Liquids solids intermolecular forces Chapter 11 1 KMT


















- Slides: 51

Liquids, solids, & intermolecular forces Chapter 11 1

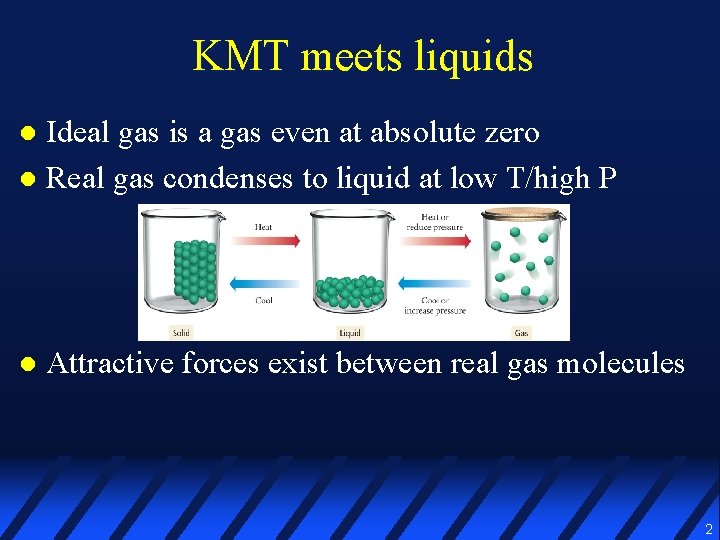
KMT meets liquids Ideal gas is a gas even at absolute zero l Real gas condenses to liquid at low T/high P l l Attractive forces exist between real gas molecules 2

Intermolecular attractions Attractive forces exist between all atoms/molecules l Relative strength of attractions indicated by l Boiling point (higher b. p. = stronger attractions) t Vapor pressure (high v. p. = weaker attractions) t ∆Hvaporization (large ∆Hvap = stronger attractions) t 3

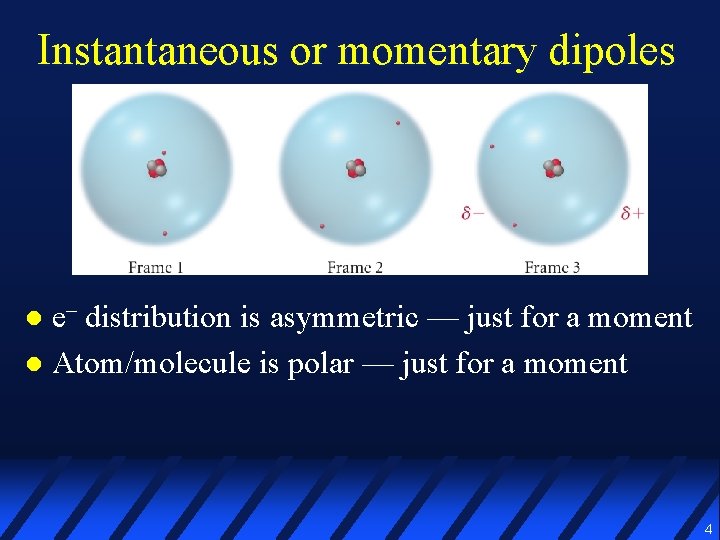
Instantaneous or momentary dipoles l e– distribution is asymmetric –– just for a moment l Atom/molecule is polar –– just for a moment 4

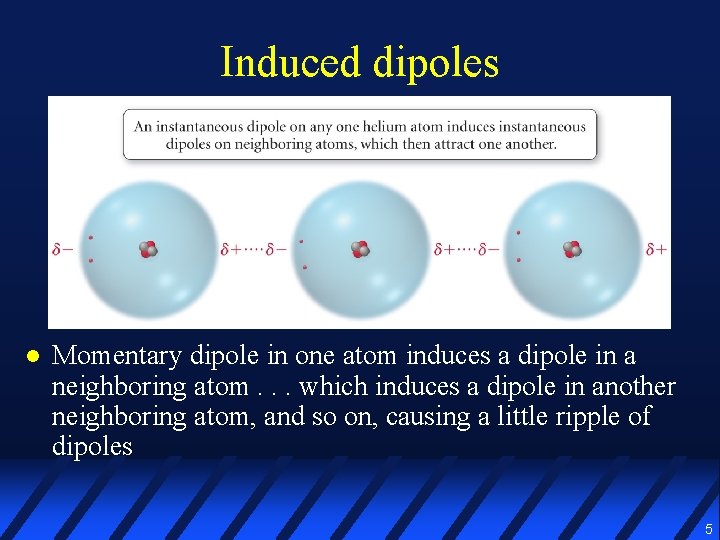
Induced dipoles l Momentary dipole in one atom induces a dipole in a neighboring atom. . . which induces a dipole in another neighboring atom, and so on, causing a little ripple of dipoles 5

Dispersion force Taken together, instantaneous & induced dipoles create an attractive force between molecules, called the dispersion force l Each dipole is tiny, but the constant ripple of countless dipoles throughout the substance makes this the primary attractive force between molecules l Even noble gas atoms show dispersion force between atoms l 6

