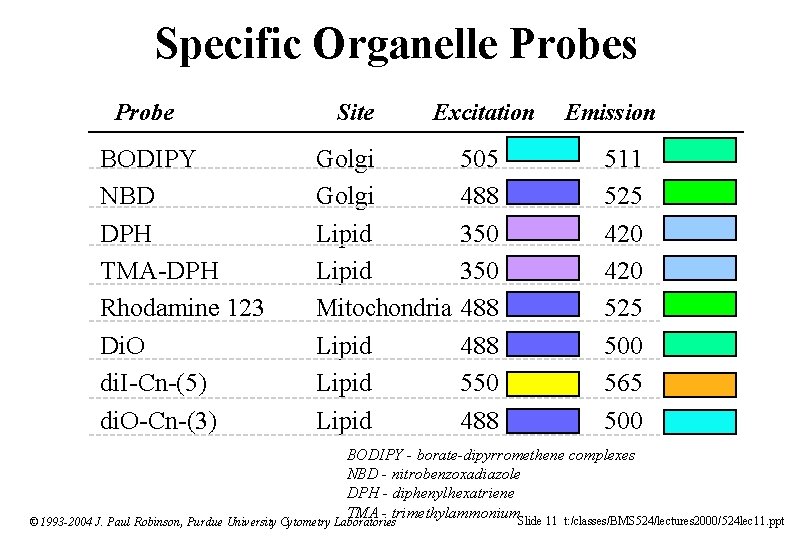
Specific Organelle Probes Probe BODIPY NBD DPH TMA-DPH Rhodamine 123 Di. O di. I-Cn-(5) di. O-Cn-(3) Site Excitation Golgi Lipid Mitochondria Lipid 505 488 350 488 550 488 Emission 511 525 420 525 500 565 500 BODIPY - borate-dipyrromethene complexes NBD - nitrobenzoxadiazole DPH - diphenylhexatriene TMA - trimethylammonium. Slide 11 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories
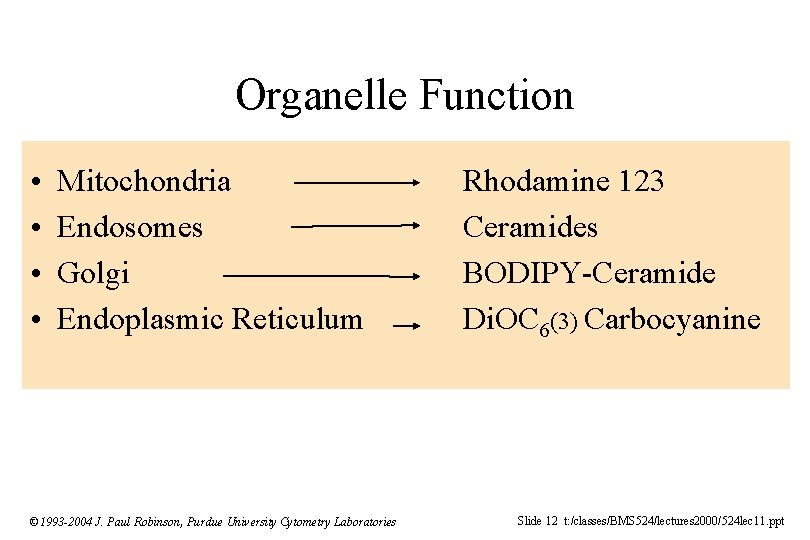
Organelle Function • • Mitochondria Endosomes Golgi Endoplasmic Reticulum © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Rhodamine 123 Ceramides BODIPY-Ceramide Di. OC 6(3) Carbocyanine Slide 12 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
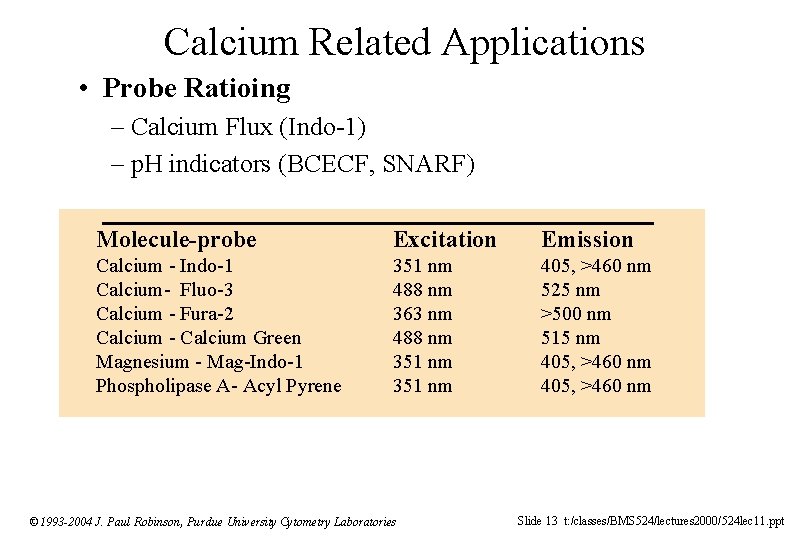
Calcium Related Applications • Probe Ratioing – Calcium Flux (Indo-1) – p. H indicators (BCECF, SNARF) Molecule-probe Excitation Emission Calcium - Indo-1 Calcium- Fluo-3 Calcium - Fura-2 Calcium - Calcium Green Magnesium - Mag-Indo-1 Phospholipase A- Acyl Pyrene 351 nm 488 nm 363 nm 488 nm 351 nm 405, >460 nm 525 nm >500 nm 515 nm 405, >460 nm © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 13 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
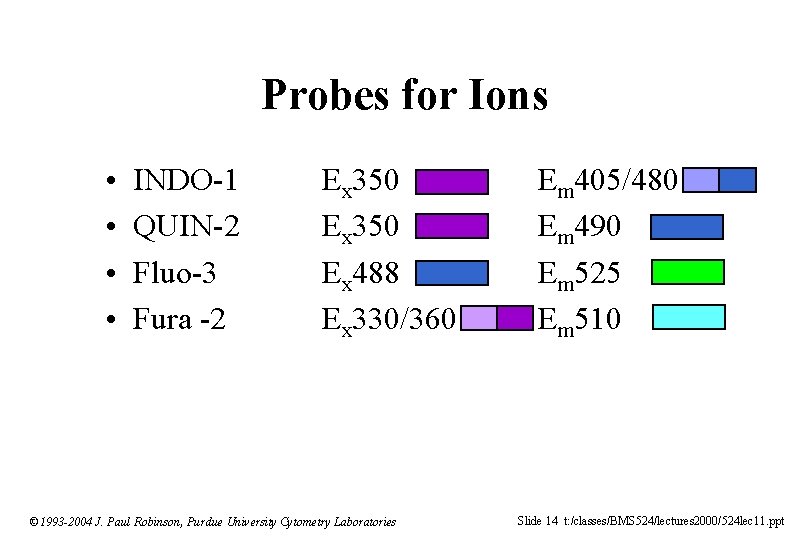
Probes for Ions • • INDO-1 QUIN-2 Fluo-3 Fura -2 Ex 350 Ex 488 Ex 330/360 © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Em 405/480 Em 490 Em 525 Em 510 Slide 14 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
