LIQUIFICATION KMT AND CONDENSED PHASES Ideal Gas Equation













- Slides: 34

LIQUIFICATION: KMT AND CONDENSED PHASES

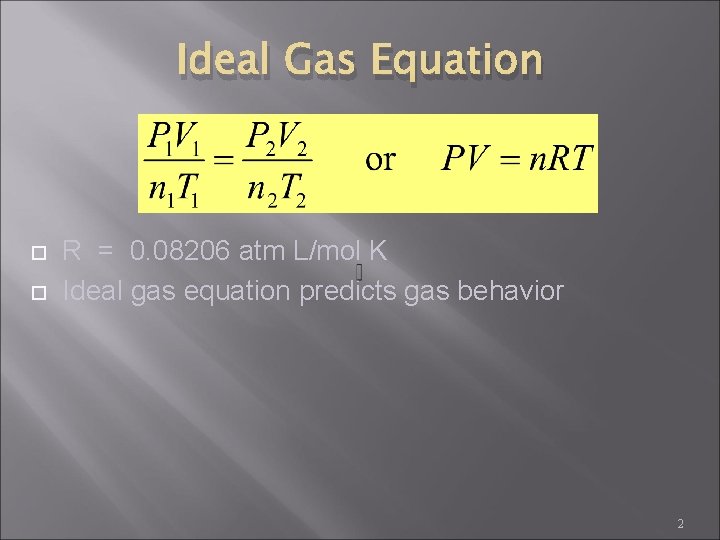
Ideal Gas Equation R = 0. 08206 atm L/mol K Ideal gas equation predicts gas behavior 2

A Model for Gas Behavior Ideal gas law describes what gases do, but not why. Kinetic Molecular Theory of Gases (KMT): model that explains gas behavior. developed in mid-1800 s based on concept of an ideal or perfect gas 3

Ideal gas Tiny particles in constant, random, straight-line motion Molecules collide w/ each other & w/ walls of container Gas molecules are points; gas volume is empty space between molecules Molecules independent of each other (no attractive or repulsive forces between them). 4

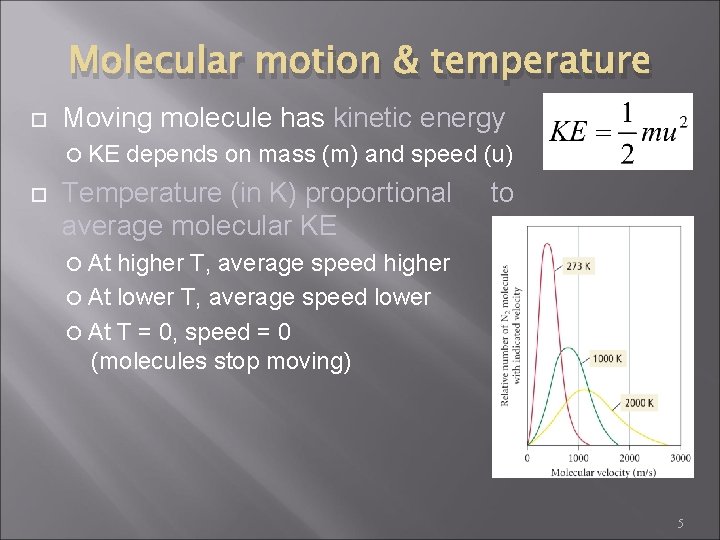
Molecular motion & temperature Moving molecule has kinetic energy KE depends on mass (m) and speed (u) Temperature (in K) proportional average molecular KE to At higher T, average speed higher At lower T, average speed lower At T = 0, speed = 0 (molecules stop moving) 5

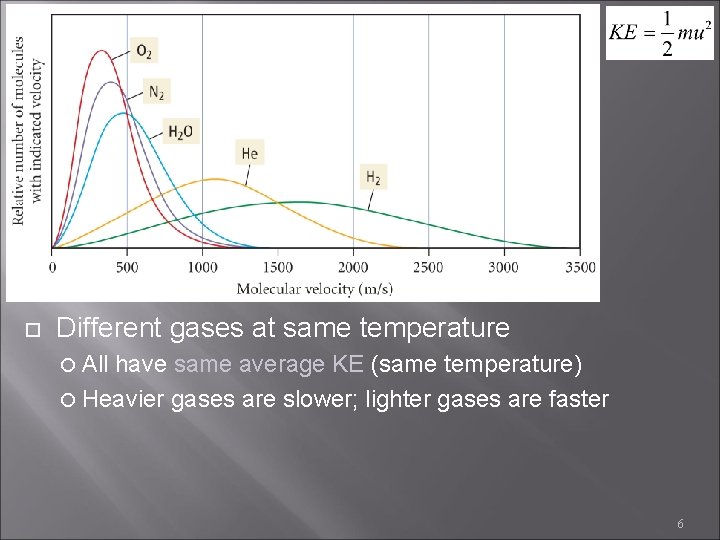
Different gases at same temperature All have same average KE (same temperature) Heavier gases are slower; lighter gases are faster 6

