Liquid Fluoride Thorium Reactors without equations x An




















- Slides: 33

Liquid Fluoride Thorium Reactors without equations ∑ x< +-= - *± An overview of liquid-fueled liquid-cooled thermal liquid spectrum Thorium breeder reactors and why they matter. Rob Morse Science Engineer- Nuclear Option 2012 1

Outline § § § Motivations for Nuclear Power LFTR overview Nuclear Chemistry Myths Questions 2

Power makes a difference. LFTR wo Equations. Thorium power conference, Oct 2009 3

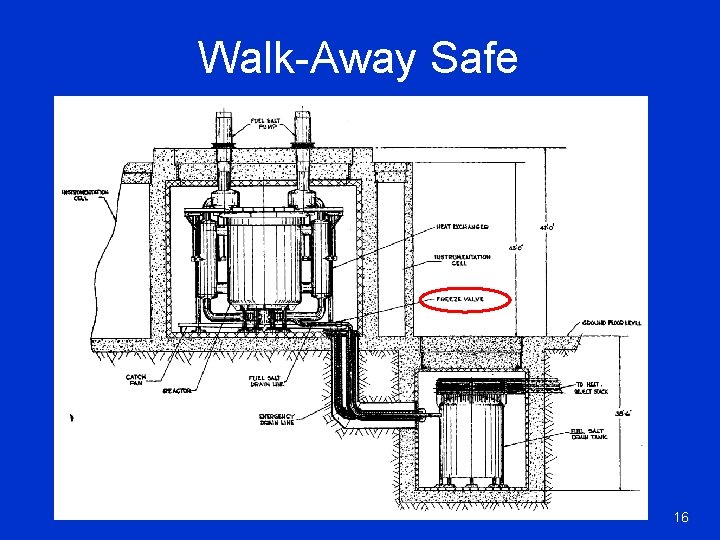
The Necessities A power source must be§ Self regulating when it is ON § “Walk away” safe when it is OFF § Inherently safe in case of an ACCIDENT LFTR wo Equations. Thorium power conference, Oct 2009 4

Fuel for a lifetime? You consume a ball of coal 10 meters in diameter… …or a ball or thorium 37 mm in diameter. LFTR wo Equations. Thorium power conference, Oct 2009 5

Outline § § § Motivations for Nuclear Power LFTR overview Nuclear Chemistry Myths Questions 6

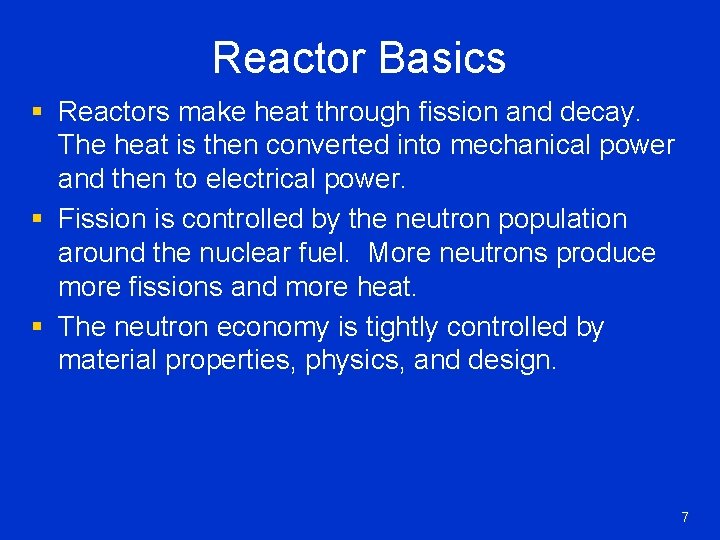
Reactor Basics § Reactors make heat through fission and decay. The heat is then converted into mechanical power and then to electrical power. § Fission is controlled by the neutron population around the nuclear fuel. More neutrons produce more fissions and more heat. § The neutron economy is tightly controlled by material properties, physics, and design. 7

