Liquids Forces Between Liquid Molecules and their Effect

Liquids Forces Between Liquid Molecules and their Effect on the Properties of Liquids

Types of Forces n n Intramolecular-forces within a moleculebonding types examples: ionic and covalent Intermolecular-forces between molecules examples: ionic, dispersion, dipole-dipole, and hydrogen bonding

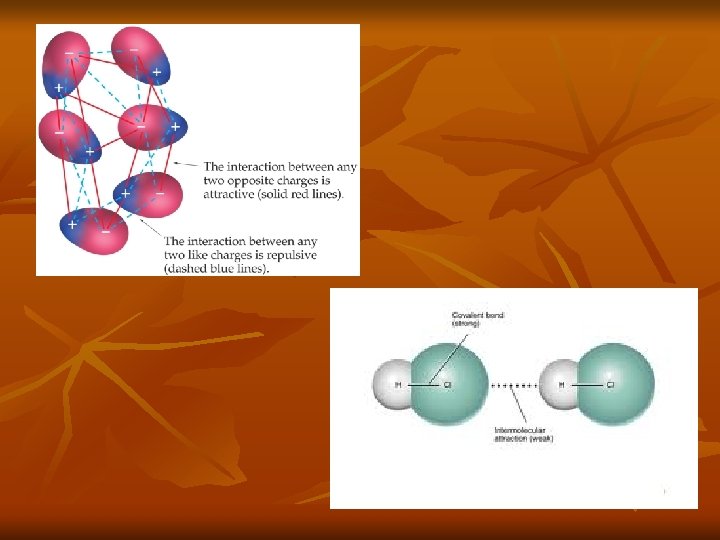
Ionic Forces n n Exist between “molecules” that contain ionic bonds. Result from the electrostatic charges within the compound. Opposite charges attract. Strongest type of intermolecular force.
London Dispersion Forces n n n Exist between noble gas atoms and nonpolar molecules Weakest Temporary-as electrons move around the nucleus, a momentary asymmetrical distribution of electrons can occur (provides a temporary dipole)

London Dispersion Forces n The instantaneous dipole can induce a similar dipole in a neighboring atom

London Dispersion Forces (continued) n n Large atoms with many electrons have stronger London Dispersion Forces Larger nonpolar molecules have stronger LD forces

Which has the stronger LD force? Cl 2 Br 2 1. 2. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20
Which has the stronger LD force? CH 4 C 2 H 6 C 4 H 10 1. 2. 3. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Which has the stronger LD force He Ne Ar Xe 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Dipole-Dipole Forces n n n Exist between polar molecules Permanent Stronger than London Dispersion Forces (1% as strong as ionic/covalent bonds) Created through an unequal sharing of electrons which results in a partial negative and positive charge Positive end of one molecule attracts the negative end of another molecule
Which of the following molecules have dipole-dipole forces present? NH 3 CH 4 Both 1. 2. 3. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Which of the following molecules have dipole-dipole forces? CO 2 H 20 Both 1. 2. 3. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20
Which of the following molecules have dipole-dipole forces present? SF 4 SF 6 Both 1. 2. 3. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

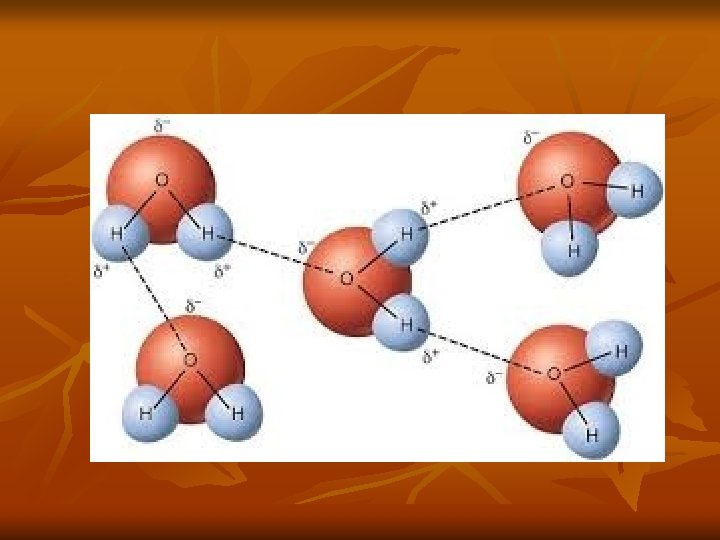
Hydrogen Bonding n n n A specific type of dipole-dipole force Occurs when hydrogen atoms are bonded to highly electronegative atoms such as N, O, or F. Created because of high levels of polarity and small size of hydrogen atoms (especially strong dipole-dipole attraction occurs)
Which of the following compounds has hydrogen bonding present? CH 4 CH 3 OH Both 1. 2. 3. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Which of the following compounds has hydrogen bonding present? H 2 S H 2 O Both 1. 2. 3. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Which of the following compounds has hydrogen bonding present? HCl HF Both 1. 2. 3. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Review of Intermolecular Forces n Identify the most important type of interparticle forces present in each of the following substances. Ar HCl HF Ca. Cl 2 CH 4 CO Na. NO 3