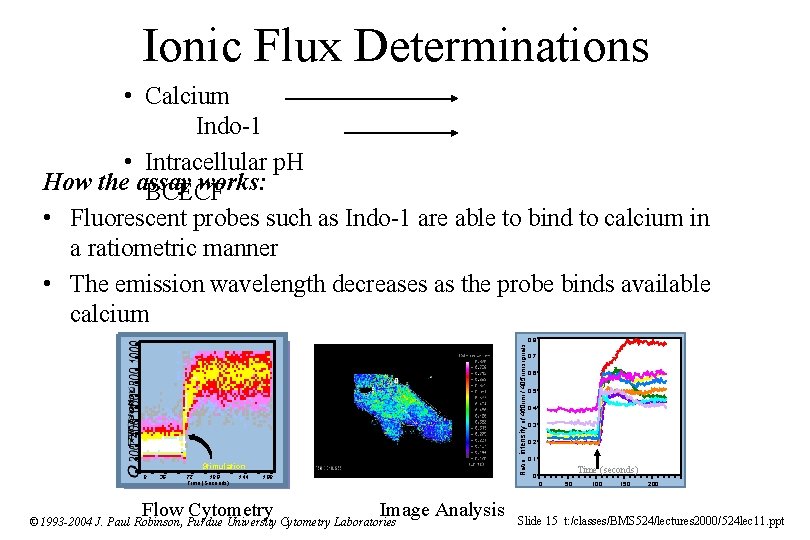
Ionic Flux Determinations • Calcium Indo-1 • Intracellular p. H How the assay works: BCECF • Fluorescent probes such as Indo-1 are able to bind to calcium in a ratiometric manner • The emission wavelength decreases as the probe binds available calcium Ratio: intensity of 460 nm / 405 nm signals 0. 8 Stimulation 0 36 72 108 Time (Seconds) 144 180 Flow Cytometry 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 Time (seconds) 0 0 Image Analysis © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories 50 100 150 200 Slide 15 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
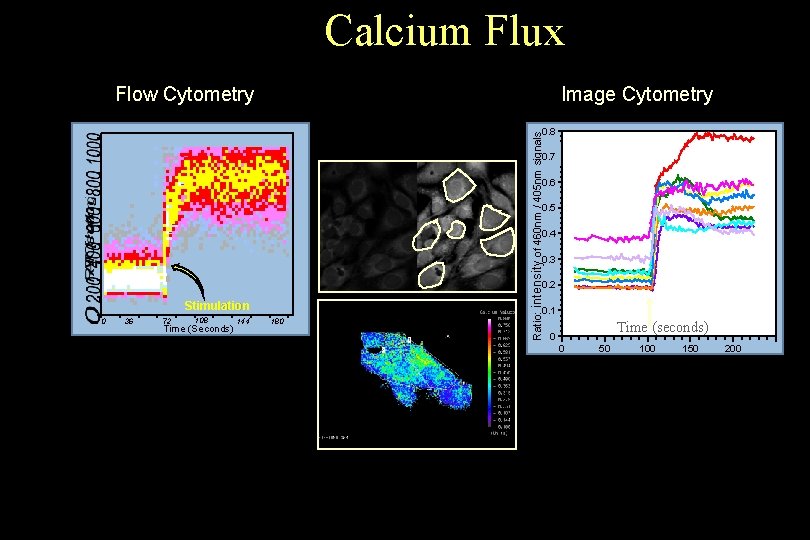
Calcium Flux Flow Cytometry Image Cytometry Ratio: intensity of 460 nm / 405 nm signals 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Stimulation 0 36 72 108 Time (Seconds) 144 180 0. 1 Time (seconds) 0 0 © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories 50 100 150 200 Slide 16 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
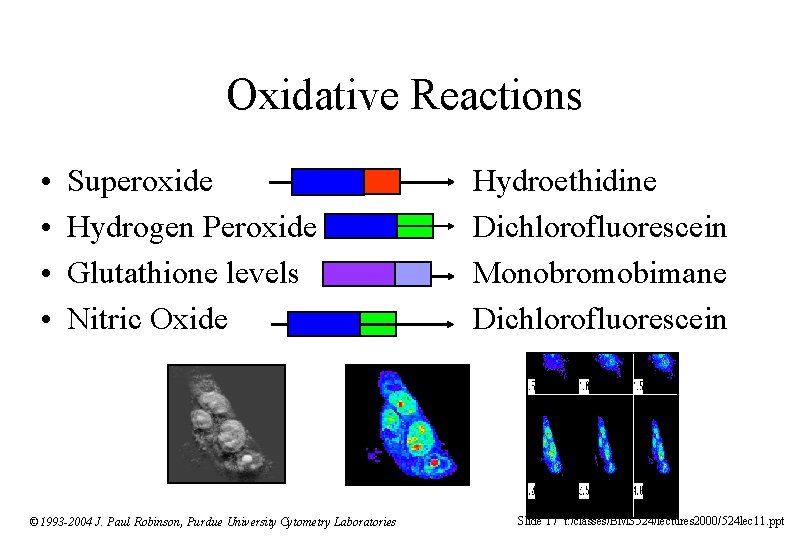
Oxidative Reactions • • Superoxide Hydrogen Peroxide Glutathione levels Nitric Oxide © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Hydroethidine Dichlorofluorescein Monobromobimane Dichlorofluorescein Slide 17 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
