LiquidLiquid Extraction CHOICE OF SOLVENT Choosing the best
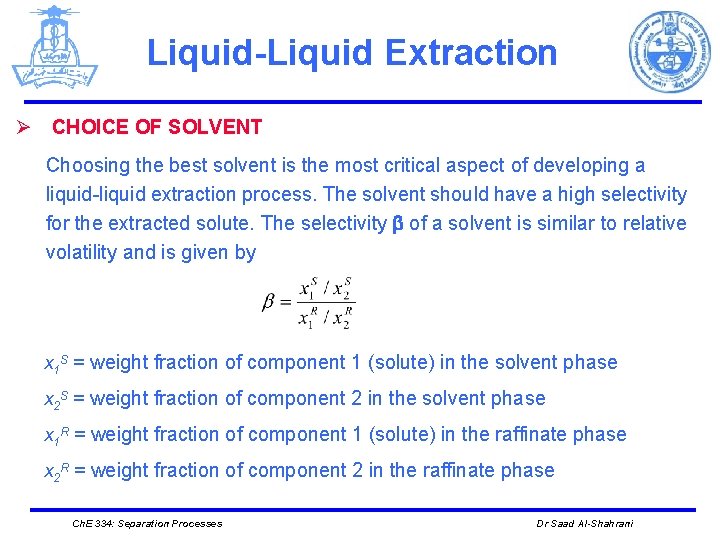
Liquid-Liquid Extraction Ø CHOICE OF SOLVENT Choosing the best solvent is the most critical aspect of developing a liquid-liquid extraction process. The solvent should have a high selectivity for the extracted solute. The selectivity of a solvent is similar to relative volatility and is given by x 1 S = weight fraction of component 1 (solute) in the solvent phase x 2 S = weight fraction of component 2 in the solvent phase x 1 R = weight fraction of component 1 (solute) in the raffinate phase x 2 R = weight fraction of component 2 in the raffinate phase Ch. E 334: Separation Processes Dr Saad Al-Shahrani
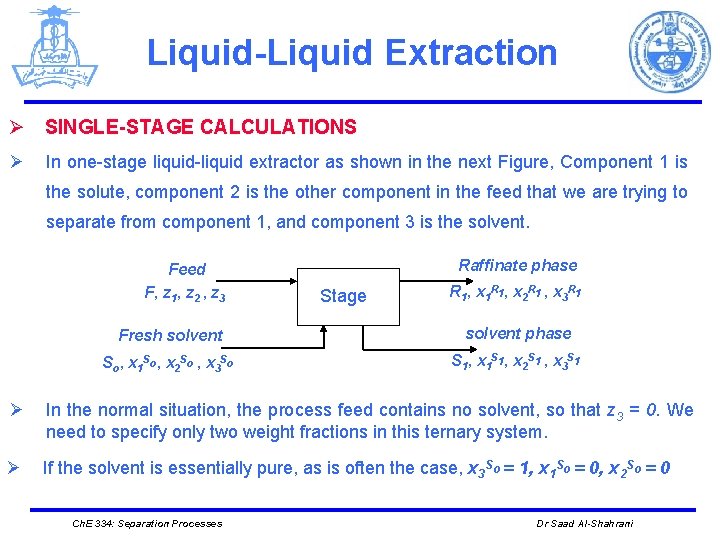
Liquid-Liquid Extraction Ø SINGLE-STAGE CALCULATIONS Ø In one-stage liquid-liquid extractor as shown in the next Figure, Component 1 is the solute, component 2 is the other component in the feed that we are trying to separate from component 1, and component 3 is the solvent. Feed F, z 1, z 2 , z 3 Raffinate phase Stage R 1 , x 1 R 1 , x 2 R 1 , x 3 R 1 Fresh solvent phase So, x 1 So, x 2 So , x 3 So S 1, x 1 S 1, x 2 S 1 , x 3 S 1 Ø In the normal situation, the process feed contains no solvent, so