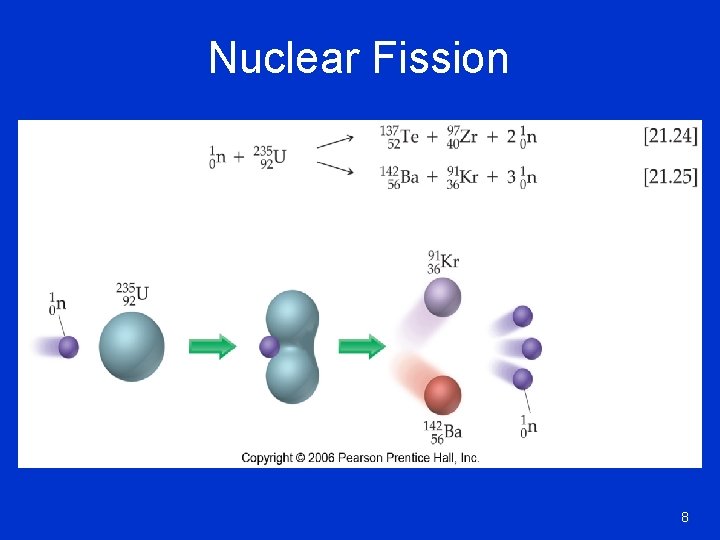
Nuclear Fission 8

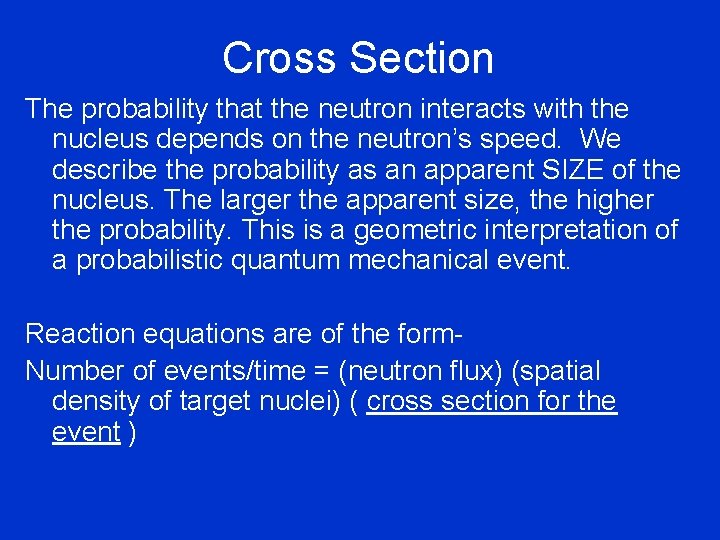
Cross Section The probability that the neutron interacts with the nucleus depends on the neutron’s speed. We describe the probability as an apparent SIZE of the nucleus. The larger the apparent size, the higher the probability. This is a geometric interpretation of a probabilistic quantum mechanical event. Reaction equations are of the form. Number of events/time = (neutron flux) (spatial density of target nuclei) ( cross section for the event )

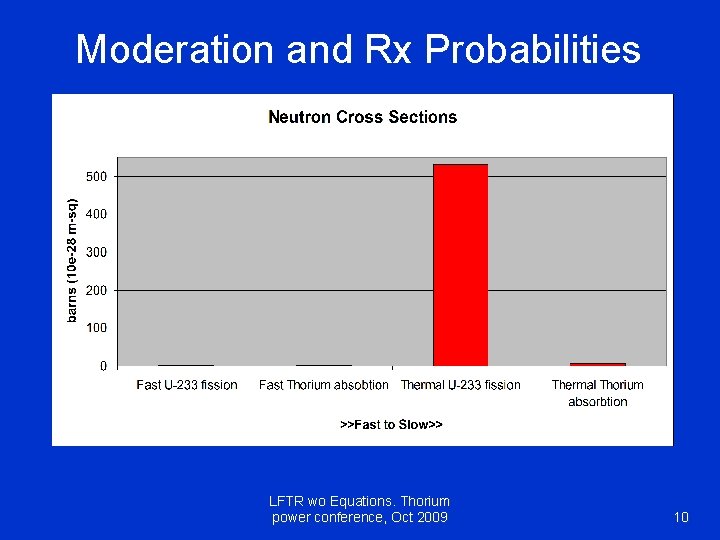
Moderation and Rx Probabilities LFTR wo Equations. Thorium power conference, Oct 2009 10