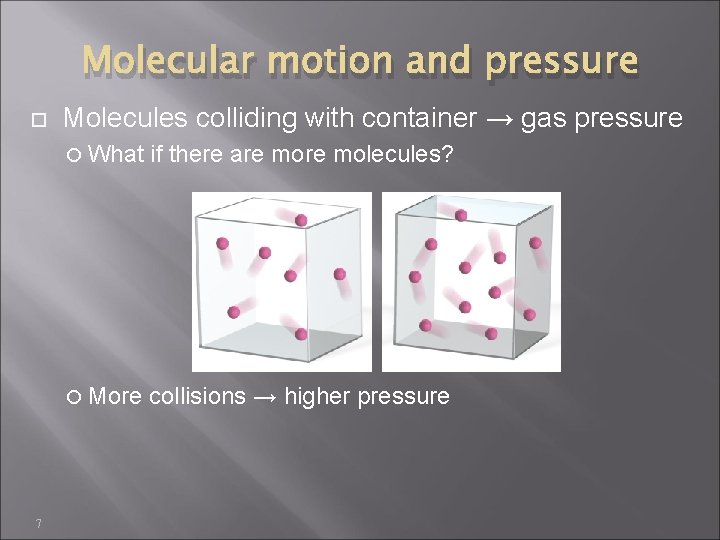
Molecular motion and pressure 7 Molecules colliding with container → gas pressure What if there are molecules? More collisions → higher pressure

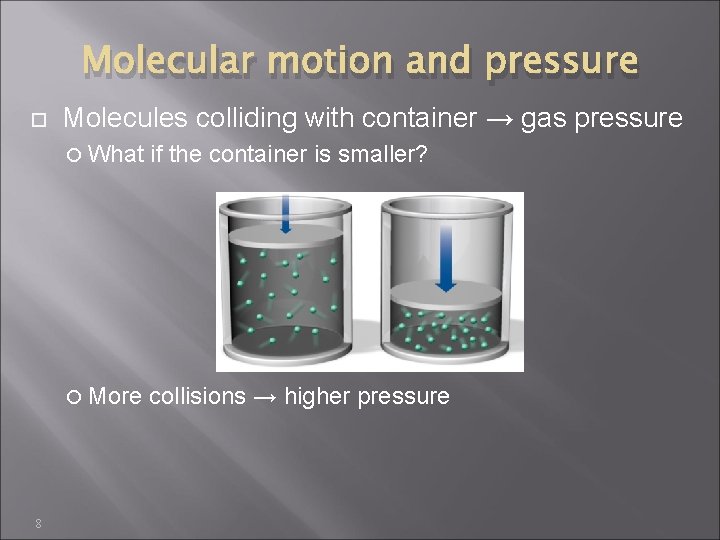
Molecular motion and pressure 8 Molecules colliding with container → gas pressure What if the container is smaller? More collisions → higher pressure

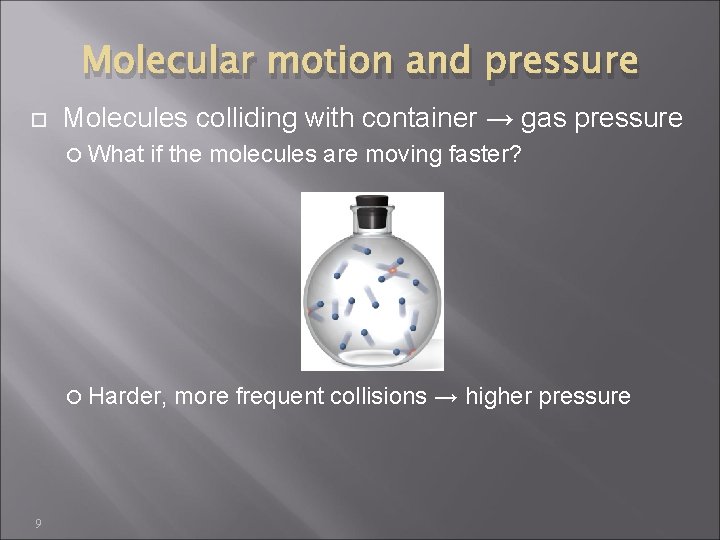
Molecular motion and pressure Molecules colliding with container → gas pressure What if the molecules are moving faster? Harder, 9 more frequent collisions → higher pressure