that z 3 = 0. We need to specify only two weight fractions in this ternary system. Ø If the solvent is essentially pure, as is often the case, x 3 So = 1, x 1 So = 0, x 2 So = 0 Ch. E 334: Separation Processes Dr Saad Al-Shahrani
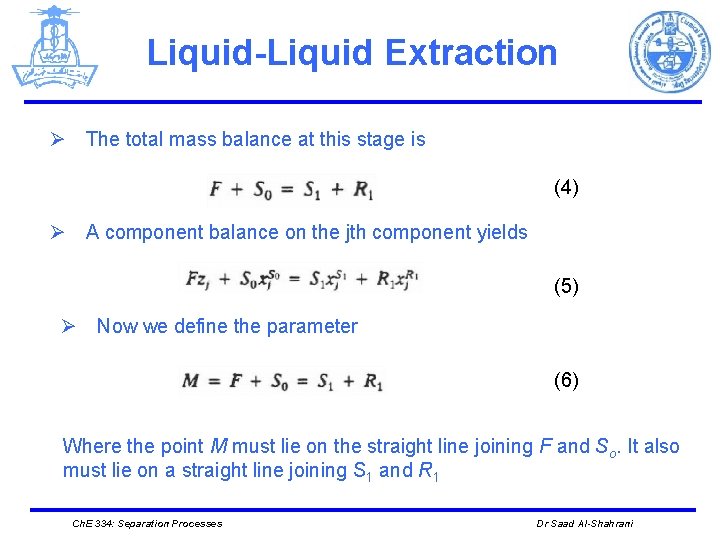
Liquid-Liquid Extraction Ø The total mass balance at this stage is (4) Ø A component balance on the jth component yields (5) Ø Now we define the parameter (6) Where the point M must lie on the straight line joining F and So. It also must lie on a straight line joining S 1 and R 1 Ch. E 334: Separation Processes Dr Saad Al-Shahrani
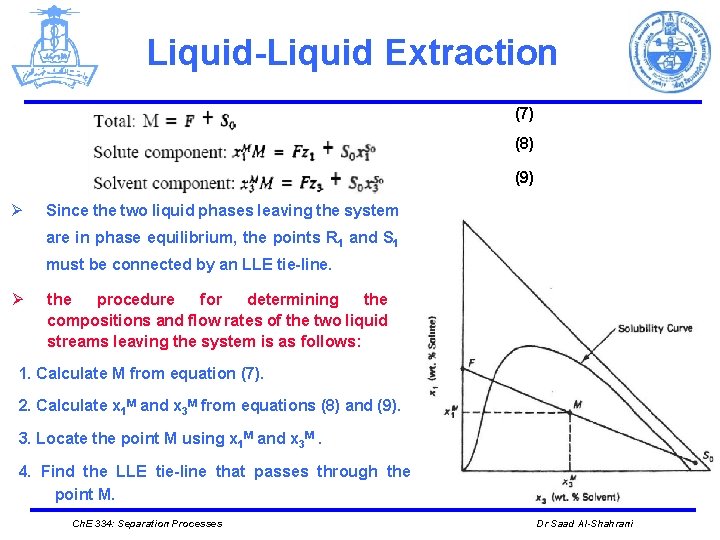
Liquid-Liquid Extraction (7) (8) (9) Ø Since the two liquid phases leaving the system are in phase equilibrium, the points R 1 and S 1 must be connected by an LLE tie-line. Ø the procedure for determining the compositions and flow rates of the two liquid streams leaving the system is as follows: 1. Calculate M from equation (7). 2. Calculate x 1 M and x 3 M from equations (8) and (9). 3. Locate the point M using x 1 M and x 3 M. 4. Find the LLE tie-line that passes through the point M. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
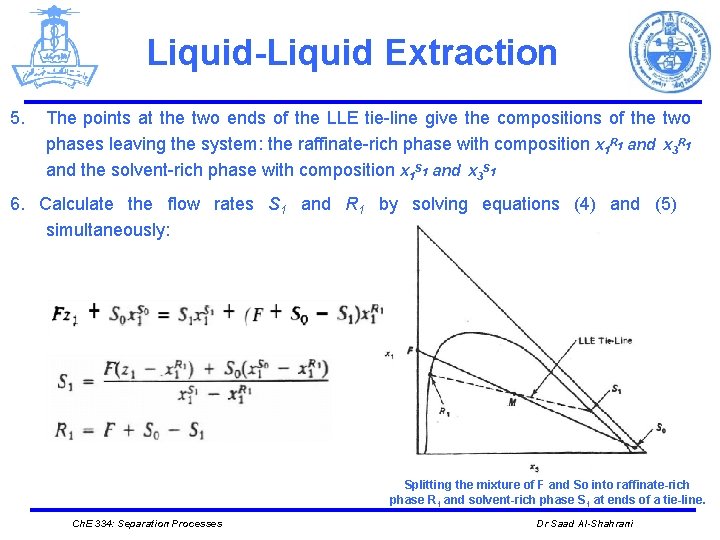