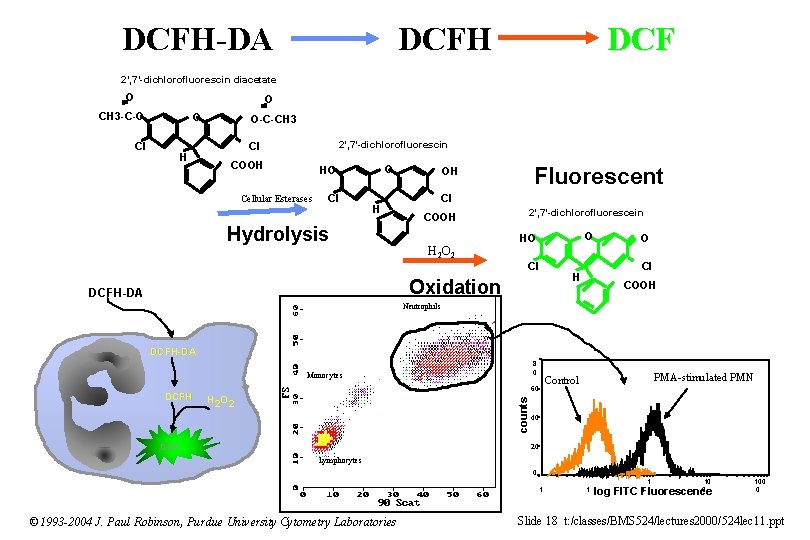
DCFH-DA DCFH DCF 2’, 7’-dichlorofluorescin diacetate O O CH 3 -C-O O Cl H O-C-CH 3 2’, 7’-dichlorofluorescin Cl COOH O HO Cellular Esterases Cl H Hydrolysis Fluorescent OH Cl COOH H 2 O 2 2’, 7’-dichlorofluorescein O HO Cl Oxidation DCFH-DA O Cl H COOH Neutrophils DCFH-DA 8 0 Monocytes H 2 O 2 counts DCFH 60 DCF PMA-stimulated PMN Control 40 20 Lymphocytes 0. 1 © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories 1 1 10 0 0 log FITC Fluorescence 100 0 Slide 18 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
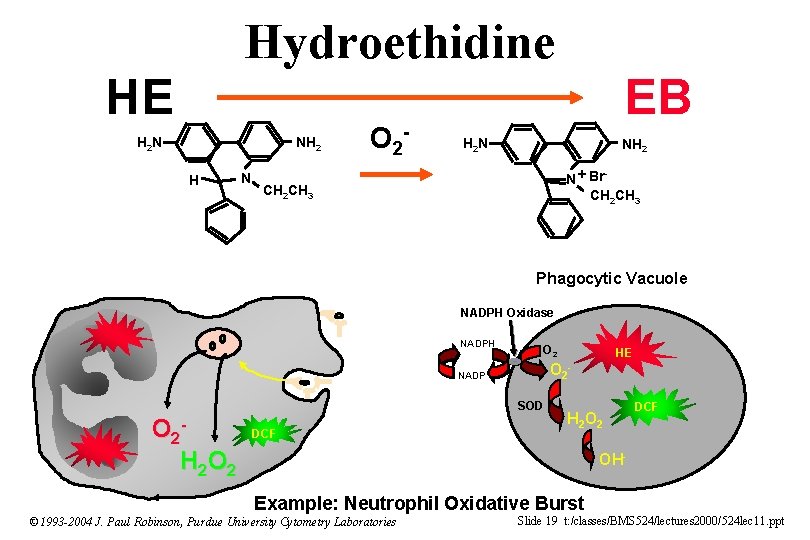
Hydroethidine HE H 2 N NH 2 H N O 2 - EB H 2 N NH 2 N + Br CH 2 CH 3 - CH 2 CH 3 Phagocytic Vacuole NADPH Oxidase NADPH O 2 NADP SOD O 2 H 2 O 2 DCF HE - H 2 O 2 DCF OH- Example: Neutrophil Oxidative Burst © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 19 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
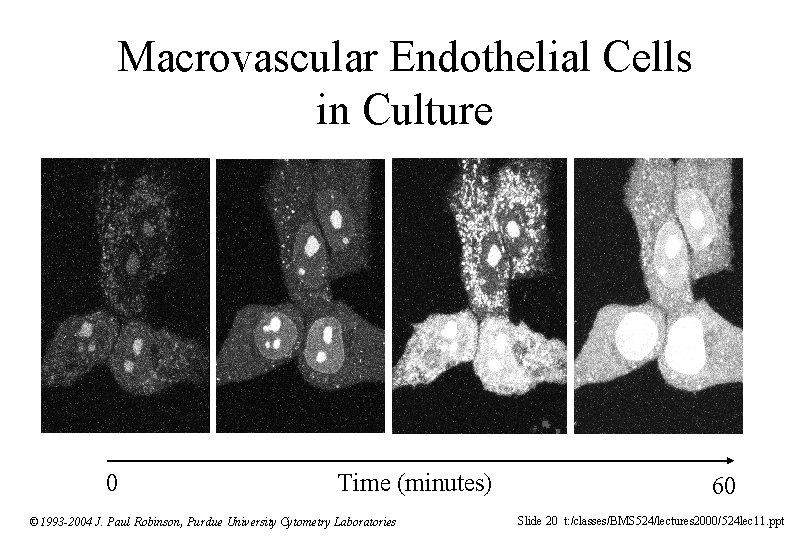
Macrovascular Endothelial Cells in Culture 0 Time (minutes) © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories 60 Slide 20 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
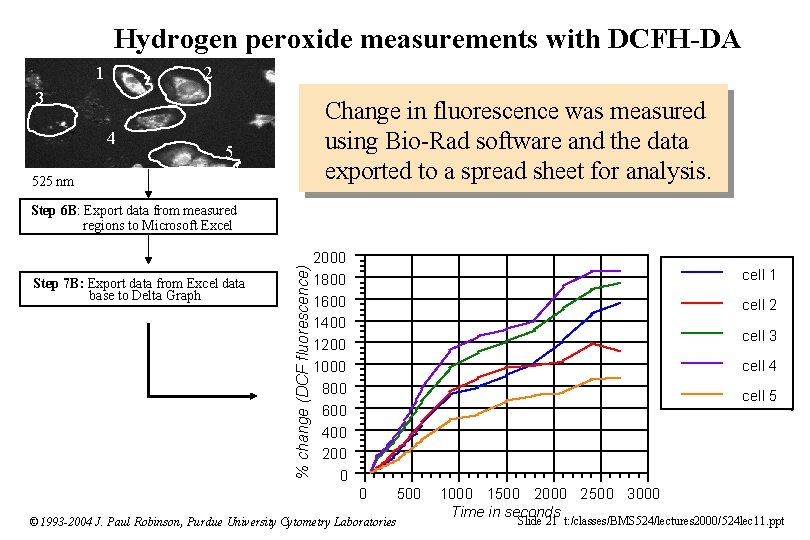