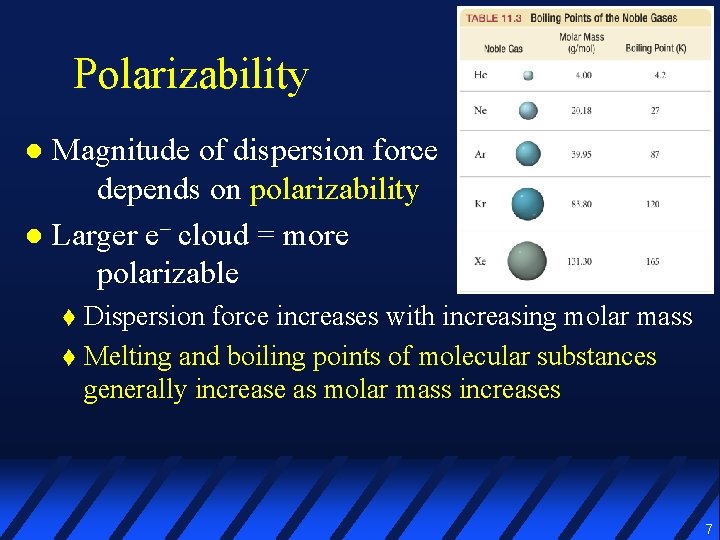
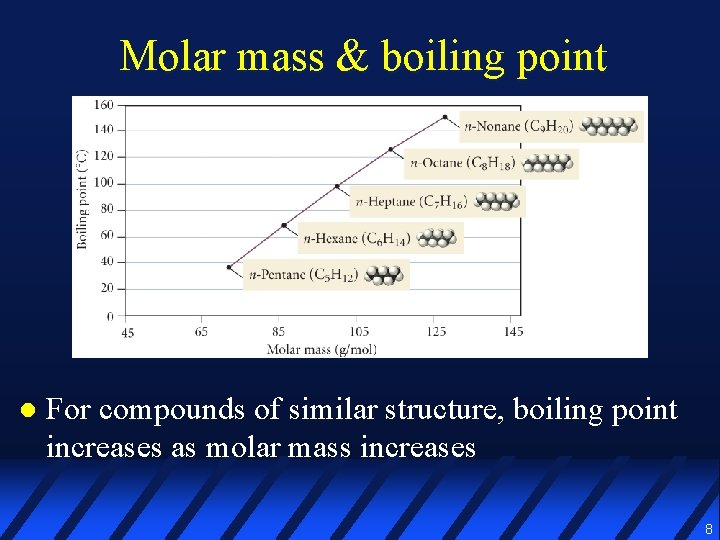
Polarizability Magnitude of dispersion force depends on polarizability l Larger e– cloud = more polarizable l Dispersion force increases with increasing molar mass t Melting and boiling points of molecular substances generally increase as molar mass increases t 7

Molar mass & boiling point l For compounds of similar structure, boiling point increases as molar mass increases 8

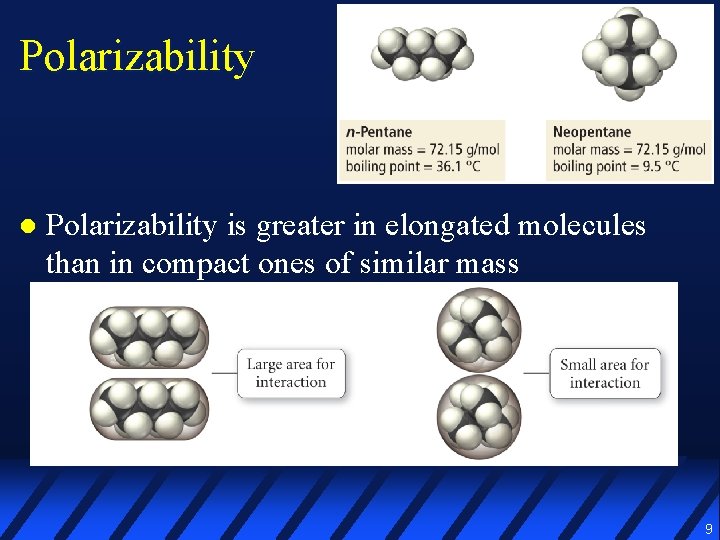
Polarizability l Polarizability is greater in elongated molecules than in compact ones of similar mass 9

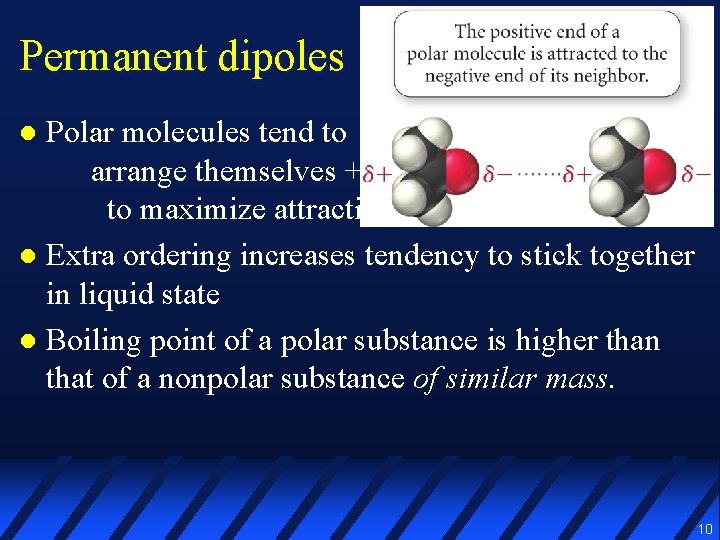
Permanent dipoles Polar molecules tend to arrange themselves +/– to maximize attractions l Extra ordering increases tendency to stick together in liquid state l Boiling point of a polar substance is higher than that of a nonpolar substance of similar mass. l 10