Liquid-Liquid Extraction 5. The points at the two ends of the LLE tie-line give the compositions of the two phases leaving the system: the raffinate-rich phase with composition x 1 R 1 and x 3 R 1 and the solvent-rich phase with composition x 1 S 1 and x 3 S 1 6. Calculate the flow rates S 1 and R 1 by solving equations (4) and (5) simultaneously: Splitting the mixture of F and So into raffinate-rich phase R 1 and solvent-rich phase S 1 at ends of a tie-line. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
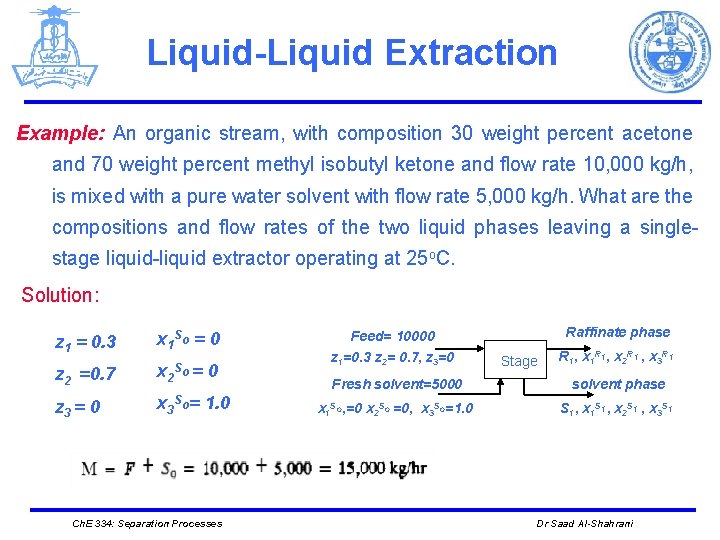
Liquid-Liquid Extraction Example: An organic stream, with composition 30 weight percent acetone and 70 weight percent methyl isobutyl ketone and flow rate 10, 000 kg/h, is mixed with a pure water solvent with flow rate 5, 000 kg/h. What are the compositions and flow rates of the two liquid phases leaving a singlestage liquid-liquid extractor operating at 25 o. C. Solution: z 1 = 0. 3 x 1 So = 0 z 2 =0. 7 x 2 So = z 3 = 0 x 3 So= 1. 0 0 Ch. E 334: Separation Processes Feed= 10000 z 1=0. 3 z 2= 0. 7, z 3=0 Raffinate phase Stage R 1, x 1 R 1, x 2 R 1 , x 3 R 1 Fresh solvent=5000 solvent phase x 1 So, =0 x 2 So =0, x 3 So=1. 0 S 1, x 1 S 1, x 2 S 1 , x 3 S 1 Dr Saad Al-Shahrani