Speed? LFTR wo Equations. Thorium power conference, Oct 2009 11

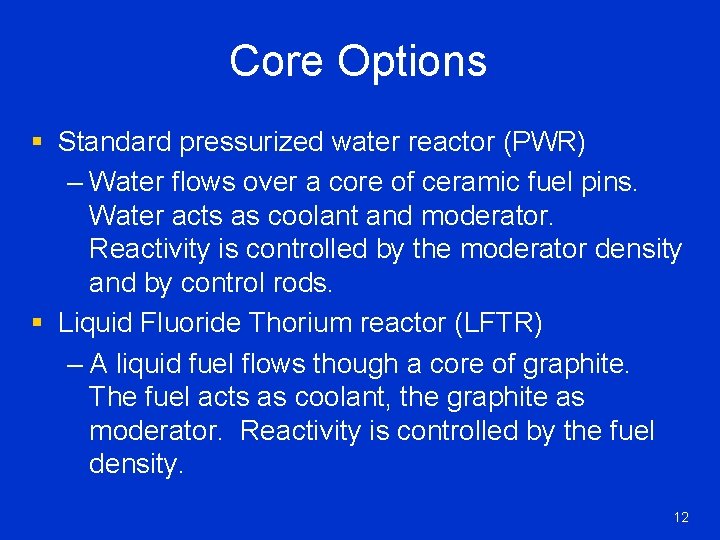
Core Options § Standard pressurized water reactor (PWR) – Water flows over a core of ceramic fuel pins. Water acts as coolant and moderator. Reactivity is controlled by the moderator density and by control rods. § Liquid Fluoride Thorium reactor (LFTR) – A liquid fuel flows though a core of graphite. The fuel acts as coolant, the graphite as moderator. Reactivity is controlled by the fuel density. 12

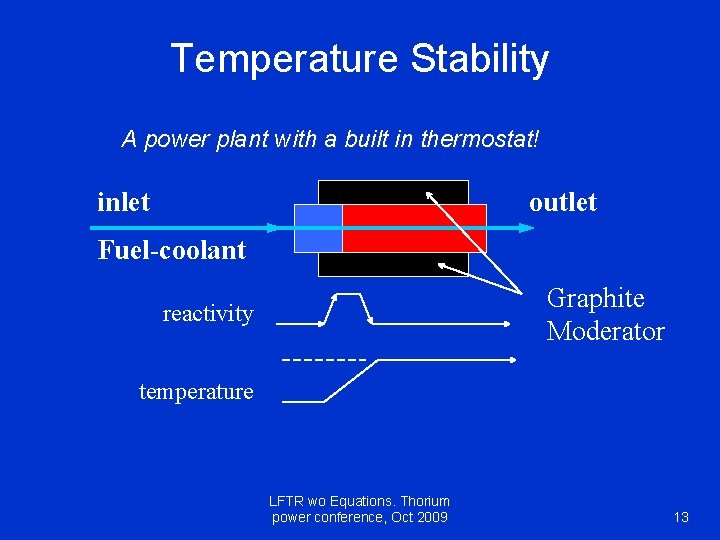
Temperature Stability A power plant with a built in thermostat! inlet outlet Fuel-coolant Graphite Moderator reactivity temperature LFTR wo Equations. Thorium power conference, Oct 2009 13

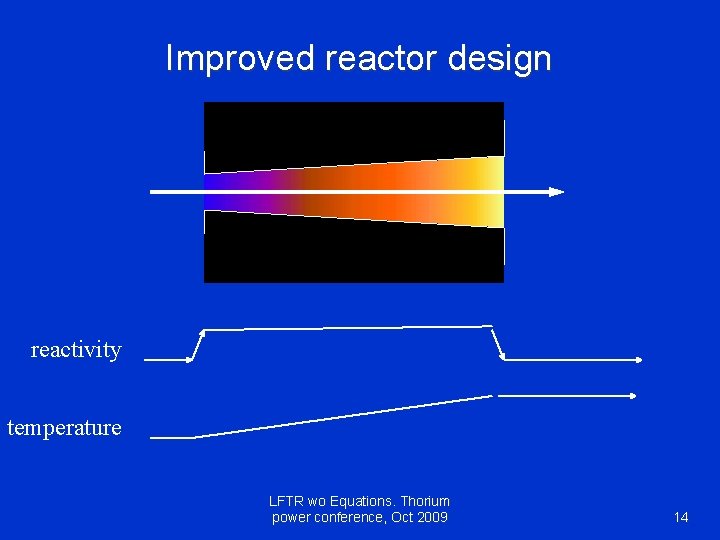
Improved reactor design reactivity temperature LFTR wo Equations. Thorium power conference, Oct 2009 14