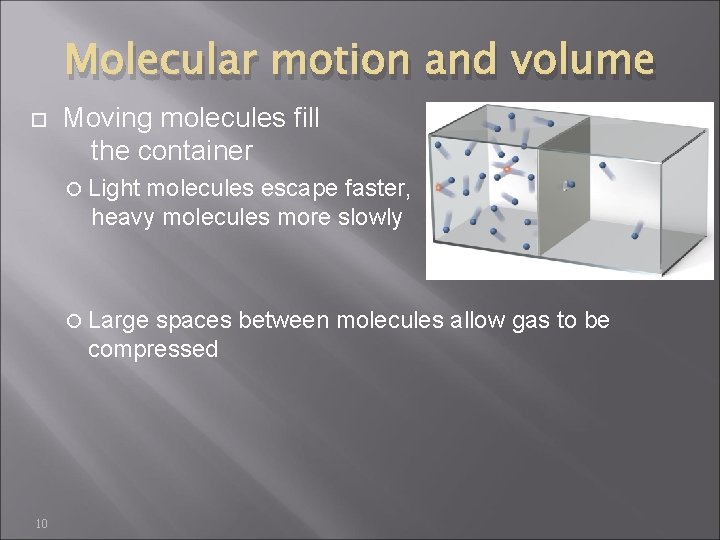
Molecular motion and volume Moving molecules fill the container Light molecules escape faster, heavy molecules more slowly Large spaces between molecules allow gas to be compressed 10

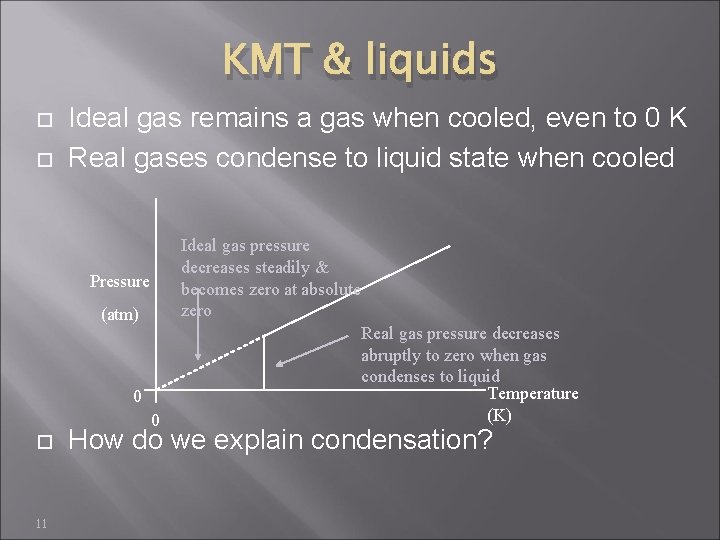
KMT & liquids Ideal gas remains a gas when cooled, even to 0 K Real gases condense to liquid state when cooled Pressure (atm) 0 0 11 Ideal gas pressure decreases steadily & becomes zero at absolute zero Real gas pressure decreases abruptly to zero when gas condenses to liquid Temperature (K) How do we explain condensation?

Condensation KMT ignores attractions between gas molecules Gas molecules are too far apart & too fast for attractions to act 12 BUT. . . attractive forces do exist between all molecules! At low enough T, attractions overcome kinetic energy & molecules stick together to form a liquid

Two opposing tendencies For every substance there are 2 opposing tendencies: Kinetic energy of the molecules, which tend to make them move apart from each other (gas-like) Attraction between molecules, which tends to make them stick together (liquid-like) At any given temp. , kinetic energy is the same for all molecules, so the attractive forces between molecules determines whether something is a liquid, solid, or gas.