Liquid-Liquid Extraction Ch. E 334: Separation Processes Dr Saad Al-Shahrani
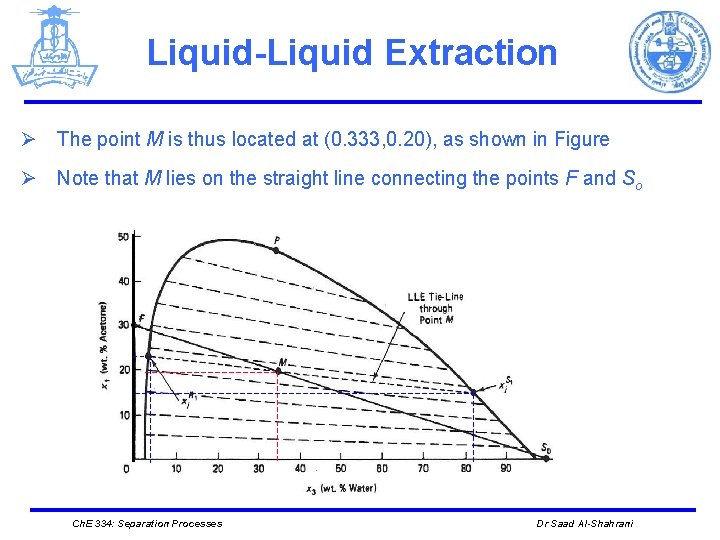
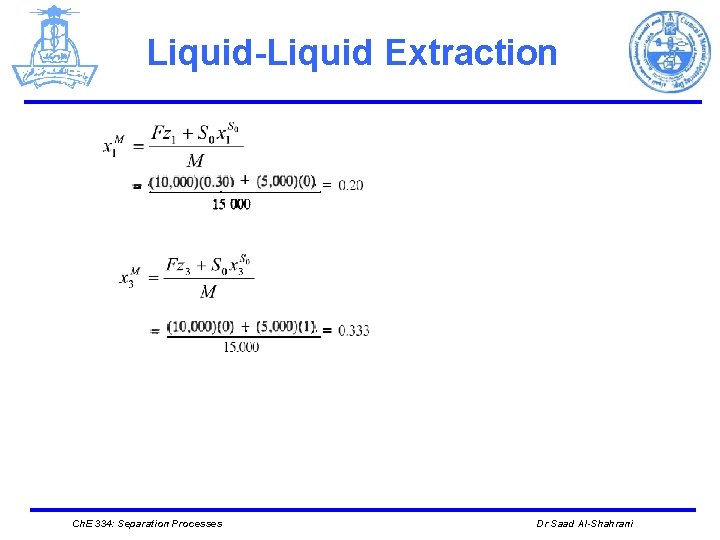
Liquid-Liquid Extraction Ø The point M is thus located at (0. 333, 0. 20), as shown in Figure Ø Note that M lies on the straight line connecting the points F and So Ch. E 334: Separation Processes Dr Saad Al-Shahrani
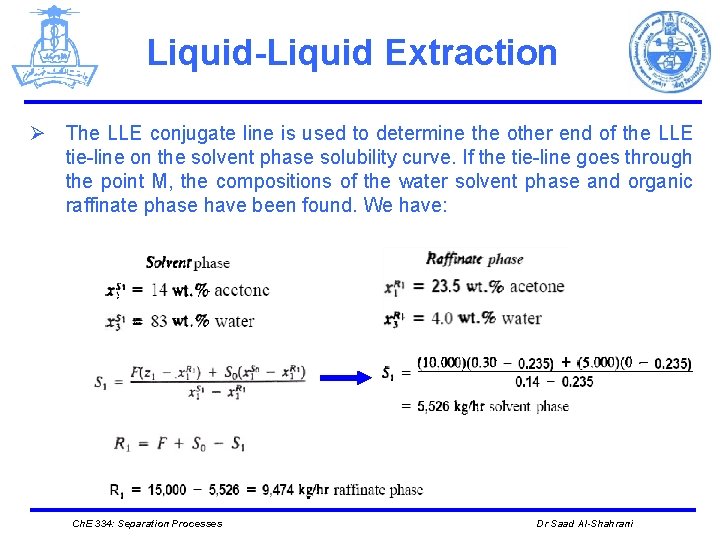
Liquid-Liquid Extraction Ø The LLE conjugate line is used to determine the other end of the LLE tie-line on the solvent phase solubility curve. If the tie-line goes through the point M, the compositions of the water solvent phase and organic raffinate phase have been found. We have: Ch. E 334: Separation Processes Dr Saad Al-Shahrani
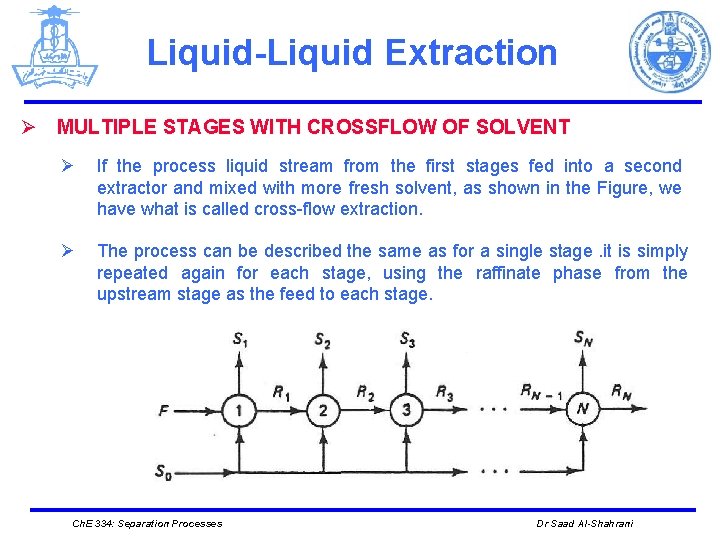
Liquid-Liquid Extraction Ø MULTIPLE STAGES WITH CROSSFLOW OF SOLVENT Ø If the process liquid stream from the first stages fed into a second extractor and mixed with more fresh solvent, as shown in the Figure, we have what is called cross-flow extraction. Ø The process can be described the same as for a single stage. it is simply repeated again for each stage, using the raffinate phase from the upstream stage as the feed to each stage. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