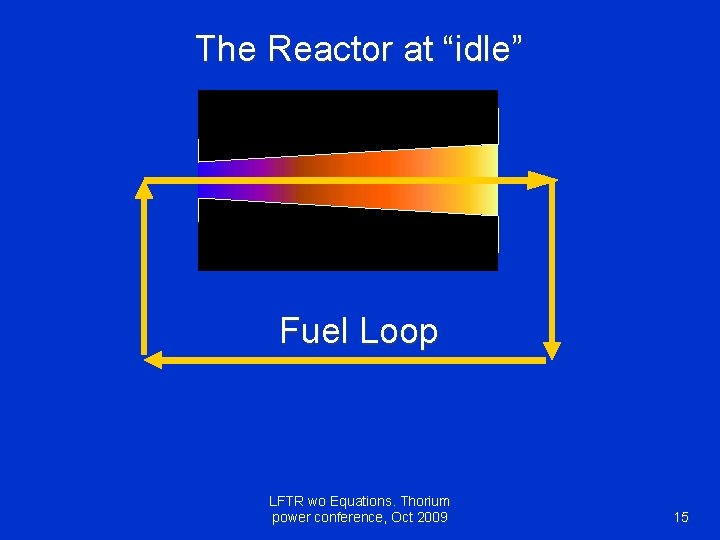
The Reactor at “idle” Fuel Loop LFTR wo Equations. Thorium power conference, Oct 2009 15

Walk-Away Safe 16

An Efficient Design Little or no radioactive waste No bomb materials Low cost per power delivered Small physical size Power on demand A wide choice of building sites. LFTR wo Equations. Thorium power conference, Oct 2009 17

Size matters! Your nuclear waste is the size of a few grains of rice! LFTR wo Equations. Thorium power conference, Oct 2009 18

Lets Make Power § This is a picture of the waste heat coming from the original LFTR test cell. § The heat transfer fluid (molten salt) is at low pressure. § We can use a gas cycle, like a jet engine, to convert heat into mechanical power. (highly efficient) § These plants can run at partial load. LFTR wo Equations. Thorium power conference, Oct 2009 19

Outline § § § Motivations for Nuclear Power LFTR overview Nuclear Chemistry Myths Questions 20

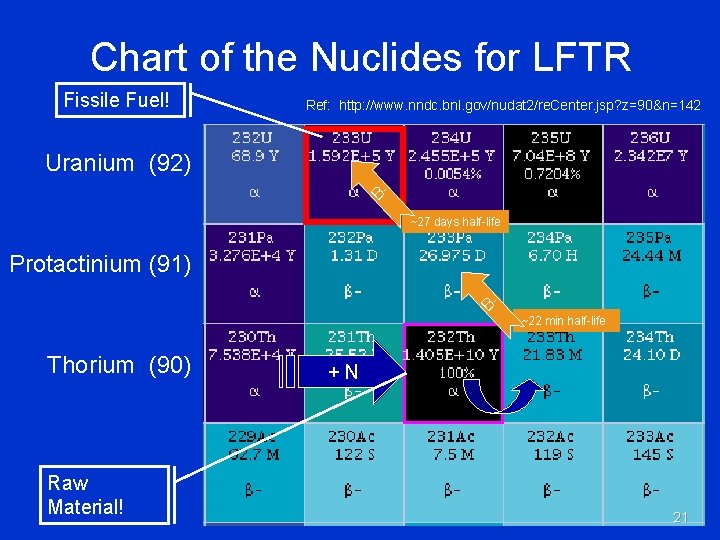
Chart of the Nuclides for LFTR Fissile Fuel! Ref: http: //www. nndc. bnl. gov/nudat 2/re. Center. jsp? z=90&n=142 Uranium (92) B~27 days half-life Protactinium (91) B- Thorium (90) Raw Material! ~22 min half-life +N 21

Uranium vs Thorium as a Fuel § 0. 7 % of Uranium is fissionable. The rest becomes nuclear waste. § Thorium is isotopically pure and converted to U 233 for fuel. § For fuel cycle side-reactions see- http: //en. wikipedia. org/wiki/Thorium_fuel_cycle 22

Fuel or Waste We want the fuel to fission rather than transmute. U 233 - 90% fissions U 235 - 83% fissions P 239 - 50% fissions P 241 - 25% fissions LFTR wo Equations. Thorium power conference, Oct 2009 23

Outline § § § Motivations for Nuclear Power LFTR overview Nuclear Chemistry Myths Questions 24

