Liquids Chemistry Mrs Coyle Liquids n n Intermolecular
















- Slides: 19

Liquids Chemistry Mrs. Coyle

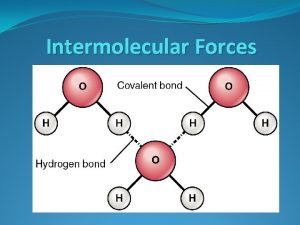
Liquids n n Intermolecular attractions hold molecules of liquids together. Incompressible, definite volume. More dense than gases. Molecules have kinetic energy.

Vaporization n Change of phase from a liquid to a gas

Evaporation n Vaporization occurring at the surface of the liquid.

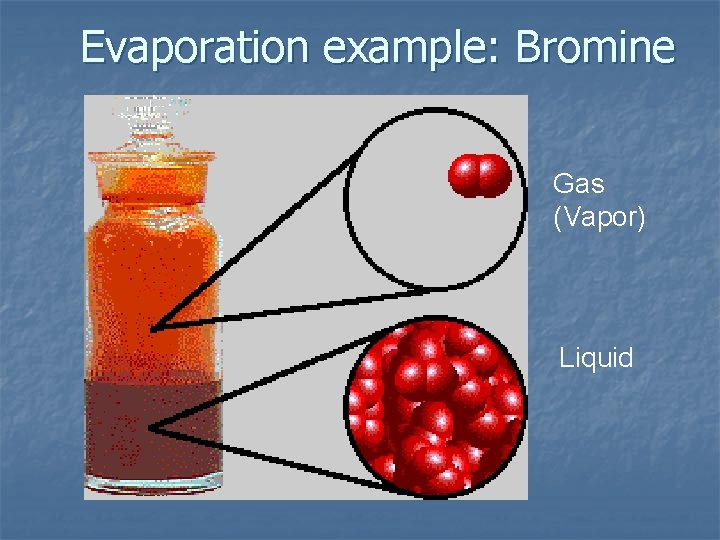
Evaporation example: Bromine Gas (Vapor) Liquid

What happens to the rate of evaporation as the liquid is heated? n The rate of evaporation increases.

Evaporation is a cooling process n n Why? The particles with the higher kinetic energy escape the liquid first.

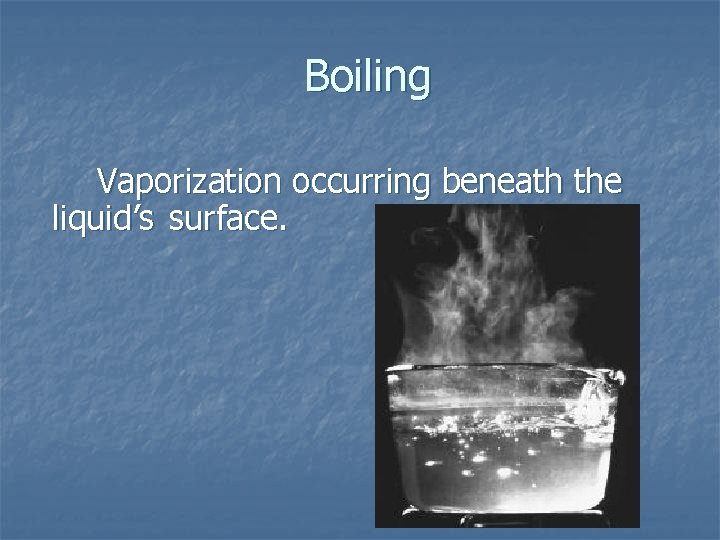
Boiling Vaporization occurring beneath the liquid’s surface.


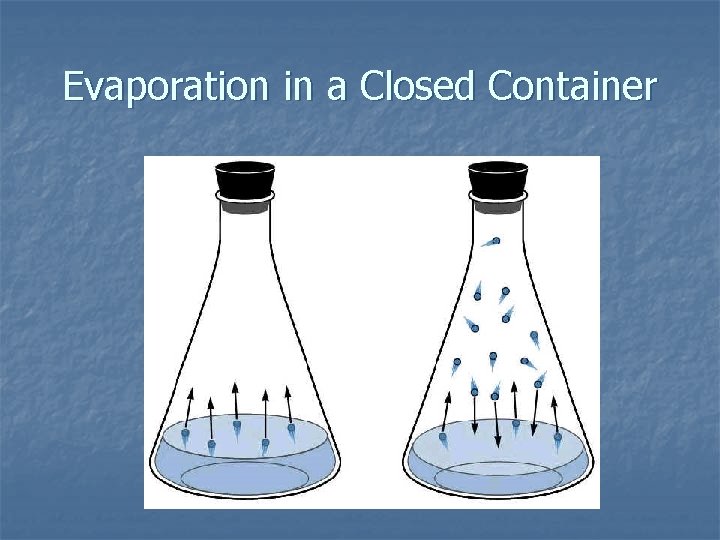
Evaporation in a Closed Container

Evaporation in a Closed Container Liquid Vapor When the rate of evaporation equals the rate of condensation the system is in Equilibrium

Vapor Pressure n The pressure of the gas that has evaporated above a liquid.

The vapor pressure increases with increasing temperature. Why? Because the kinetic energy of the liquid molecules increases and more leave the liquid and collide with the walls of the container.

Boiling Point n The temperature at which a liquid boils.

Boiling and External Pressure n When the external pressure is greater than the vapor pressure of the bubbles in the liquid the bubbles cannot come to the surface. Boiling does not happen.


Boiling and External Pressure n When the external pressure is equal to the vapor pressure of the bubbles in the liquid, boiling occurs.

Why does water boil at a lower temperature at high altitudes? n Because the external pressure is lower.

Normal Boiling Point n The boiling point at 1 atm or 101. 3 k. Pa
 Ap chemistry intermolecular forces
Ap chemistry intermolecular forces Mrs. darling was ___________ of mrs. s.
Mrs. darling was ___________ of mrs. s. They are mrs garcia and mrs castro
They are mrs garcia and mrs castro They are mrs garcia and mrs castro
They are mrs garcia and mrs castro Synthesis of ionic liquids ppt
Synthesis of ionic liquids ppt Isabelle degrange
Isabelle degrange Dr christine coyle
Dr christine coyle Aaron coyle
Aaron coyle Coyle and castello method
Coyle and castello method Coyle
Coyle Caitlin coyle
Caitlin coyle Coyle health and wellbeing
Coyle health and wellbeing Metodo coyle
Metodo coyle Do coyle clil
Do coyle clil Goethe mindmap
Goethe mindmap Gauss law ap physics c
Gauss law ap physics c Coyle method
Coyle method Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Hco2h intermolecular forces
Hco2h intermolecular forces