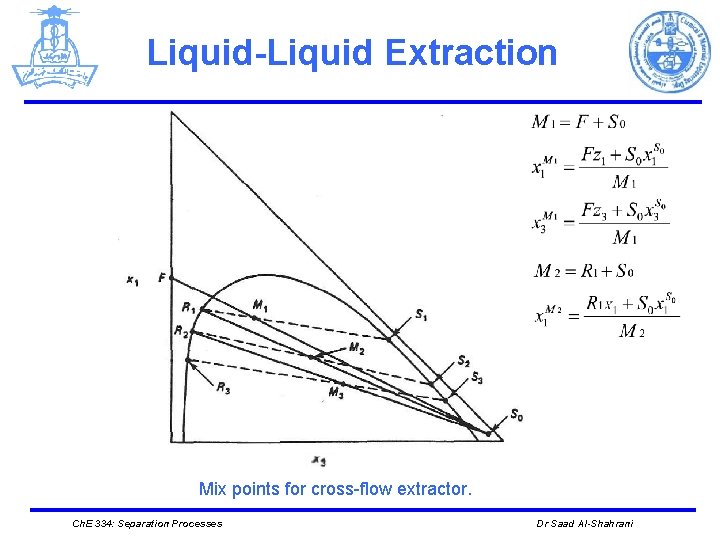
Liquid-Liquid Extraction Mix points for cross-flow extractor. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
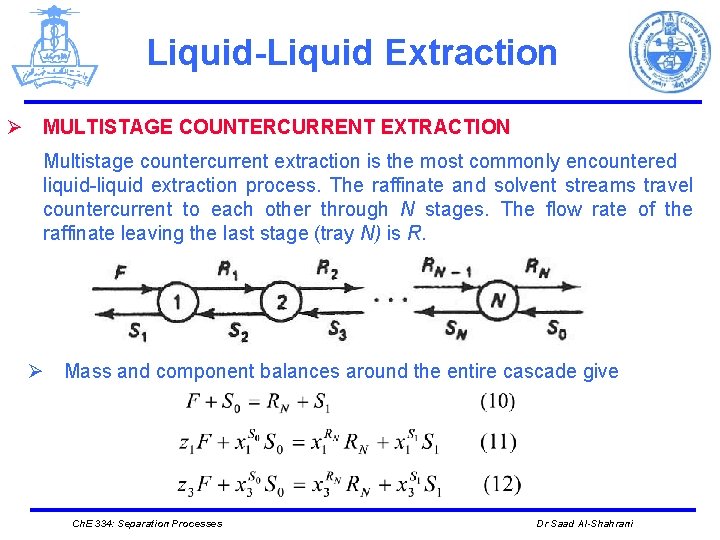
Liquid-Liquid Extraction Ø MULTISTAGE COUNTERCURRENT EXTRACTION Multistage countercurrent extraction is the most commonly encountered liquid-liquid extraction process. The raffinate and solvent streams travel countercurrent to each other through N stages. The flow rate of the raffinate leaving the last stage (tray N) is R. Ø Mass and component balances around the entire cascade give Ch. E 334: Separation Processes Dr Saad Al-Shahrani
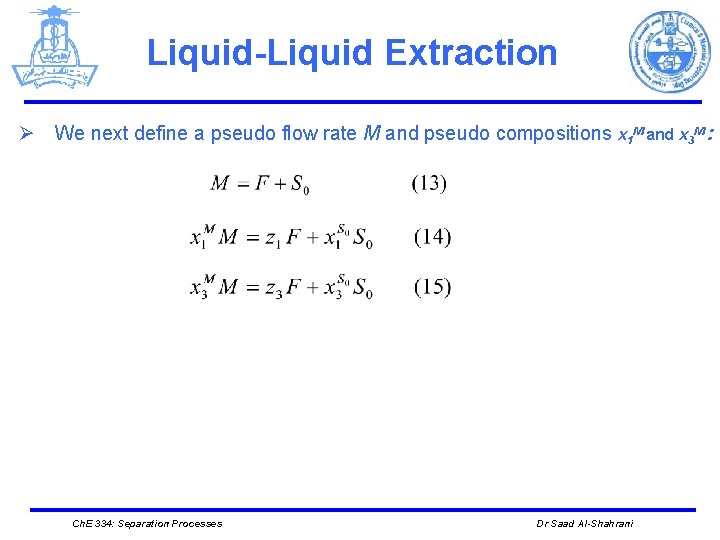
Liquid-Liquid Extraction Ø We next define a pseudo flow rate M and pseudo compositions x 1 M and x 3 M : Ch. E 334: Separation Processes Dr Saad Al-Shahrani
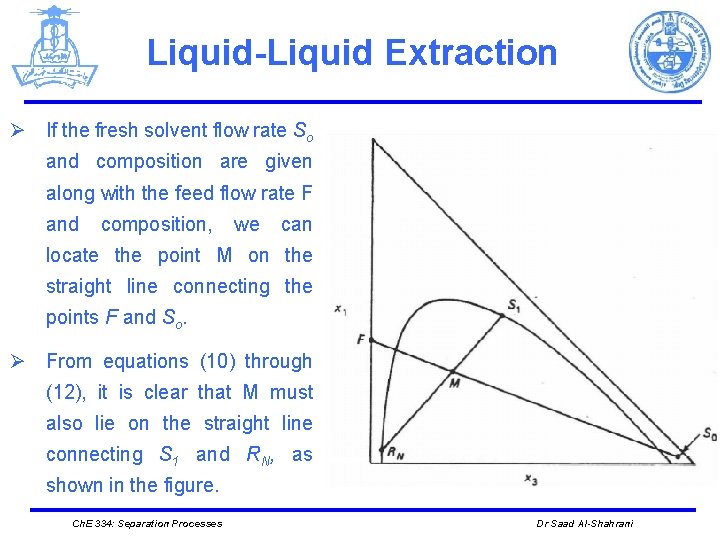
Liquid-Liquid Extraction Ø If the fresh solvent flow rate So and composition are given along with the feed flow rate F and composition, we can locate the point M on the straight line connecting the points F and So. Ø From equations (10) through (12), it is clear that M must also lie on the straight line connecting S 1 and RN, as shown in the figure. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