Intermolecular Attractions At same temp. (same KE), molecules are gases, liquids, solids. This suggests that some molecules have stronger attractions between molecules. How do you judge the strength of the attractive forces between molecules? Look at the boiling point. Low Boiling point = weak intermolecular forces (molecules are not sticky) High Boiling point = strong intermolecular forces (molecules are sticky)

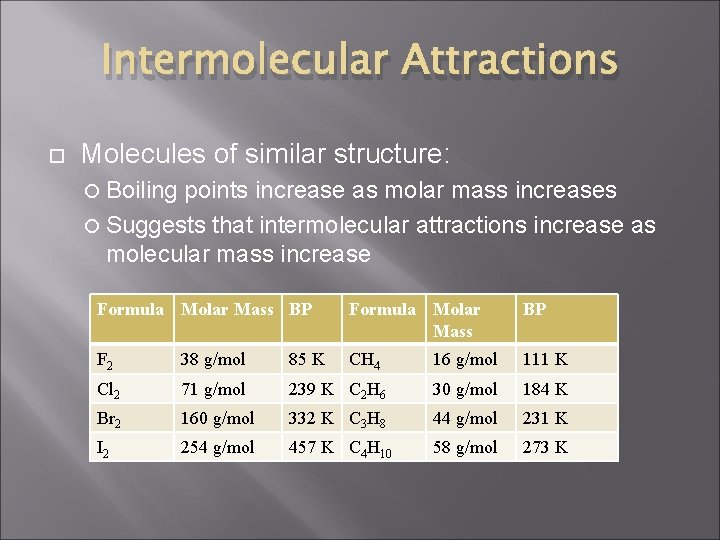
Intermolecular Attractions Molecules of similar structure: Boiling points increase as molar mass increases Suggests that intermolecular attractions increase as molecular mass increase Formula Molar Mass BP F 2 38 g/mol 85 K CH 4 16 g/mol 111 K Cl 2 71 g/mol 239 K C 2 H 6 30 g/mol 184 K Br 2 160 g/mol 332 K C 3 H 8 44 g/mol 231 K I 2 254 g/mol 457 K C 4 H 10 58 g/mol 273 K

Let’s Practice… Predict which would have a higher boiling point, and why? Na or K F 2 or Br 2

Phase Changes and Temperature As you heat a solid, the temp. increases until the solid melts. Temp. will remain constant until solid melts completely. You observe the same pattern when a gas is cooled until it changes into a solid Temp. solid will remain constant until gas changes into