What is the most importance type of interparticle force present in Ar? Ionic London Dispersion Dipole-Dipole Hydrogen Bonding 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20

What is the most important type of interparticle force present in HCl? Ionic London Dispersion Dipole-Dipole Hydrogen Bonding 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20

What is the most important type of interparticle force present in HF? Ionic London Dispersion Dipole-Dipole Hydrogen Bonding 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20

What is the most important type of interparticle force present in Ca. Cl 2? Ionic London Dispersion Dipole-Dipole Hydrogen Bonding 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20

What is the most important type of interparticle force present in CH 4? Ionic London Dispersion Dipole-Dipole Hydrogen Bonding 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20

What is the most important type of interparticle force present in CO? Ionic London Dispersion Dipole-Dipole Hydrogen Bonding 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20

What is the most important type of interparticle force present in Na. NO 3? Ionic London Dispersion Dipole-Dipole Hydrogen Bonding 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 10 13 14 15 16 17 18 19 20
Arrange the following molecules in order of decreasing intermolecular interaction: SO 2, Cl 2, CH 3 OH, CH 3 NH 2 CH 3 OH > CH 3 NH 2>SO 2>Cl 2>SO 2>CH 3 OH>CH 3 NH 2 SO 2>CH 3 NH 2>CH 3 OH>Cl 2 CH 3 NH 2>CH 3 OH>SO 2>Cl 2 1. 2. 3. 4. 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 10 16 17 18 19 20

Properties of Liquids

Surface Tension n Resistance of a liquid to increase in surface area Measure of the inward forces Liquids with high intermolecular forces have high surface tensions

Viscosity n n A resistance to flow Strong intermolecular forces result in high viscosity Large molecules also have high viscosity due to greater LD forces As temperature increases, viscosity decreases

Vapor Pressure n n n Pressure of vapor above the surface of a liquid Caused when the molecules on the surface break away and go into the gas phase In order to break away, the molecules must possess a minimum amount of kinetic energy High intermolecular forces result in low vapor pressures Volatile liquids are liquids that evaporate rapidly resulting in high vapor pressures

Vapor Pressure (continued) n n Substances with weak intermolecular forces tend to be volatile As temperature increases, vapor pressure increases (more molecules possess the minimum kinetic energy and the rate of evaporation increases)

Calculating Vapor Pressure n The equation used to calculate the vapor pressure is: ln (Pvap. T 1) = ∆Hvap(1 - 1) (Pvap. T 2) = R (T 2 - T 1) ∆H is the heat of vaporization in J/mol, T is Kelvin temperature, R is 8. 314 J/Kmol, and Pvap is vapor pressure

Practice Problem n In Breckenridge , Colorado, the typical atmospheric pressure is 520. torr. What is the boiling point of water (∆Hvap = 40. 7 k. J/mol) in Breckenridge?

Capillary Action n n Spontaneous rising of a liquid in a narrow tube Caused by cohesive and adhesive forces cohesive forces-intermolecular forces among liquid molecules adhesive forces- force between the liquid molecules and the container; occur when the container is made of a polar substance

Example of Capillary Action n n When water is placed in a glass graduated cylinder, the meniscus is concave adhesive forces > cohesive forces (glass is polar) When mercury is placed in the same graduated cylinder, the meniscus is convex cohesive forces > adhesive forces (mercury contains only dispersion forces-nonpolar)

Melting Point and Boiling Points n n n Melting point- temperature at which the solid and liquid have the same vapor pressure Boiling point-temperature at which the vapor pressure is equal to 1 atm (atmospheric pressure) Melting and boiling points are higher when the intermolecular forces are stronger

Which of the following has the highest boiling point? H 2 O HF HI HBr 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Which of the following liquids will be the most viscous? C 3 H 8 C 6 H 6 CH 4 C 2 H 6 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Arrange the following liquids, A, B, C, with vapor pressures at room temperature of 88, 680, and 155, respectively, in order of decreasing boiling points. B>C>A A>B>C A>C>B C>A>B 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20
Knowing that solutes with a certain polarity are best dissolved in solutions with similar polarity, which of the following solvents would be optimal for the solvation of CH 3 COOH? CH 4 CH 3 OH C 2 H 6 C 6 H 6 1. 2. 3. 4. 10 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20
- Slides: 42