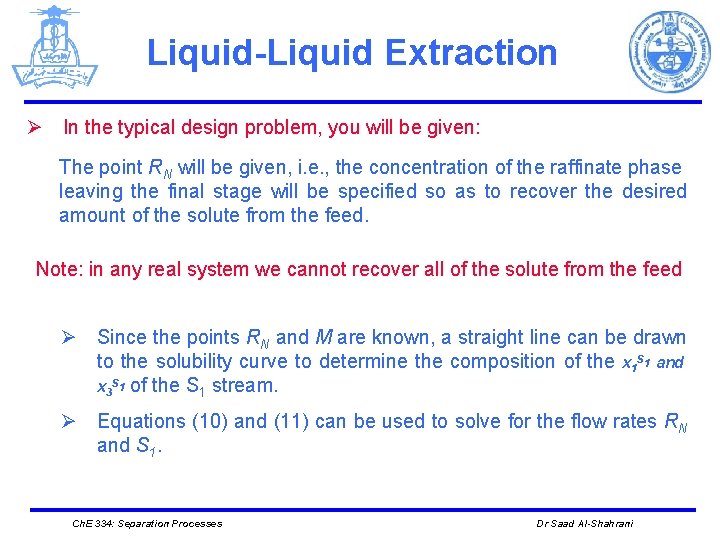
Liquid-Liquid Extraction Ø In the typical design problem, you will be given: The point RN will be given, i. e. , the concentration of the raffinate phase leaving the final stage will be specified so as to recover the desired amount of the solute from the feed. Note: in any real system we cannot recover all of the solute from the feed Ø Since the points RN and M are known, a straight line can be drawn to the solubility curve to determine the composition of the x 1 S 1 and x 3 S 1 of the S 1 stream. Ø Equations (10) and (11) can be used to solve for the flow rates RN and S 1. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
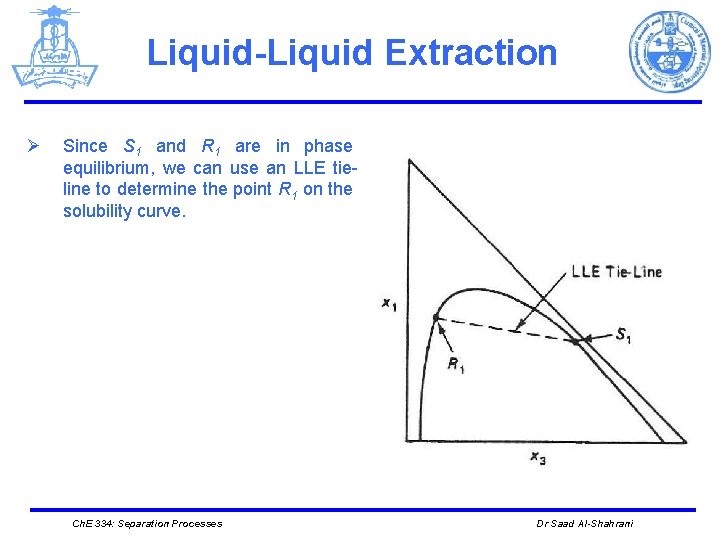
Liquid-Liquid Extraction Ø Since S 1 and R 1 are in phase equilibrium, we can use an LLE tieline to determine the point R 1 on the solubility curve. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
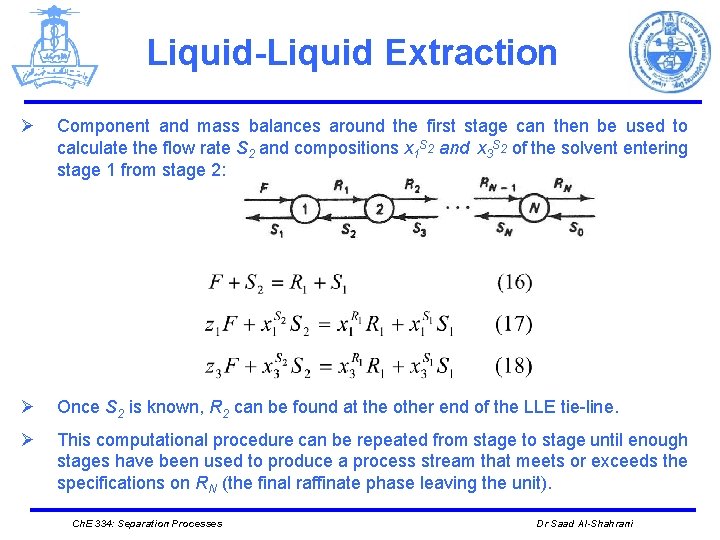
Liquid-Liquid Extraction Ø Component and mass balances around the first stage can then be used to calculate the flow rate S 2 and compositions x 1 S 2 and x 3 S 2 of the solvent entering stage 1 from stage 2: Ø Once S 2 is known, R 2 can be found at