Hydrogen peroxide measurements with DCFH-DA 1 2 3 4 Change in fluorescence was measured using Bio-Rad software and the data exported to a spread sheet for analysis. 5 525 nm Step 7 B: Export data from Excel data base to Delta Graph % change (DCF fluorescence) Step 6 B: Export data from measured regions to Microsoft Excel 2000 1800 1600 1400 1200 1000 800 600 400 200 0 cell 1 cell 2 cell 3 cell 4 cell 5 0 500 © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories 1000 1500 2000 2500 3000 Time in seconds Slide 21 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
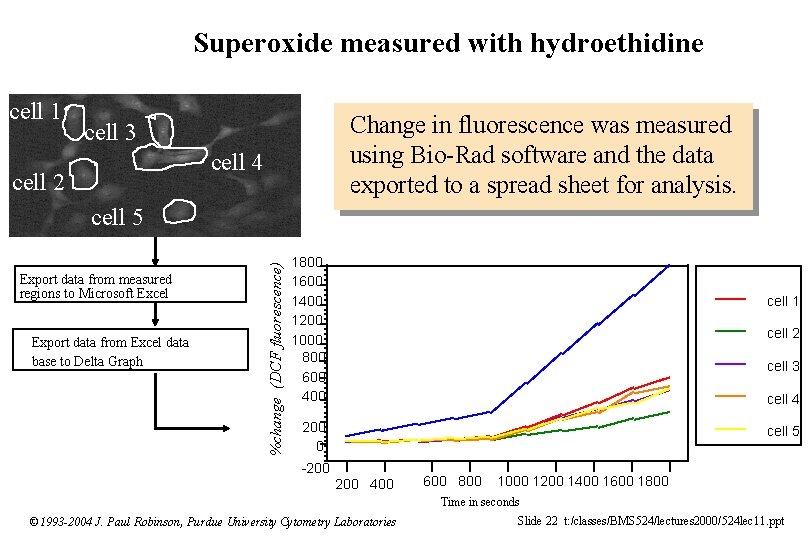
Superoxide measured with hydroethidine cell 1 Change in fluorescence was measured using Bio-Rad software and the data exported to a spread sheet for analysis. cell 3 cell 4 cell 2 Export data from measured regions to Microsoft Excel Export data from Excel data base to Delta Graph %change (DCF fluorescence) cell 5 1800 1600 1400 1200 1000 800 600 400 cell 1 cell 2 cell 3 cell 4 200 0 cell 5 -200 400 600 800 1000 1200 1400 1600 1800 Time in seconds © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 22 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
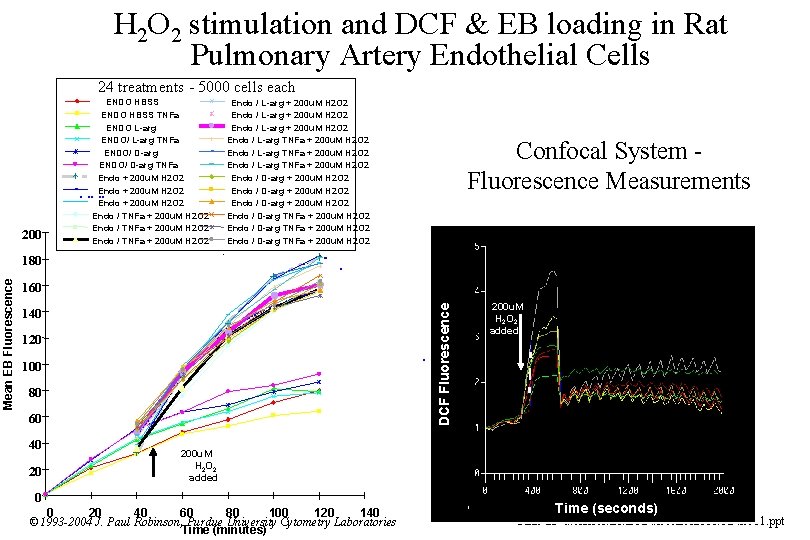
H 2 O 2 stimulation and DCF & EB loading in Rat Pulmonary Artery Endothelial Cells 24 treatments - 5000 cells each 200 ENDO HBSS TNFa ENDO L-arg ENDO/ L-arg TNFa ENDO/ D-arg TNFa Endo + 200 u. M H 2 O 2 Endo / TNFa + 200 u. M H 2 O 2 Confocal System Fluorescence Measurements . 