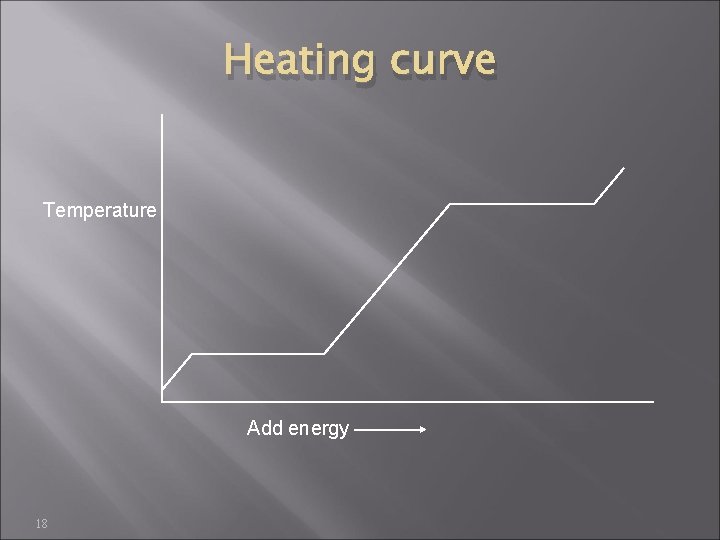
Heating curve Temperature Add energy 18

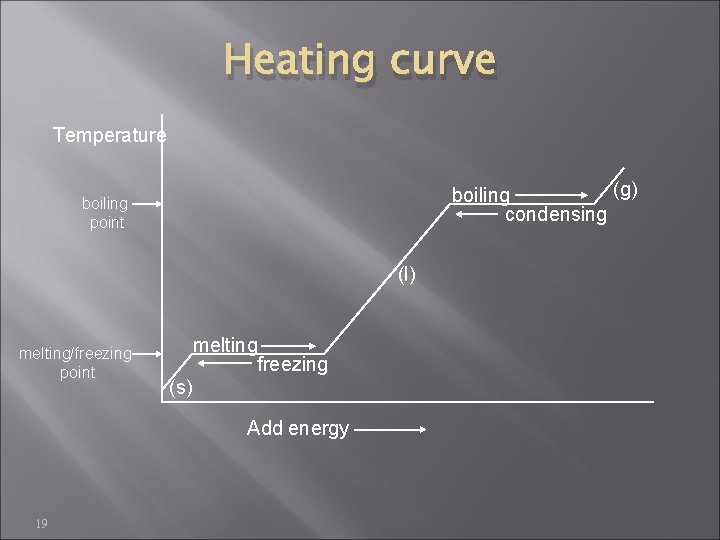
Heating curve Temperature (g) boiling condensing boiling point (l) melting/freezing point melting freezing (s) Add energy 19

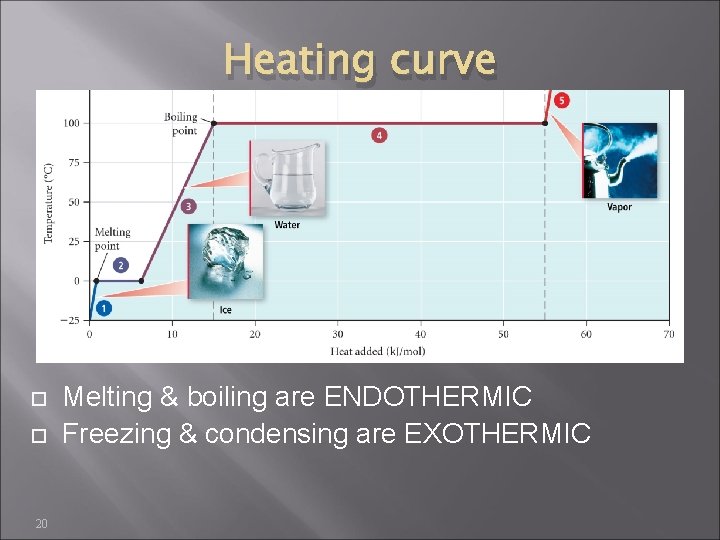
Heating curve 20 Melting & boiling are ENDOTHERMIC Freezing & condensing are EXOTHERMIC

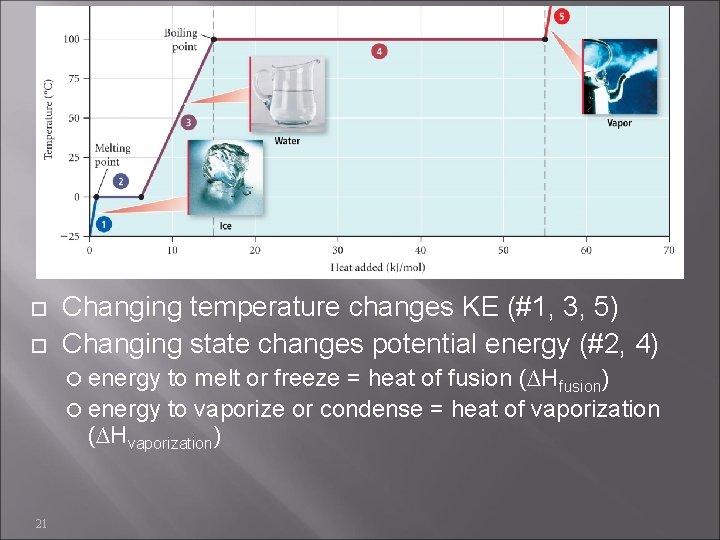
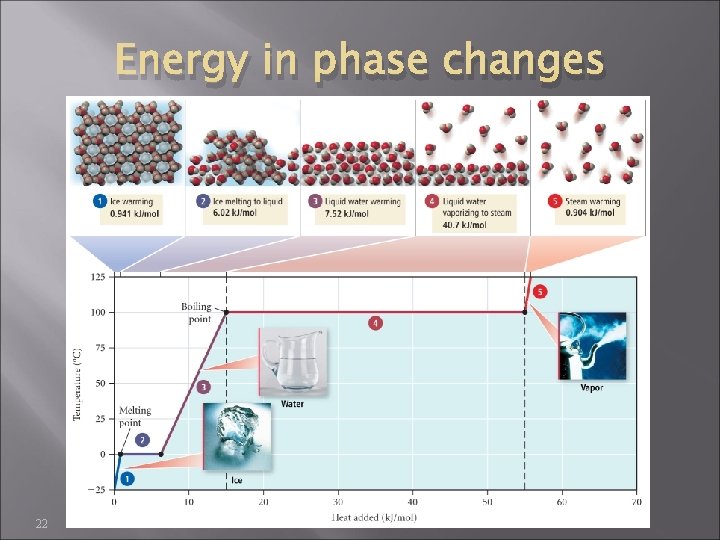
Changing temperature changes KE (#1, 3, 5) Changing state changes potential energy (#2, 4) energy to melt or freeze = heat of fusion (∆Hfusion) energy to vaporize or condense = heat of vaporization (∆Hvaporization) 21