the other end of the LLE tie-line. Ø This computational procedure can be repeated from stage to stage until enough stages have been used to produce a process stream that meets or exceeds the specifications on RN (the final raffinate phase leaving the unit). Ch. E 334: Separation Processes Dr Saad Al-Shahrani
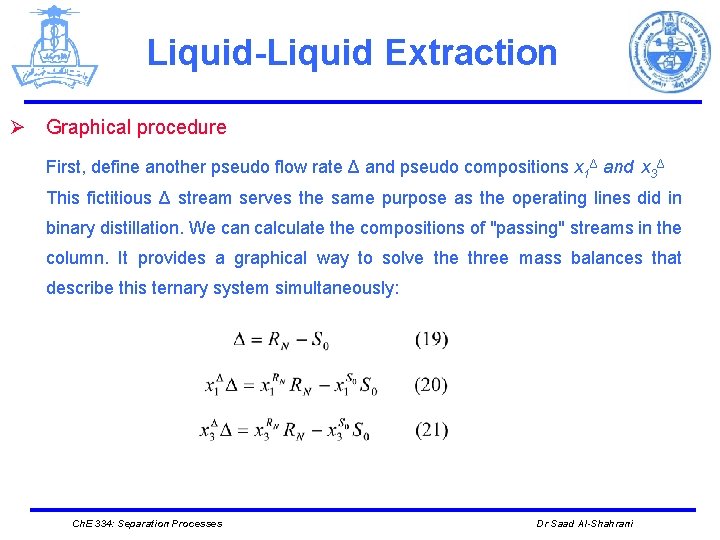
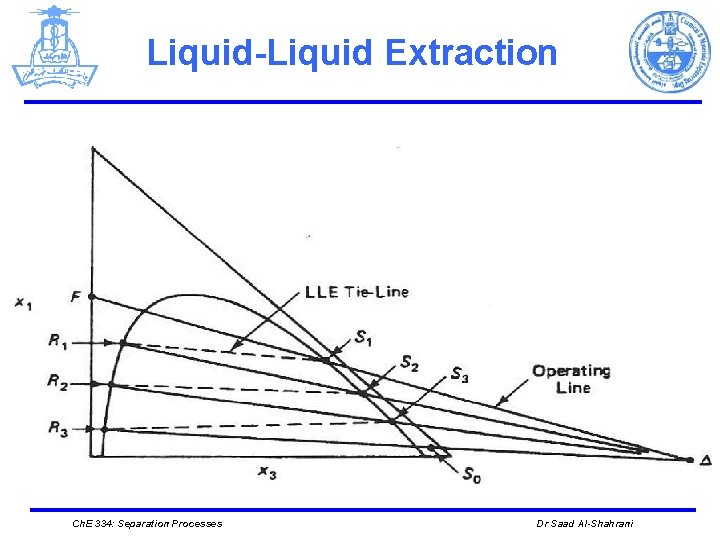
Liquid-Liquid Extraction Ø Graphical procedure First, define another pseudo flow rate Δ and pseudo compositions x 1Δ and x 3Δ This fictitious Δ stream serves the same purpose as the operating lines did in binary distillation. We can calculate the compositions of "passing" streams in the column. It provides a graphical way to solve three mass balances that describe this ternary system simultaneously: Ch. E 334: Separation Processes Dr Saad Al-Shahrani
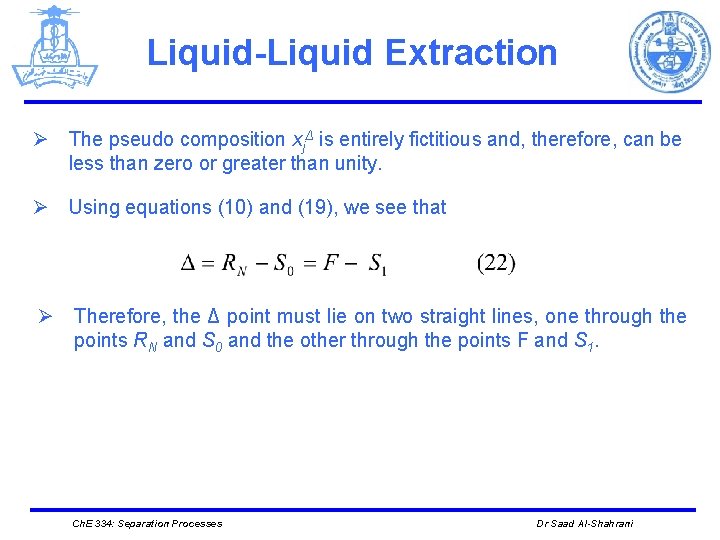
Liquid-Liquid Extraction Ø The pseudo composition xjΔ is entirely fictitious and, therefore, can be less than zero or greater than unity. Ø Using equations (10) and (19), we see that Ø Therefore, the Δ point must lie on two straight lines, one through the points RN and S 0 and the other through the points F and S 1. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Liquid-Liquid Extraction Ø Δ can lie either to the left or to the right of the phase diagram Ch. E 334: Separation Processes Dr Saad Al-Shahrani