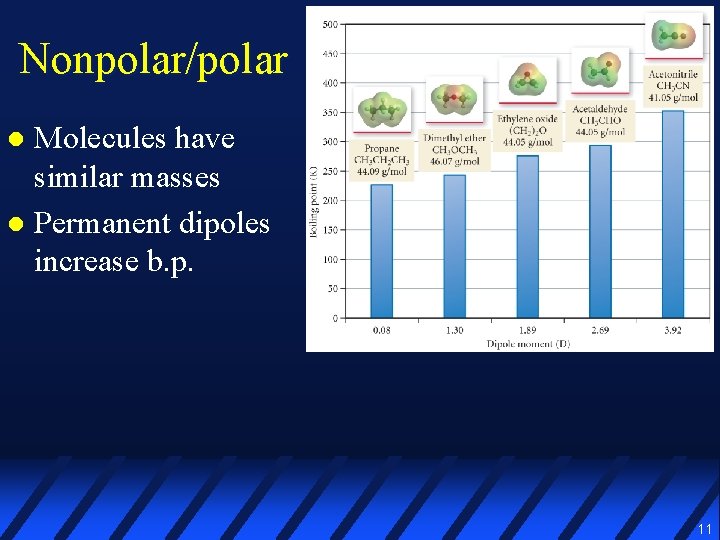
Nonpolar/polar Molecules have similar masses l Permanent dipoles increase b. p. l 11

The van der Waals forces l Together, dispersion and pemanent dipole forces are known as the van der Waals forces When comparing substances of comparable mass (± 10%), the presence of a permanent dipole increases boiling point significantly t When comparing substances of different molar masses, the dispersion force (related to mass) is more important than the permanent dipole t 13

Examples l Which would you expect to have the highest boiling point, and why: C 3 H 8, CO 2, CH 3 CN 14

Examples l Which would you expect to have the highest boiling point, and why: C 3 H 8, CO 2, CH 3 CN masses similar (C 3 H 8 = 44, CO 2 = 44, CH 3 CN = 41) t CH 3 CN polar = highest bp t Actual values: C 3 H 8 = 231 K, CO 2 = 195 K, CH 3 CN = t 15

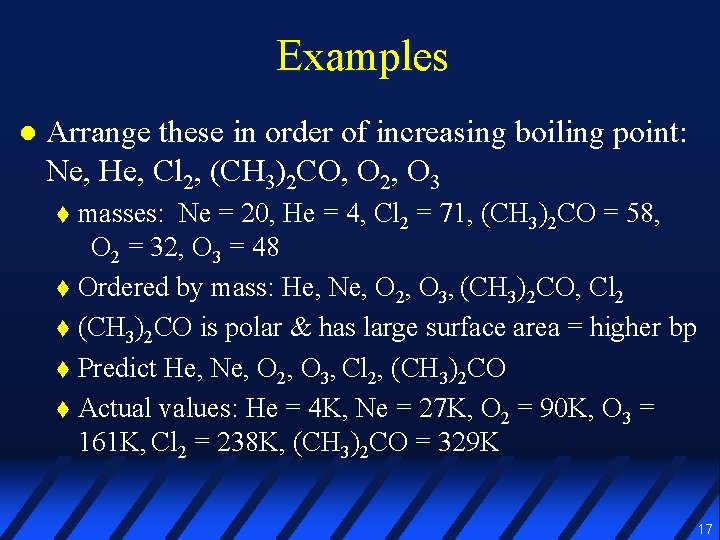
Examples l Arrange these in order of increasing boiling point: Ne, He, Cl 2, (CH 3)2 CO, O 2, O 3 16

Examples l Arrange these in order of increasing boiling point: Ne, He, Cl 2, (CH 3)2 CO, O 2, O 3 masses: Ne = 20, He = 4, Cl 2 = 71, (CH 3)2 CO = 58, O 2 = 32, O 3 = 48 t Ordered by mass: He, Ne, O 2, O 3, (CH 3)2 CO, Cl 2 t (CH 3)2 CO is polar & has large surface area = higher bp t Predict He, Ne, O 2, O 3, Cl 2, (CH 3)2 CO t Actual values: He = 4 K, Ne = 27 K, O 2 = 90 K, O 3 = 161 K, Cl 2 = 238 K, (CH 3)2 CO = 329 K t 17