Energy in phase changes 22

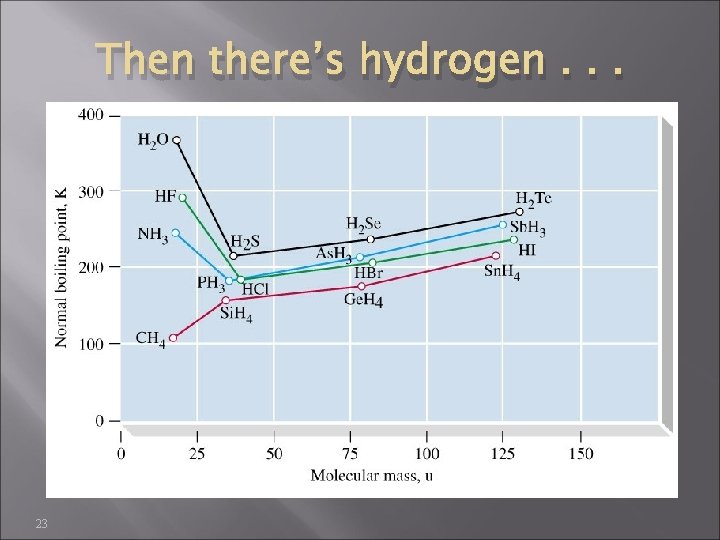
Then there’s hydrogen. . . 23

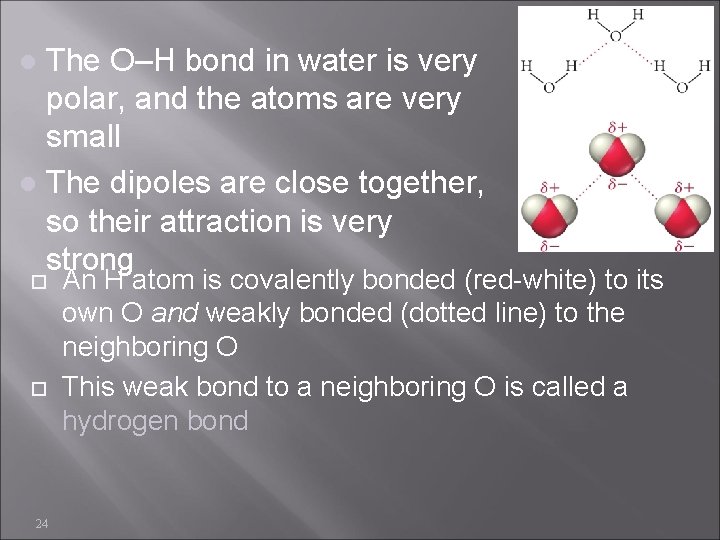
The O–H bond in water is very polar, and the atoms are very small l The dipoles are close together, so their attraction is very strong l 24 An H atom is covalently bonded (red-white) to its own O and weakly bonded (dotted line) to the neighboring O This weak bond to a neighboring O is called a hydrogen bond

Hydrogen bonding 25 Hydrogen bonding occurs only between molecules containing N–H, O–H, and F–H bonds Hydrogen bonding is much stronger than ordinary intermolecular attractions ⇒ very high boiling points for their mass Hydrogen bonds are not as strong as covalent bonds (15 -40 k. J/mol, vs >150 k. J/mol)

Heat of Fusion and Vaporization Heat of Fusion- change in energy when a solid substance melts Heat of Vaporization- change in energy when liquid substance vaporizes (evaporates)

Let’s practice Given that the heat of fusion (ice) is 6. 0 kj/mol, how much energy is needed to melt an ice cube with a mass of 42 g? Given that the heat of vaporization (water) is 41 kj/mol, how much energy is need to convert 24 g of water into steam?