180 160 DCF Fluorescence Mean EB Fluorescence Endo / L-arg + 200 u. M H 2 O 2 Endo / L-arg TNFa + 200 u. M H 2 O 2 Endo / D-arg TNFa + 200 u. M H 2 O 2 140 120 100 80 60 40 20 200 u. M H 2 O 2 added 0 0 20 40 60 80 100 120 140 © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Time (minutes) Time (seconds) Slide 23 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
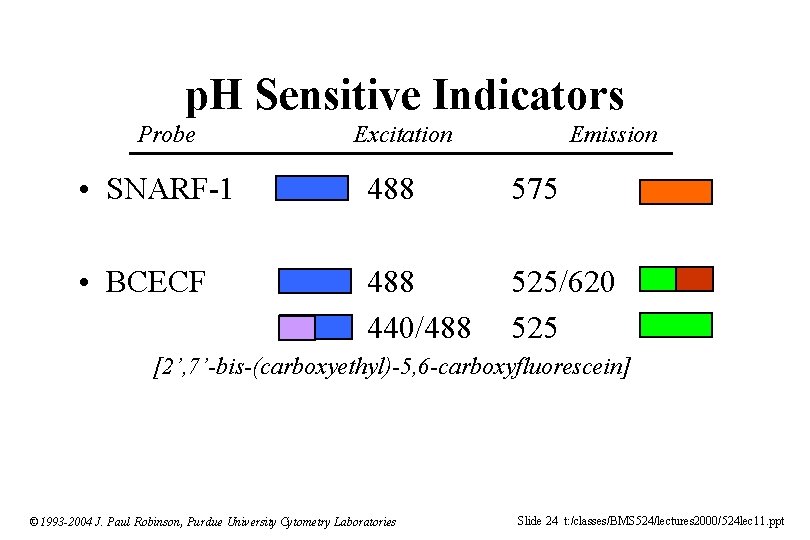
p. H Sensitive Indicators Probe Excitation Emission • SNARF-1 488 575 • BCECF 488 440/488 525/620 525 [2’, 7’-bis-(carboxyethyl)-5, 6 -carboxyfluorescein] © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 24 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
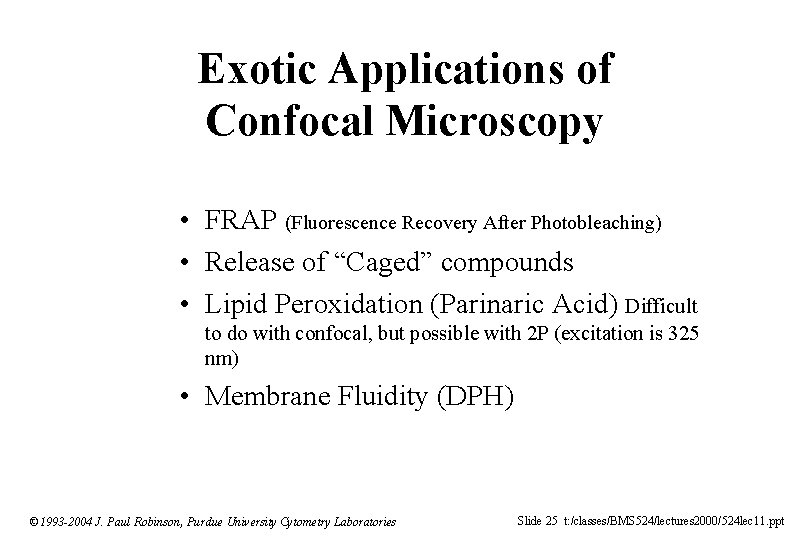
Exotic Applications of Confocal Microscopy • FRAP (Fluorescence Recovery After Photobleaching) • Release of “Caged” compounds • Lipid Peroxidation (Parinaric Acid) Difficult to do with confocal, but possible with 2 P (excitation is 325 nm) • Membrane Fluidity (DPH) © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 25 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
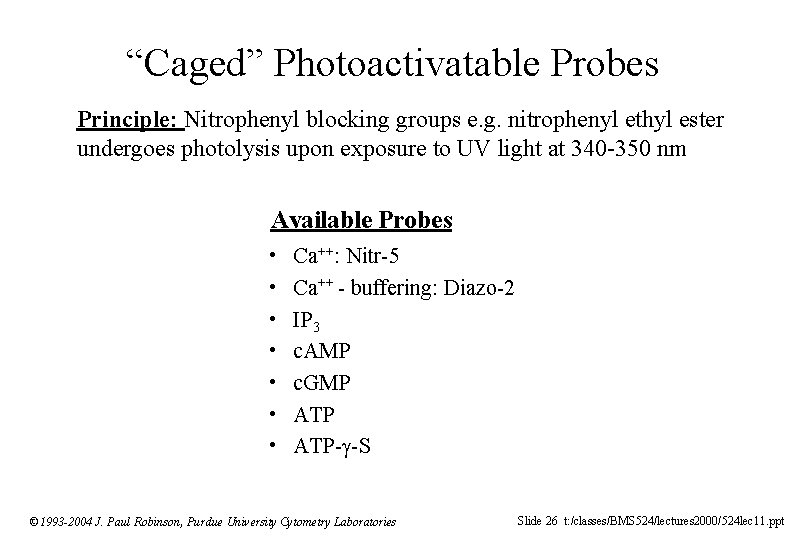
“Caged” Photoactivatable Probes Principle: Nitrophenyl blocking groups e. g. nitrophenyl ethyl ester undergoes photolysis upon exposure to UV light at 340 -350 nm Available Probes • • Ca++: Nitr-5 Ca++ - buffering: Diazo-2 IP 3 c. AMP c. GMP ATP- -S © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 26 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