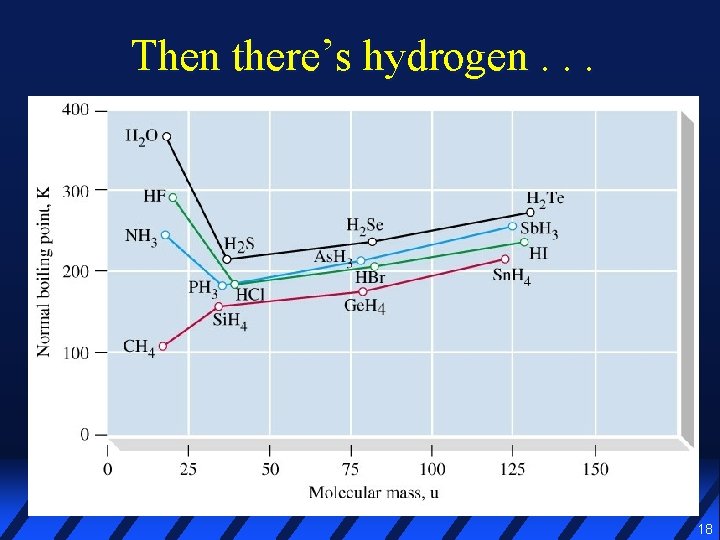
Then there’s hydrogen. . . 18

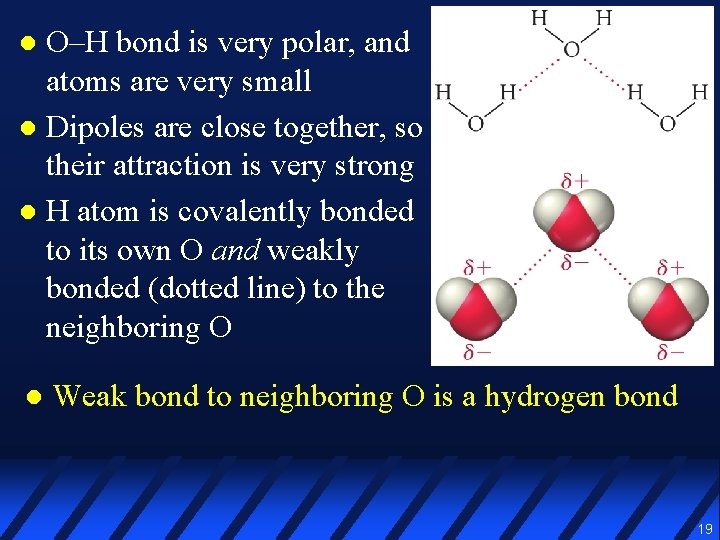
O–H bond is very polar, and atoms are very small l Dipoles are close together, so their attraction is very strong l H atom is covalently bonded to its own O and weakly bonded (dotted line) to the neighboring O l l Weak bond to neighboring O is a hydrogen bond 19

Hydrogen bonding occurs only between molecules containing N–H, O–H, and F–H bonds l Hydrogen bonding is much stronger than ordinary dispersion/dipole → much higher boiling points than expected for their mass l Hydrogen bonds are not as strong as covalent bonds (15 -40 k. J/mol, vs >150 k. J/mol) l 20

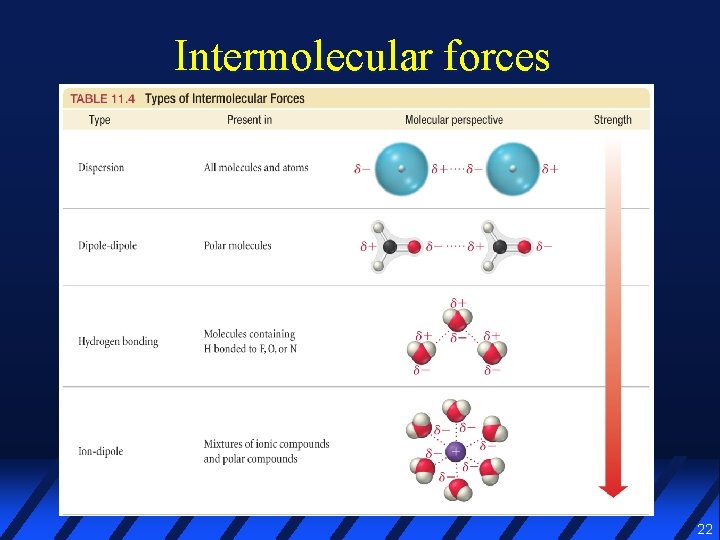
Intermolecular forces 22

Substances that are not molecular l Ionic substances Held together by lattice energy t Generally high mp & bp t l Metallic substances Metal cations in sea of electrons t Generally high mp & bp t l Network covalent solids (e. g. diamond) Melting = disrupt covalent bonds t VERY high mp & bp t 23

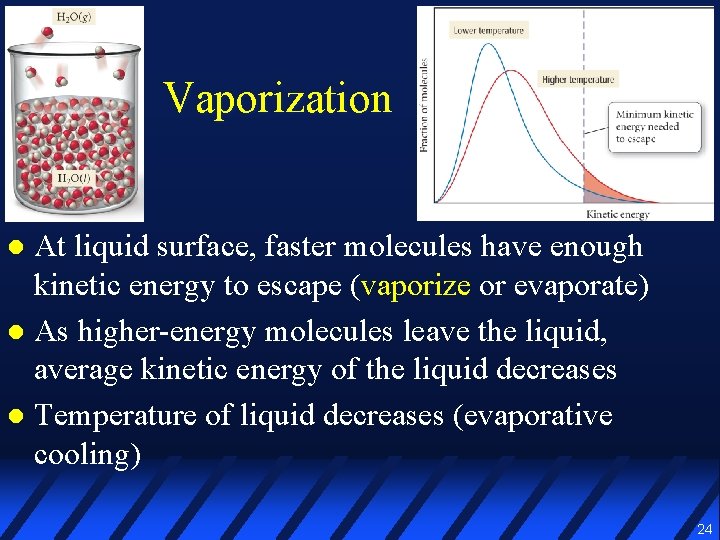
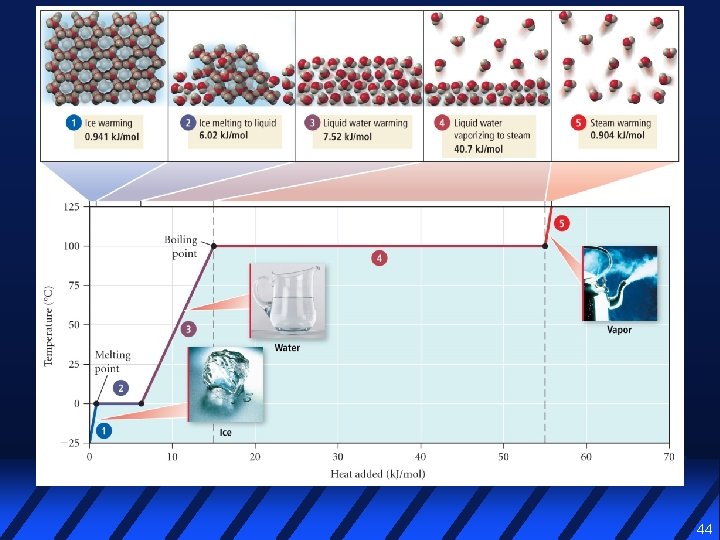
Vaporization At liquid surface, faster molecules have enough kinetic energy to escape (vaporize or evaporate) l As higher-energy molecules leave the liquid, average kinetic energy of the liquid decreases l Temperature of liquid decreases (evaporative cooling) l 24