Phase change: Liquid to gas A quick look into the past… At any given temp. molecules have the same avg. KE Light molecules = fast Heavy molecules = slow Having the same avg. KE means some molecules are moving faster than avg. , and some slower.

Phase change: Liquid to gas Consider if you will… A container of water at 25 o. C (room temp. ) All the water molecules have the same avg. KE Water is not hot, but a few fast molecules will leave and become water vapor. Vapor molecules exert pressure = vapor pressure

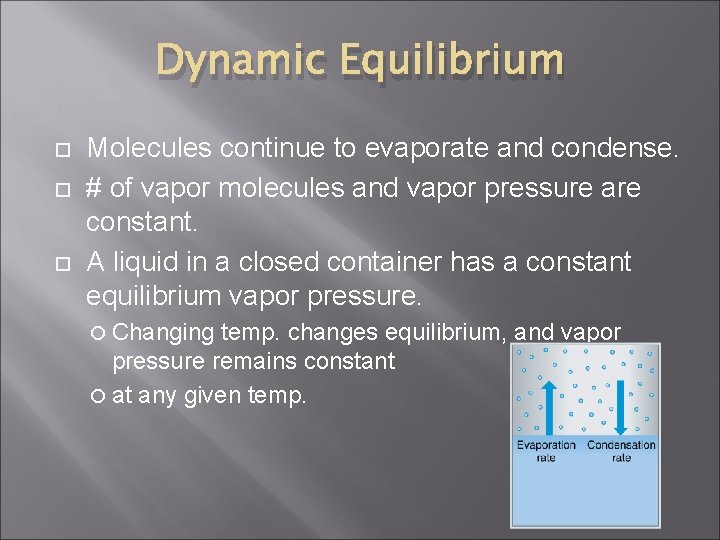
Phase change: Liquid to gas Now, consider this… Water molecules can escape from open container, but not a closed container. Energy is transferred by collisions w/ other vapor molecules. Some vapor molecules will be slow enough to return to liquid. Eventually, rate of evaporation = rate of condensing This is called DYNAMIC EQUILIBRIUM

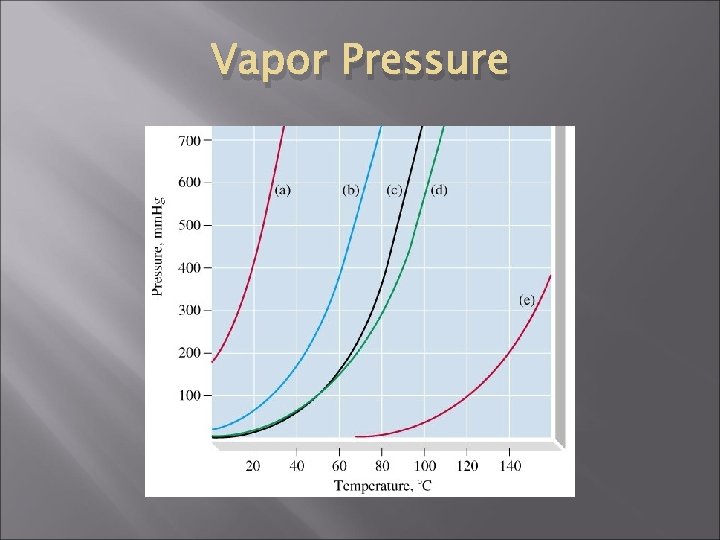
Dynamic Equilibrium Molecules continue to evaporate and condense. # of vapor molecules and vapor pressure are constant. A liquid in a closed container has a constant equilibrium vapor pressure. Changing temp. changes equilibrium, and vapor pressure remains constant at any given temp.

Dynamic Equilibrium Imagine this… Open container and heat it. KE increases, more molecules evaporate Bubbles of water form throughout liquid, rise to surface and break… boiling begins

Dynamic Equilibrium As temp. increases, vapor pressure increases A liquid boils when the vapor pressure matches the external pressure. In an open container, external pressure is atmospheric pressure

Vapor Pressure