Lithium ion Battery theoretical capacity calculation ELECTRON ION

Lithium ion Battery theoretical capacity calculation

ELECTRON, ION AND COULOMBIC ENERGY The Motion of an ion in the electrolyte or of an electron in a wire is a transfer of definite amount of electricity. The actual amount of electricity transported by a single electron or a monovalent ion is very small. . 1. 60186 x 10 -19 Coulombs According to Faraday’s law, 1 gram equivalent of any substance is equal to 96500 Coulombs, I. e. , if the ion is monovalent the total charge is 96500 Coulombs and if bivalent the charge is twice of this amount.
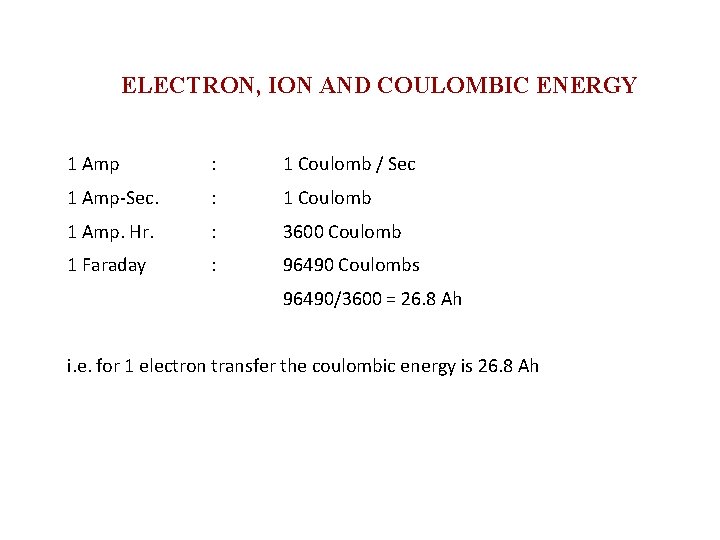
ELECTRON, ION AND COULOMBIC ENERGY 1 Amp : 1 Coulomb / Sec 1 Amp-Sec. : 1 Coulomb 1 Amp. Hr. : 3600 Coulomb 1 Faraday : 96490 Coulombs 96490/3600 = 26. 8 Ah i. e. for 1 electron transfer the coulombic energy is 26. 8 Ah

ELECTRON, ION AND COULOMBIC ENERGY • Lead acid • Li-ion For Lead Acid Battery System For Li-ion Battery System No. of electrons transfer : 2 Pos. Pb. O 2 Molecular Wt : 239 g Neg. Pb Molecular Wt. : 207 g For a 2 e- transfer, Coulombic energy is 2 x 26. 8 : 53. 6 Ah : 1 For a 1 e- transfer, Coulombic energy is 26. 8 Ah
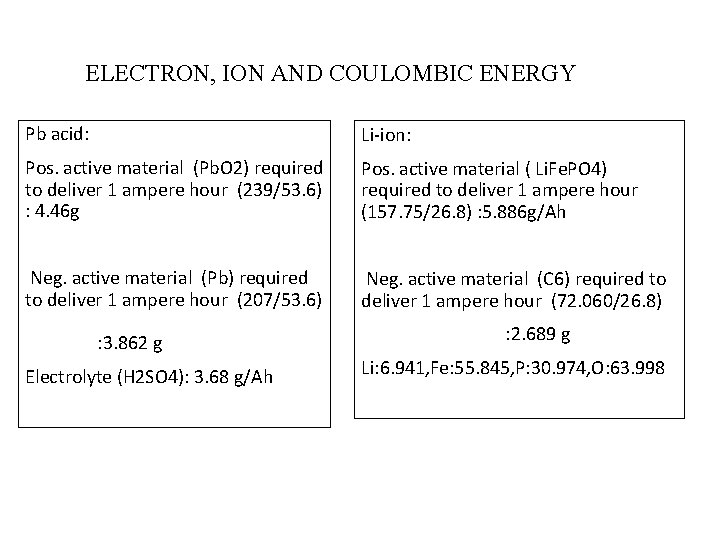
ELECTRON, ION AND COULOMBIC ENERGY Pb acid: Li-ion: Pos. active material (Pb. O 2) required to deliver 1 ampere hour (239/53. 6) : 4. 46 g Pos. active material ( Li. Fe. PO 4) required to deliver 1 ampere hour (157. 75/26. 8) : 5. 886 g/Ah Neg. active material (Pb) required to deliver 1 ampere hour (207/53. 6) Neg. active material (C 6) required to deliver 1 ampere hour (72. 060/26. 8) : 3. 862 g Electrolyte (H 2 SO 4): 3. 68 g/Ah : 2. 689 g Li: 6. 941, Fe: 55. 845, P: 30. 974, O: 63. 998
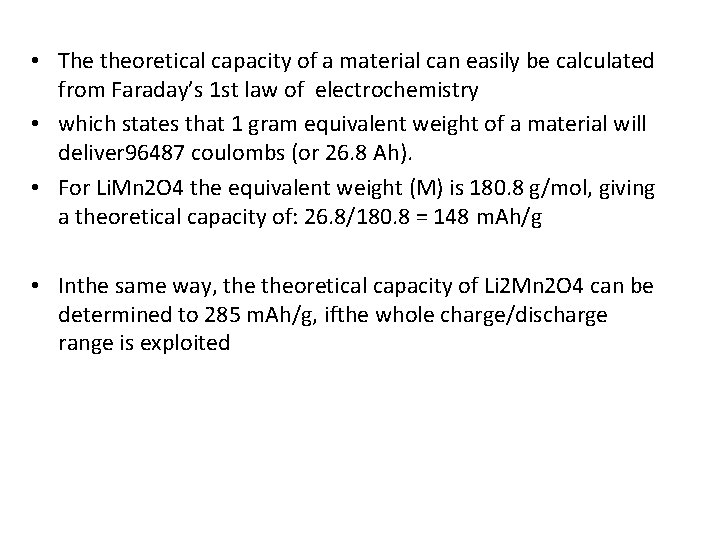
• The theoretical capacity of a material can easily be calculated from Faraday’s 1 st law of electrochemistry • which states that 1 gram equivalent weight of a material will deliver 96487 coulombs (or 26. 8 Ah). • For Li. Mn 2 O 4 the equivalent weight (M) is 180. 8 g/mol, giving a theoretical capacity of: 26. 8/180. 8 = 148 m. Ah/g • Inthe same way, theoretical capacity of Li 2 Mn 2 O 4 can be determined to 285 m. Ah/g, ifthe whole charge/discharge range is exploited