Vaporization For liquid temperature to remain constant during evaporation, liquid must absorb energy from surroundings l Amount of energy liquid must absorb to keep temperature constant during evaporation = enthalpy (heat) of vaporization (∆Hvaporization) l Vaporization is endothermic, so ∆Hvap is positive l 25

Example l How much energy is required to vaporize 2. 35 g of diethyl ether, (C 2 H 5)2 O, at 298 K? ∆Hvap for diethyl ether at 298 K is 29. 1 k. J/mol. 26

Liquid-vapor equilibrium When rate of vaporization = rate of condensation in a closed sysem, system has reached equilibrium 27

Vapor Pressure l l Pressure exerted by vapor in dynamic equilibrium w its liquid = vapor pressure of that liquid Vapor pressure depends only on type of liquid & temperature As long as both phases are present, amount of liquid in container does not affect vapor pressure Liquids with high vapor pressure at room temperature are volatile (evaporate easily) 28

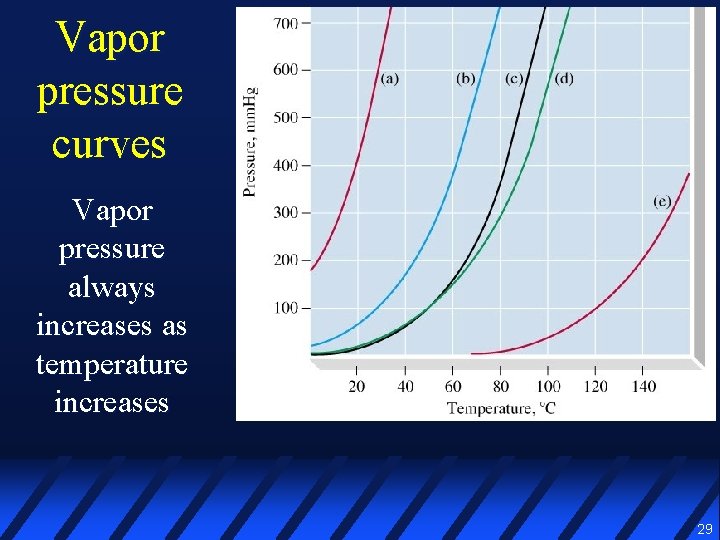
Vapor pressure curves Vapor pressure always increases as temperature increases 29

Vapor pressure and boiling l l In open container, evaporation occurs only at surface As temperature increases, evaporation increases l. At some point, evaporation begins to occur throughout the liquid instead of just at the surface: boiling! 30

Vapor pressure & boiling l l l Vapor bubbles form throughout liquid Bubbles rise to surface, burst, release vapor All energy is used to convert liquid to vapor, so temperature remains constant while liquid boils 31

Boiling point l l l Boiling begins when the liquid’s vapor pressure matches the external pressure of the atmosphere The temperature at which this occurs is the boiling point When the external atmospheric pressure = 1 atm, the boiling point is called the normal boiling point 32

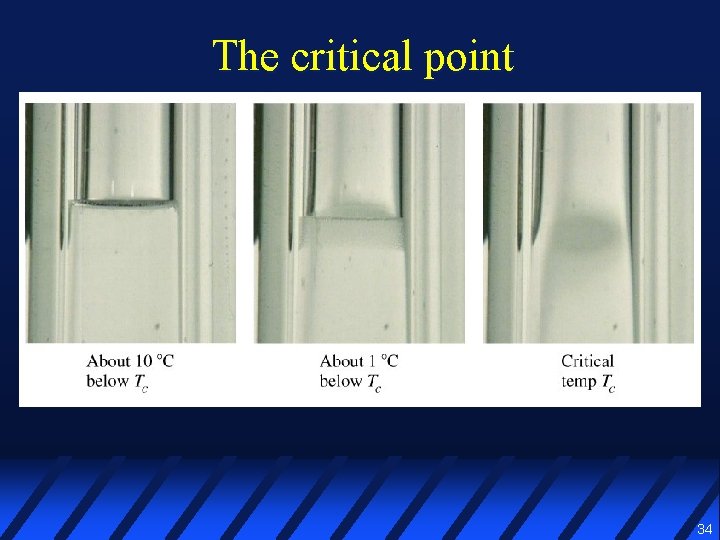
The critical point l Liquid heated in a rigid sealed container does not boil t t t l Vapor pressure and vapor density increase Liquid density decreases Vapor & liquid densities become equal & meniscus disappears This point is called the critical point 33

The critical point 34

Vapor pressure and temperature l Clausius-Clapeyron equation shows relationship between vapor pressure and temperature 35

Clausius-Clapeyron equation P (vapor pressure) can be in any unit l R must be 8. 3145 J/mol K l ∆Hvaporization is usually given in k. J/mol but must be converted to J/mol to agree with R l T is in Kelvins (duh) l 36

Example l The vapor pressure of methanol is 100 mm Hg at 21. 2 °C. What is its vapor pressure at 25. 0 °C? ∆Hvap for methanol is 38. 0 k. J/mol. 37

Example l The normal boiling point of isooctane is 99. 2 °C and its ∆Hvap is 35. 76 k. J/mol. What is the vapor pressure of isooctane at 25. 0 °C? 38