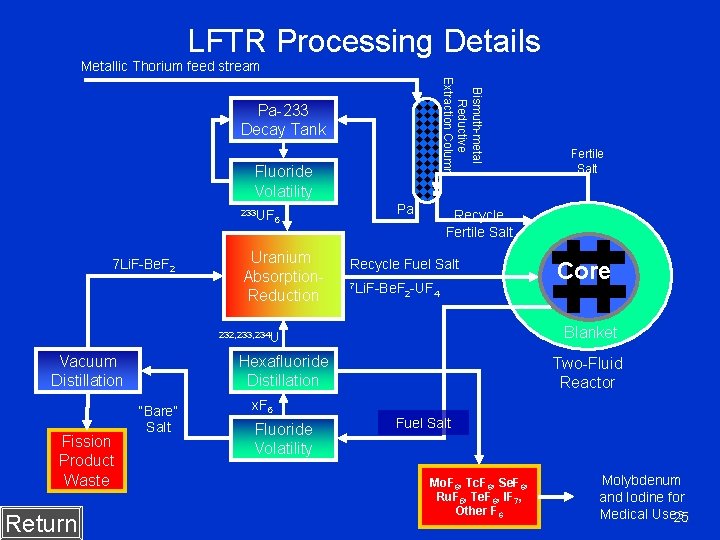
LFTR Processing Details Metallic Thorium feed stream Bismuth-metal Reductive Extraction Column Pa-233 Decay Tank Fluoride Volatility 233 UF 7 Li. F-Be. F 2 6 Uranium Absorption. Reduction Pa Recycle Fertile Salt Recycle Fuel Salt 7 Li. F-Be. F 2 -UF 4 F 6 Hexafluoride Distillation Fission Product Waste Return “Bare” Salt Core Blanket 232, 233, 234 U Vacuum Distillation Fertile Salt Two-Fluid Reactor x. F 6 Fluoride Volatility Fuel Salt Mo. F 6, Tc. F 6, Se. F 6, Ru. F 5, Te. F 6, IF 7, Other F 6 Molybdenum and Iodine for Medical Uses 25

Metal Reduction Column 26

Outline § § § Motivations for Nuclear Power LFTR overview Nuclear Chemistry Myths Questions 27

Myth of Half Life § What makes a radioactive material dangerous? – Even low energy beta decays can break organic bonds. § Is a material with a 1 second half life dangerous? – It is very dangerous. . today. Tomorrow it is inert. § Is a 15 billion year half life dangerous? (Half of it has decayed since the big bang. ) It has a very low activity, and we used it for hundreds of years. § How about a thousand year half life? It is both active and persistent. 28

Myth of Creating Radioactivity § If radiation is dangerous then we should do what we can to eliminate it from the environment. That is exactly what nuclear reactors do. They are the nuclear analog to chemical catalysis reactors; they accelerate natural processes. § The only way to “create” nuclear radiation is with particle accelerators. Conventional reactors simply accelerate decay towards stable elements. 29

Myth of Concentration Thorium and uranium are dispersed in nature and they decay naturally. We concentrate them, and so concentrate their natural toxicity. For safe disposal, should we re-disperse them and lower their effective activity, or sequester them and decrease our contact? 30

References http: //en. wikipedia. org/wiki/Liquid_fluoride_thorium_reactor http: //www. scribd. com/doc/59204103/Thorium-presentation-Green-Energy -Forum-2008 -07 -25 http: //www. thoriumenergyalliance. com/Thorium. Site/resources. html http: //moltensalt. org/references/static/downloads/pdf/NAT_MSBRrecycle. p df Thorium in 5 minutes (remix video) http: //www. youtube. com/watch? v=u. K 367 T 7 h 6 ZY 31

Outline § § § Motivations for Nuclear Power LFTR overview Nuclear Chemistry Myths Questions 32

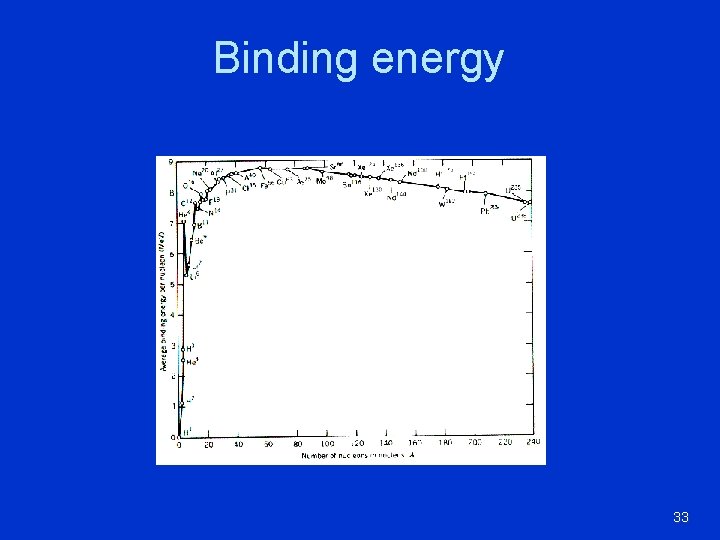
Binding energy 33