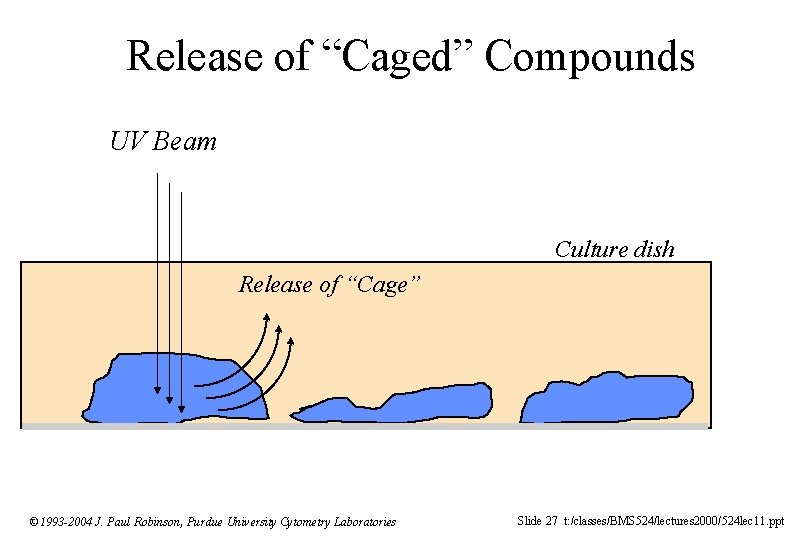
Release of “Caged” Compounds UV Beam Culture dish Release of “Cage” © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 27 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
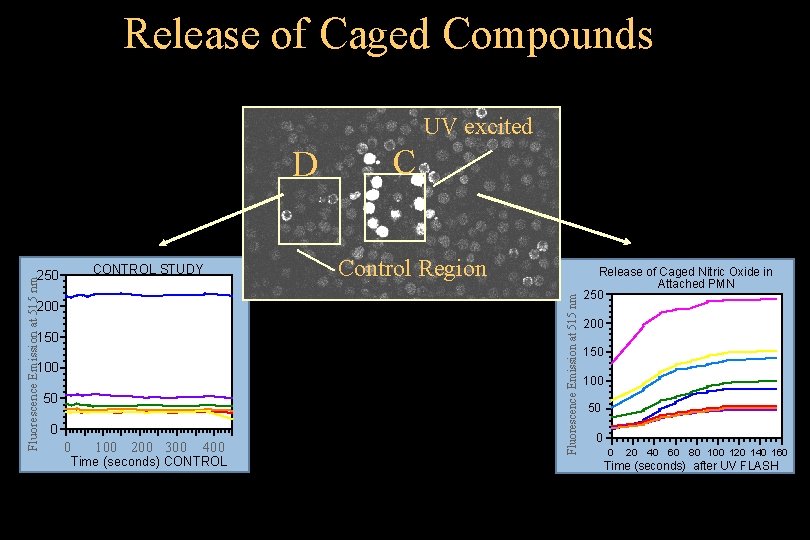
Release of Caged Compounds UV excited CONTROL STUDY Fluorescence Emission at 515 nm 250 C Control Region 200 150 100 50 0 0 100 200 300 400 Time (seconds) CONTROL © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Fluorescence Emission at 515 nm D Release of Caged Nitric Oxide in Attached PMN 250 200 150 100 50 0 0 20 40 60 80 100 120 140 160 Time (seconds) after UV FLASH Slide 28 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
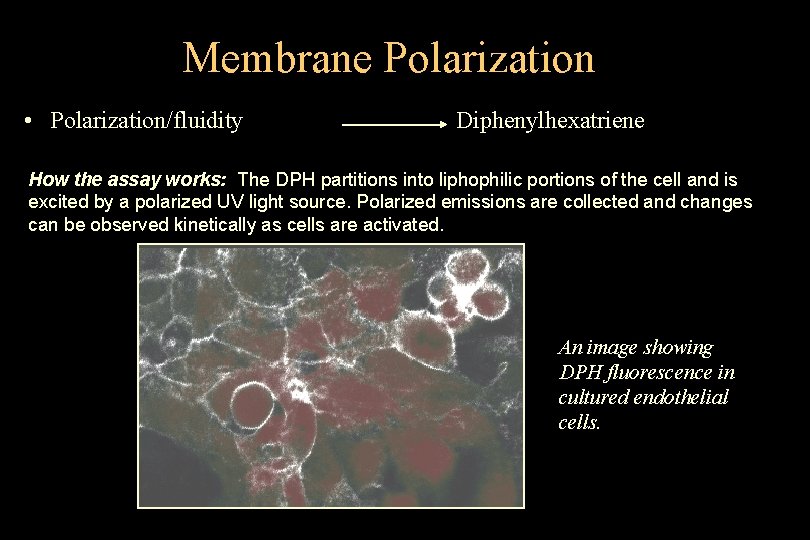
Membrane Polarization • Polarization/fluidity Diphenylhexatriene How the assay works: The DPH partitions into liphophilic portions of the cell and is excited by a polarized UV light source. Polarized emissions are collected and changes can be observed kinetically as cells are activated. An image showing DPH fluorescence in cultured endothelial cells. © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 29 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
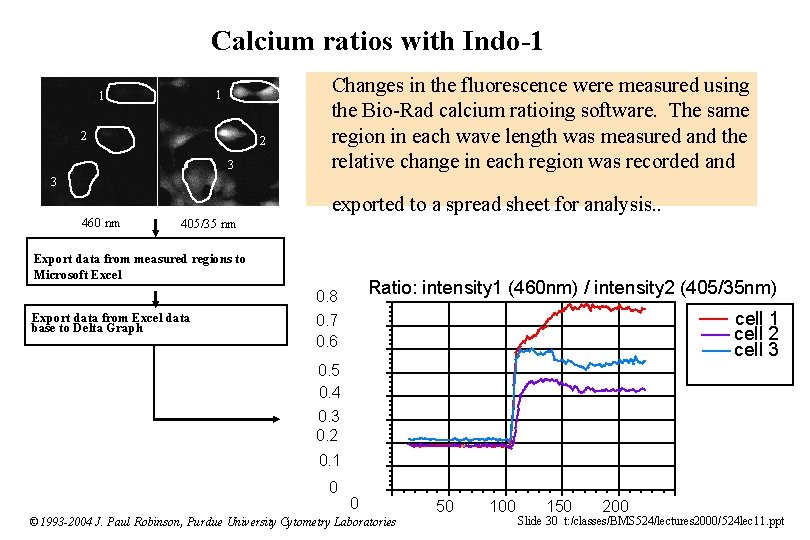