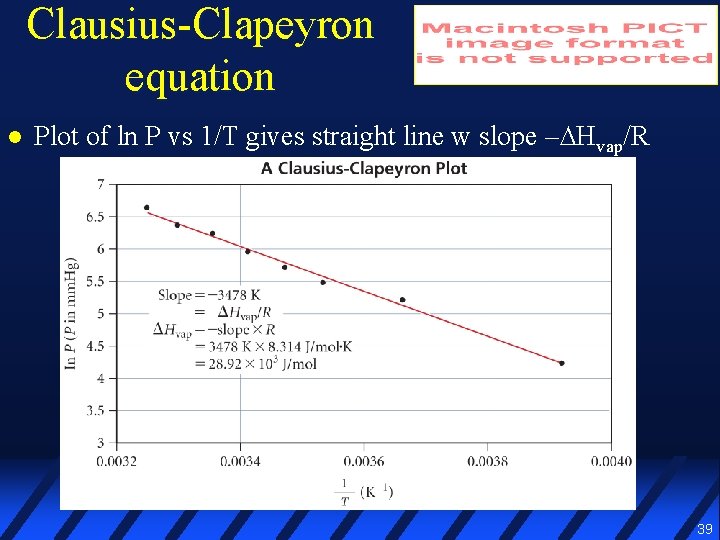
Clausius-Clapeyron equation l Plot of ln P vs 1/T gives straight line w slope –∆Hvap/R 39

Changes of state l Liquid ↔ gas t l Solid ↔ liquid t l Vaporization/boiling and condensation Melting (fusion) and freezing Solid ↔ gas t Sublimation and deposition 40

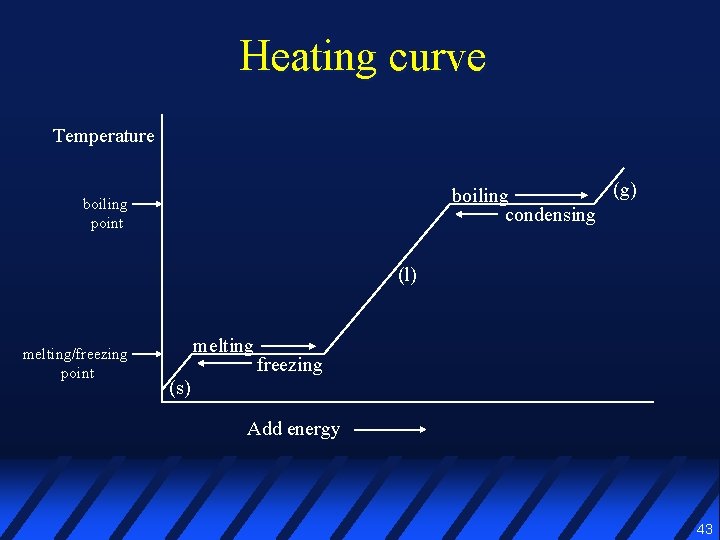
Heating curve Temperature Add energy 42

Heating curve Temperature (g) boiling condensing boiling point (l) melting/freezing point melting freezing (s) Add energy 43

44

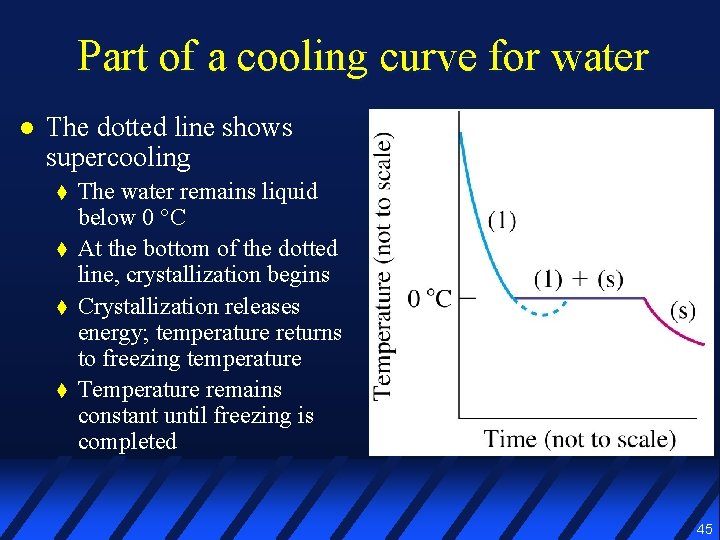
Part of a cooling curve for water l The dotted line shows supercooling t t The water remains liquid below 0 °C At the bottom of the dotted line, crystallization begins Crystallization releases energy; temperature returns to freezing temperature Temperature remains constant until freezing is completed 45

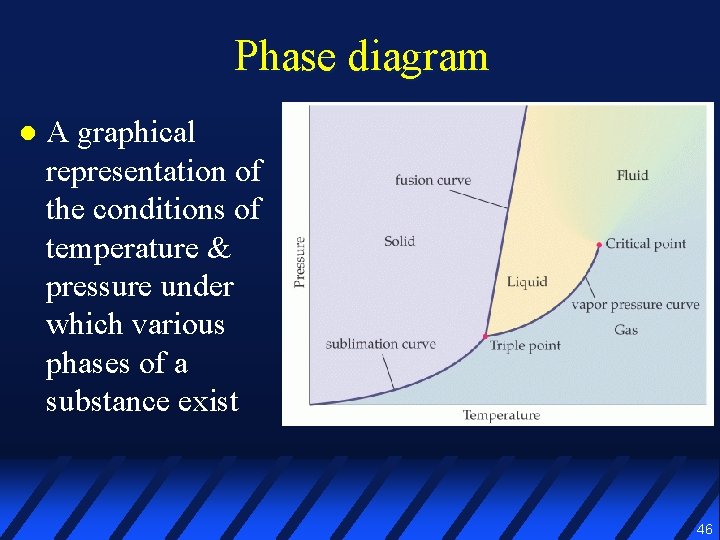
Phase diagram l A graphical representation of the conditions of temperature & pressure under which various phases of a substance exist 46