The thermodynamic quantity describing the change in energy as a function of changes in Li concentration in the host matrix is the chemical potential ( µ ), defined as µ= ƏG Əx where G is the Gibbs free energy and x is the number of inserted Li atoms. The change in free energy can also be expressed as • • where n is the number of electrons in both electrode-reactions δ in the cell-reaction above), F is Faraday’s constant, and E is the potential difference between the electrodes. By combining (1) and (2), we get the relation between electrical and chemical energy in the system: ΔG = -n. FE = -δFE=µc --µa • where µc c and µa are the chemical potentials of the lithium ions in the cathode and anode, respectively.

Ampere-hour (Ah) capacity is the total charge that can be discharged from a fully charged battery under specified conditions. People also use Wh (or k. Wh) capacity to represent a battery capacity. The rated Wh capacity is defined as Rated Wh Capacity = Rated Ah Capacity × Rated Battery Voltage: Power : energy by second , watt W=A×V=Current × Voltage Energy : power multiplied by time WH=Ah × V=Capacity × Voltage Capacity : Current × time Ah

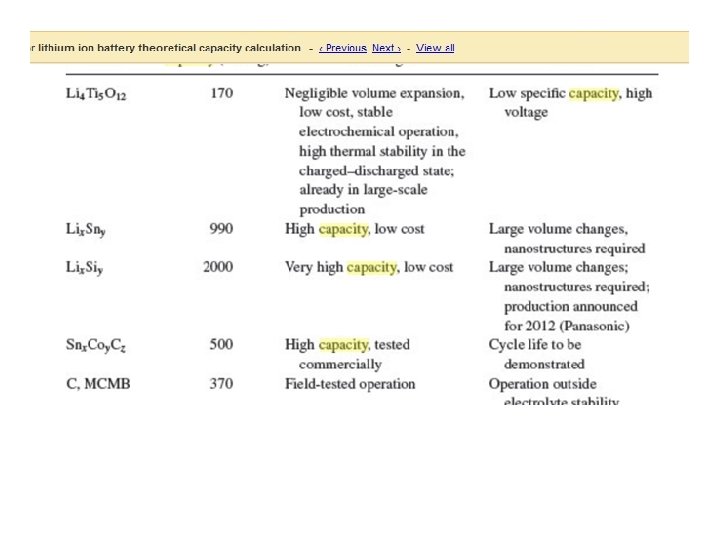
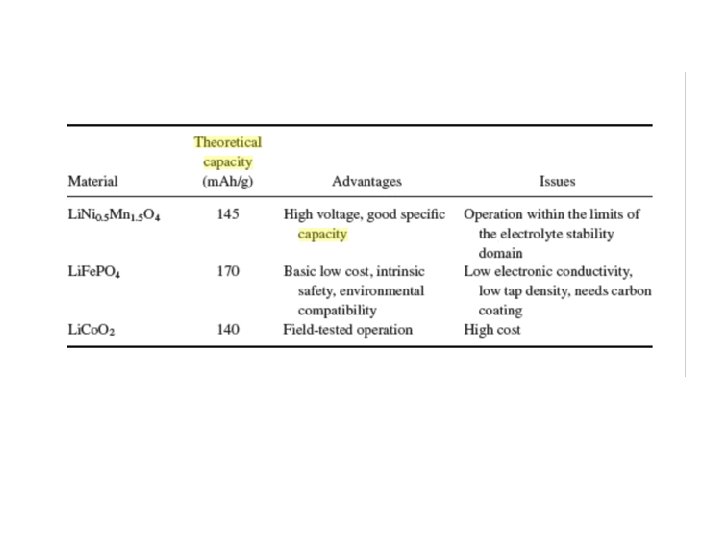
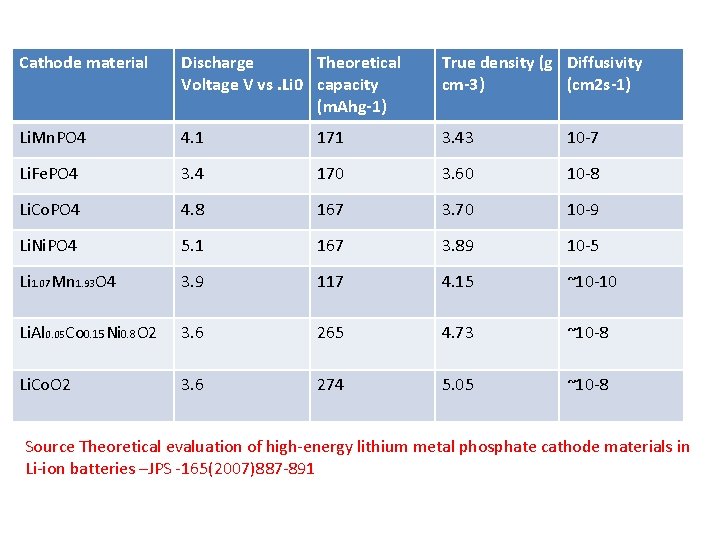
Cathode material Discharge Theoretical Voltage V vs. Li 0 capacity (m. Ahg-1) True density (g Diffusivity cm-3) (cm 2 s-1) Li. Mn. PO 4 4. 1 171 3. 43 10 -7 Li. Fe. PO 4 3. 4 170 3. 60 10 -8 Li. Co. PO 4 4. 8 167 3. 70 10 -9 Li. Ni. PO 4 5. 1 167 3. 89 10 -5 Li 1. 07 Mn 1. 93 O 4 3. 9 117 4. 15 ~10 -10 Li. Al 0. 05 Co 0. 15 Ni 0. 8 O 2 3. 6 265 4. 73 ~10 -8 Li. Co. O 2 3. 6 274 5. 05 ~10 -8 Source Theoretical evaluation of high-energy lithium metal phosphate cathode materials in Li-ion batteries –JPS -165(2007)887 -891
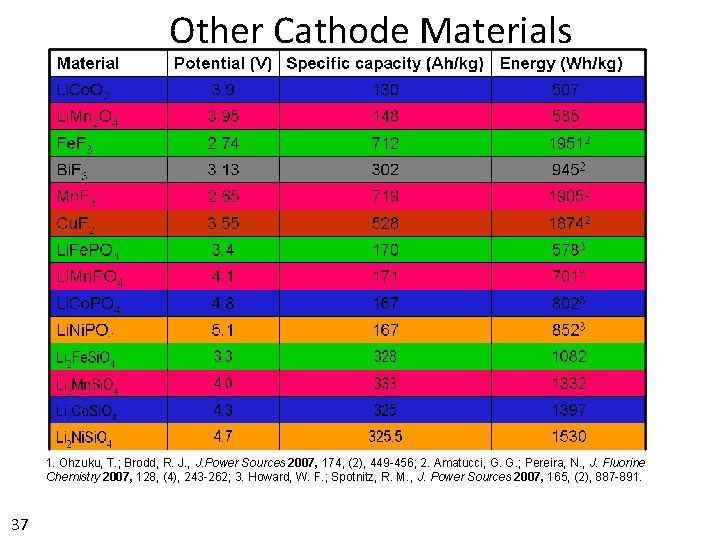
Other Cathode Materials 1. Ohzuku, T. ; Brodd, R. J. , J. Power Sources 2007, 174, (2), 449 -456; 2. Amatucci, G. G. ; Pereira, N. , J. Fluorine Chemistry 2007, 128, (4), 243 -262; 3. Howard, W. F. ; Spotnitz, R. M. , J. Power Sources 2007, 165, (2), 887 -891. 37
- Slides: 13