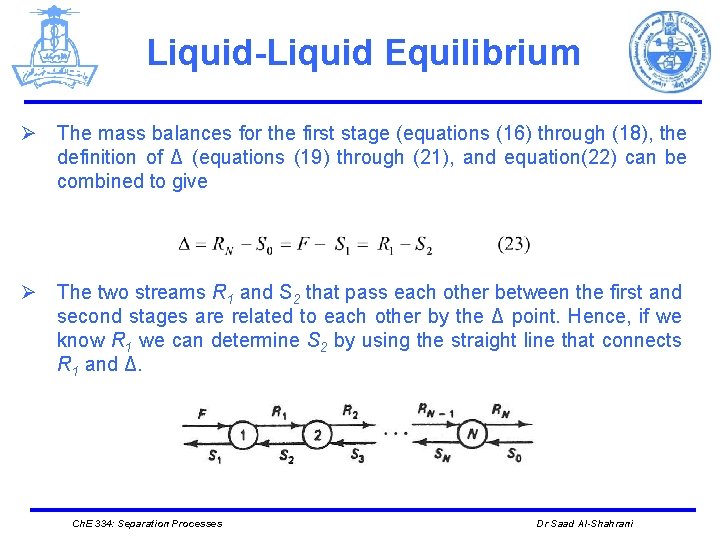
Liquid-Liquid Equilibrium Ø The mass balances for the first stage (equations (16) through (18), the definition of Δ (equations (19) through (21), and equation(22) can be combined to give Ø The two streams R 1 and S 2 that pass each other between the first and second stages are related to each other by the Δ point. Hence, if we know R 1 we can determine S 2 by using the straight line that connects R 1 and Δ. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
Liquid-Liquid Extraction Ch. E 334: Separation Processes Dr Saad Al-Shahrani
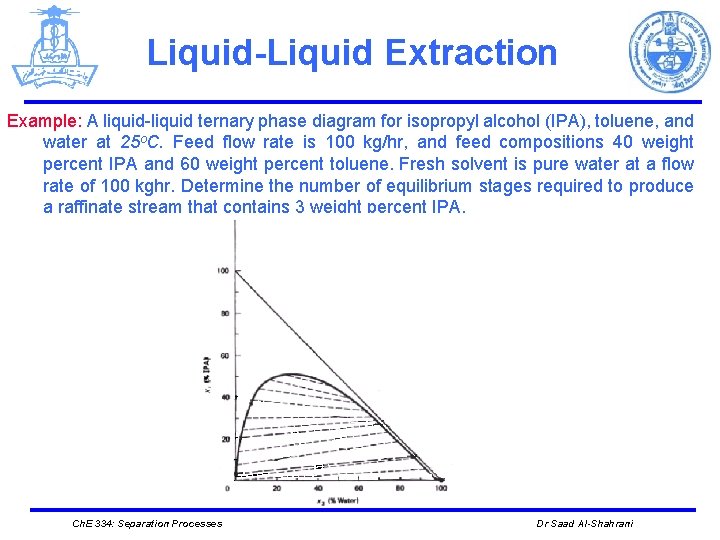
Liquid-Liquid Extraction Example: A liquid-liquid ternary phase diagram for isopropyl alcohol (IPA), toluene, and water at 25 o. C. Feed flow rate is 100 kg/hr, and feed compositions 40 weight percent IPA and 60 weight percent toluene. Fresh solvent is pure water at a flow rate of 100 kghr. Determine the number of equilibrium stages required to produce a raffinate stream that contains 3 weight percent IPA. Ch. E 334: Separation Processes Dr Saad Al-Shahrani
- Slides: 23