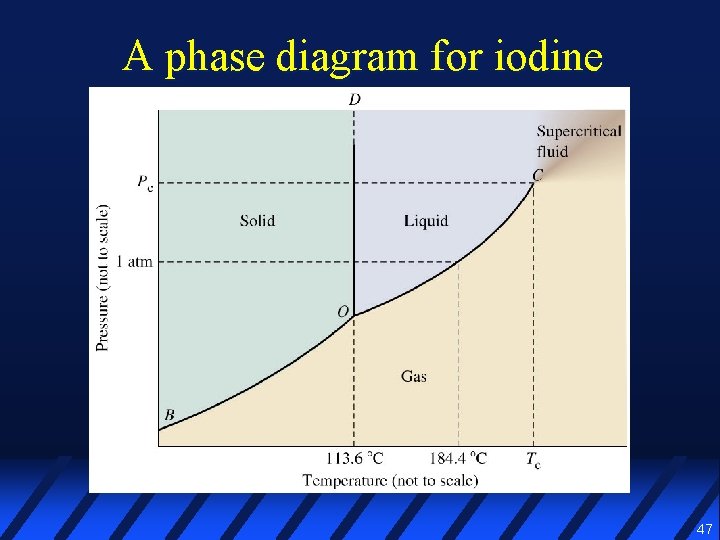
A phase diagram for iodine 47

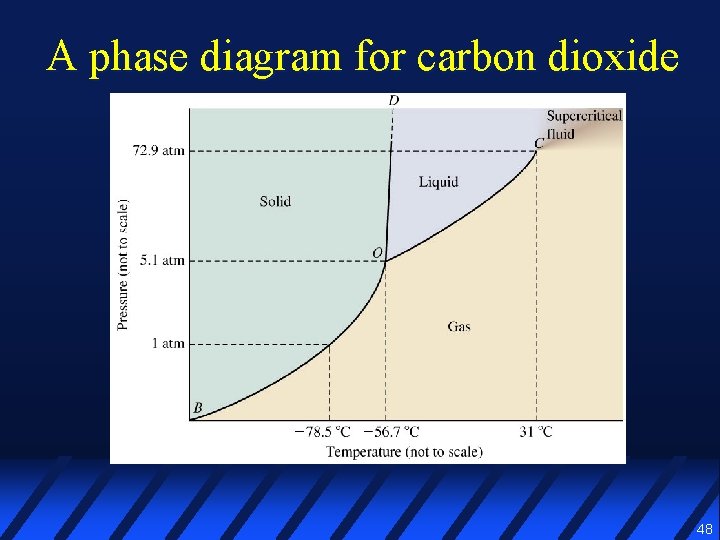
A phase diagram for carbon dioxide 48

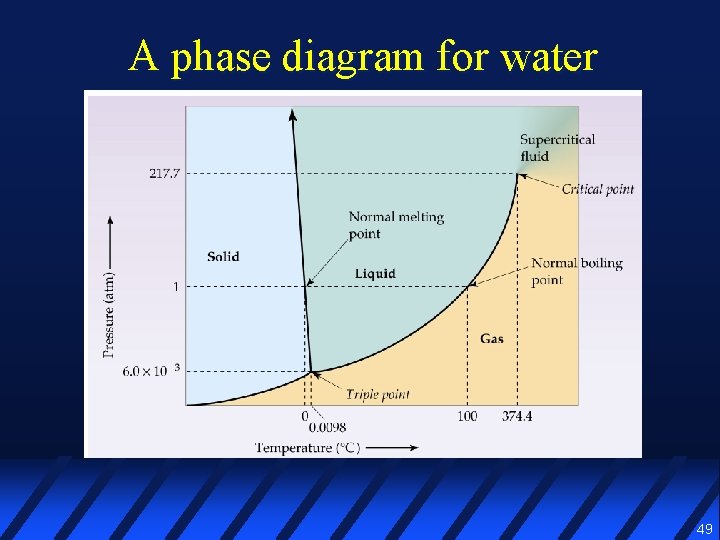
A phase diagram for water 49

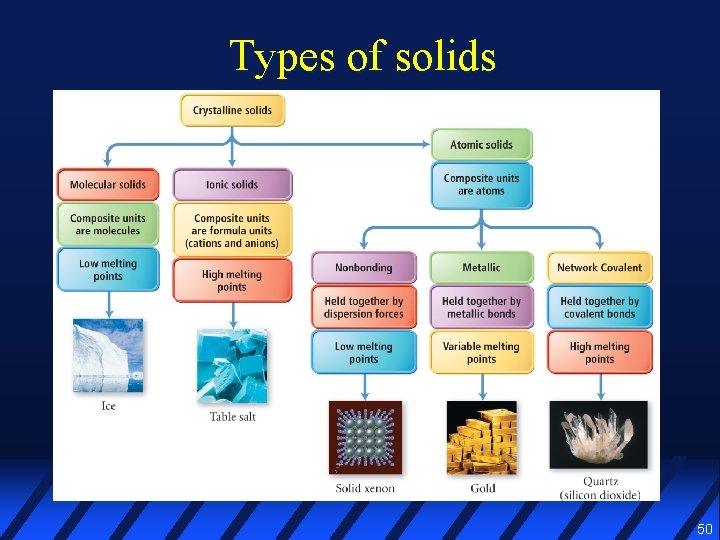
Types of solids 50

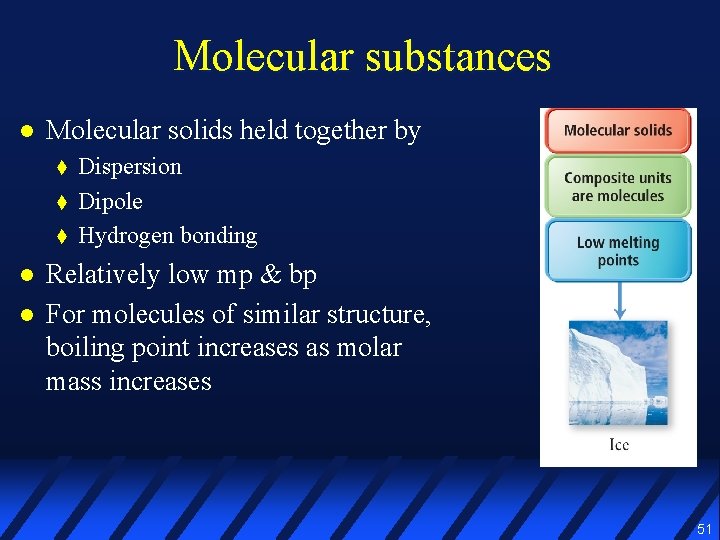
Molecular substances l Molecular solids held together by t t t l l Dispersion Dipole Hydrogen bonding Relatively low mp & bp For molecules of similar structure, boiling point increases as molar mass increases 51

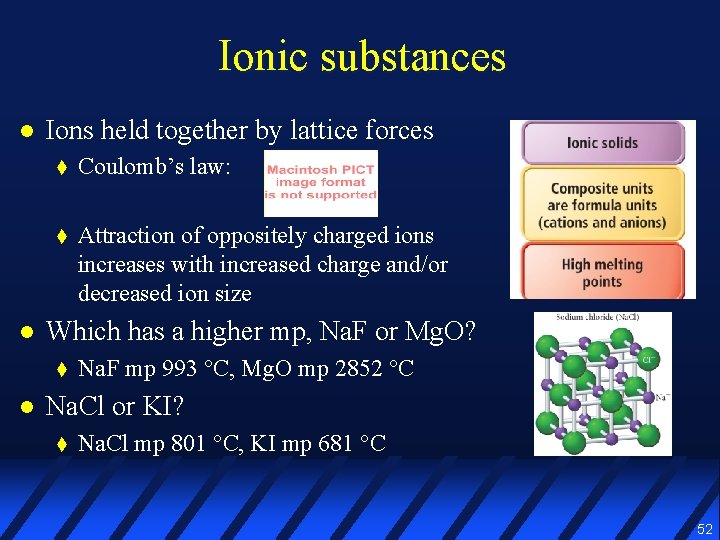