Calcium ratios with Indo-1 1 1 2 2 3 Changes in the fluorescence were measured using the Bio-Rad calcium ratioing software. The same region in each wave length was measured and the relative change in each region was recorded and 3 460 nm exported to a spread sheet for analysis. . 405/35 nm Export data from measured regions to Microsoft Excel Ratio: intensity 1 (460 nm) / intensity 2 (405/35 nm) 0. 8 Export data from Excel data base to Delta Graph cell 1 cell 2 cell 3 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 0 © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories 50 100 150 200 Slide 30 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
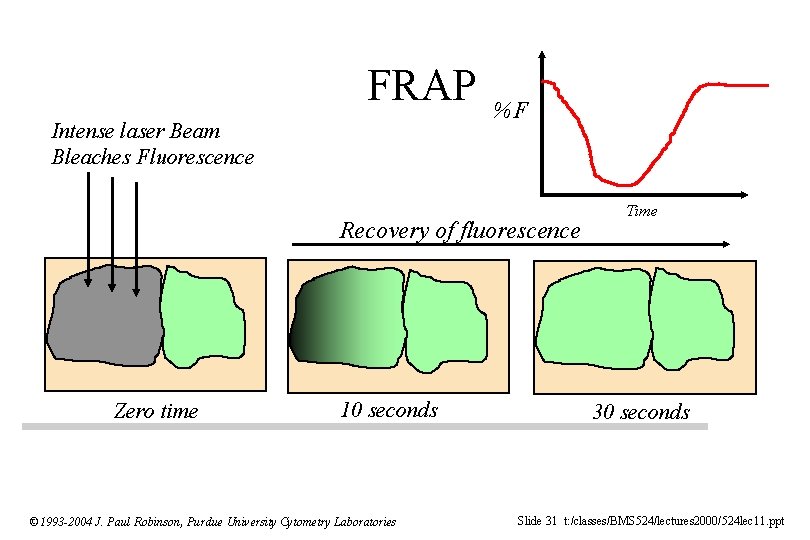
FRAP Intense laser Beam Bleaches Fluorescence %F Recovery of fluorescence Zero time 10 seconds © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Time 30 seconds Slide 31 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
4 D confocal microscopy • Time vs 3 D sections • Used when evaluating kinetic changes in tissue or cells • Requires fast 3 D sectioning • Difficult to evaluate © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 32 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
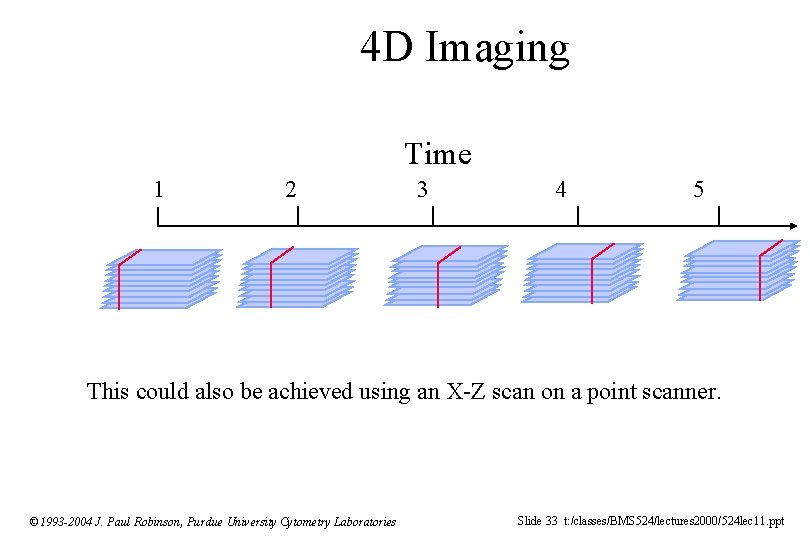
4 D Imaging Time 1 2 3 4 5 This could also be achieved using an X-Z scan on a point scanner. © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 33 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
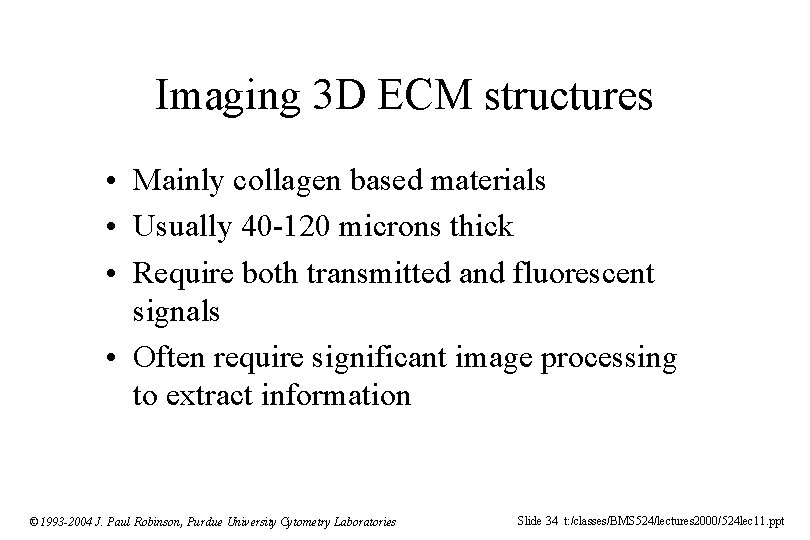
Imaging 3 D ECM structures • Mainly collagen based materials • Usually 40 -120 microns thick • Require both transmitted and fluorescent signals • Often require significant image processing to extract information © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 34 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
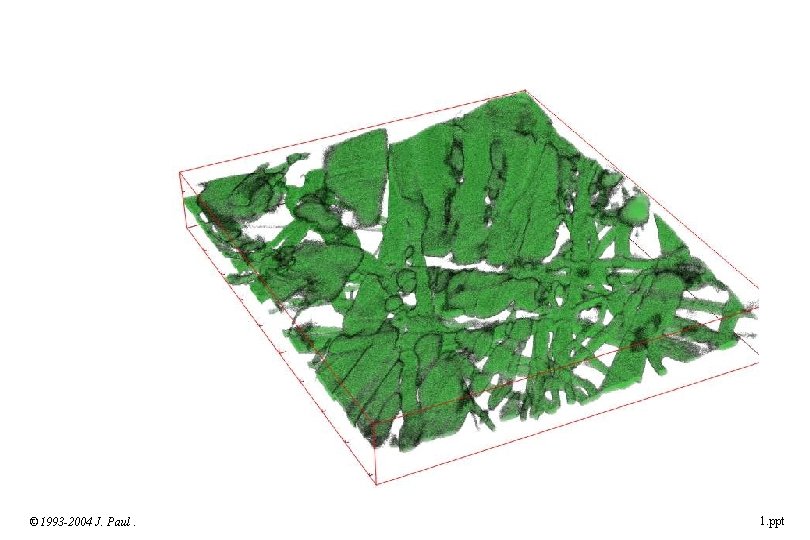
© 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 35 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
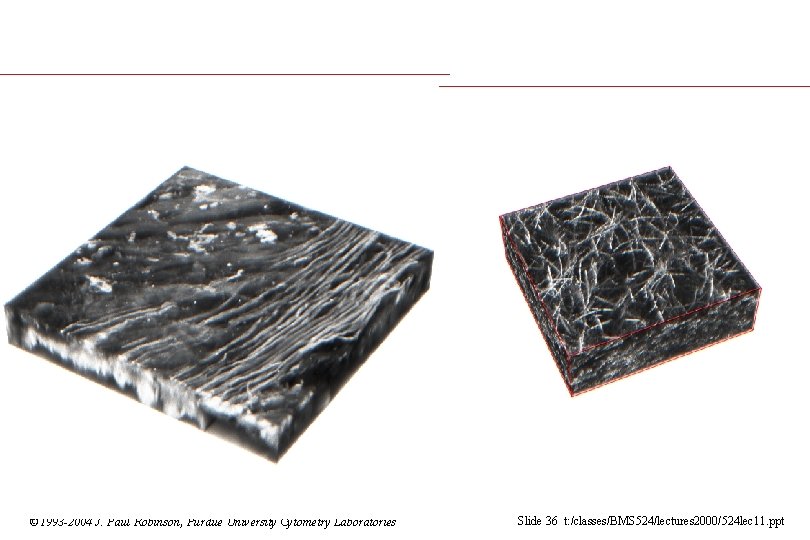
© 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 36 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
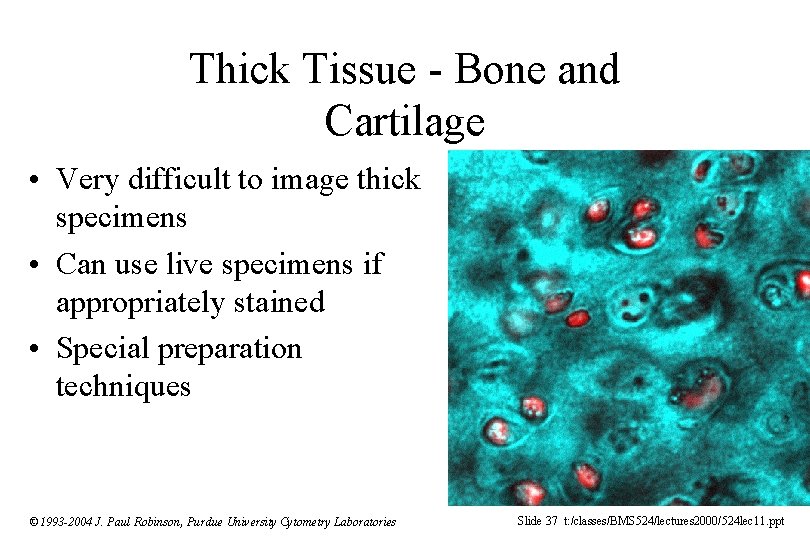
Thick Tissue - Bone and Cartilage • Very difficult to image thick specimens • Can use live specimens if appropriately stained • Special preparation techniques © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 37 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
Lecture Summary • Live cell applications are relatively common using confocal microscopy • Correct use of fluorescent probes necessary • Temperature and atmosphere control may be required • Thick specimens often require advanced image processing • Exotic applications are potentially useful • A limited window of time is available to image live cells before cells deteriorate © 1993 -2004 J. Paul Robinson, Purdue University Cytometry Laboratories Slide 38 t: /classes/BMS 524/lectures 2000/524 lec 11. ppt
- Slides: 37