Ionic substances l l Ions held together by lattice forces t Coulomb’s law: t Attraction of oppositely charged ions increases with increased charge and/or decreased ion size Which has a higher mp, Na. F or Mg. O? t l Na. F mp 993 °C, Mg. O mp 2852 °C Na. Cl or KI? t Na. Cl mp 801 °C, KI mp 681 °C 52

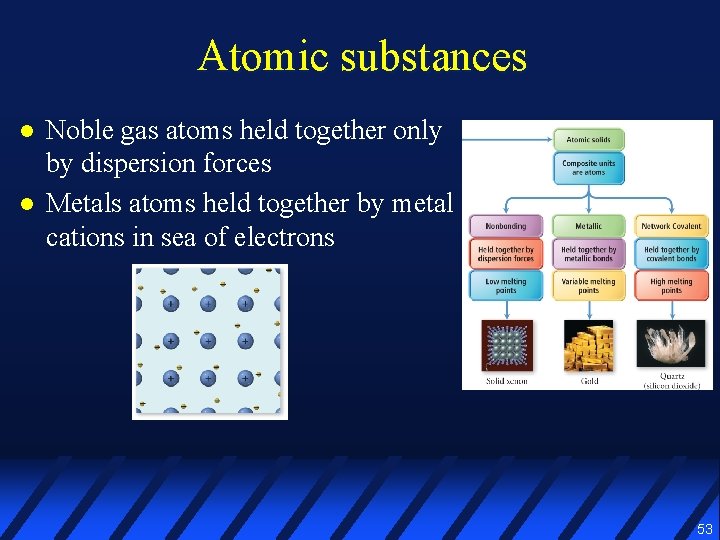
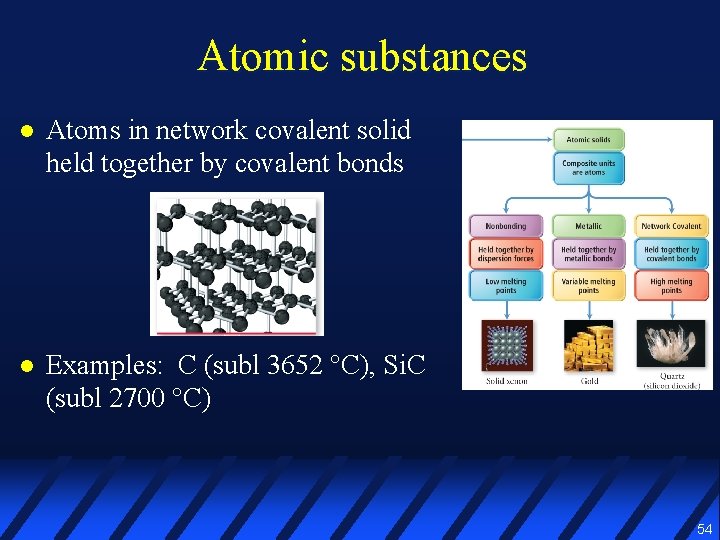
Atomic substances l l Noble gas atoms held together only by dispersion forces Metals atoms held together by metal cations in sea of electrons 53

Atomic substances l Atoms in network covalent solid held together by covalent bonds l Examples: C (subl 3652 °C), Si. C (subl 2700 °C) 54