Liquid Crystal Devices Dr Sally E Day s













![Post Aligned Bistable Device (PABN) 3 -D Modelling Window [1], [2] • Device under Post Aligned Bistable Device (PABN) 3 -D Modelling Window [1], [2] • Device under](https://slidetodoc.com/presentation_image_h/a5fe9cdcaadc86dd3b9c7c866a77e12b/image-81.jpg)



- Slides: 86

Liquid Crystal Devices Dr. Sally E. Day s. day@ee. ucl. ac. uk 1

Abstract: Liquid Crystals Displays (LCD) – very common, low power, light-weight displays, as well as larger area flat panel displays for monitors and TV applications. Liquid Crystals have a remarkable electro-optic coefficient, a large birefringence is switched with a very low voltage. Newer displays require complex structures with careful control of small features in the liquid crystal. This makes them of interest in other applications besides displays. This tutorial will cover the physical properties essential for the operation of liquid crystal devices including displays and non-display applications. 2

Contents: 1. Structure-property relationships in liquid crystals a. b. c. d. Phases of liquid crystals Order parameter in liquid crystals Anisotropy in a liquid: Dielectric, optical and viscoelastic properties Molecular structure and influence on the physical properties 2. Optical properties of liquid crystals a. Birefringence b. Polarisation of light c. Control of polarisation 3. Structure of liquid crystal devices (LCD) a. Alignment b. Basic construction of LCDs 4. Optical properties of display and other devices a. Twisted nematic, In-plane switching, Vertically aligned nematic b. Holograms and Beam steering c. Micro and nano-structures and liquid crystals 3

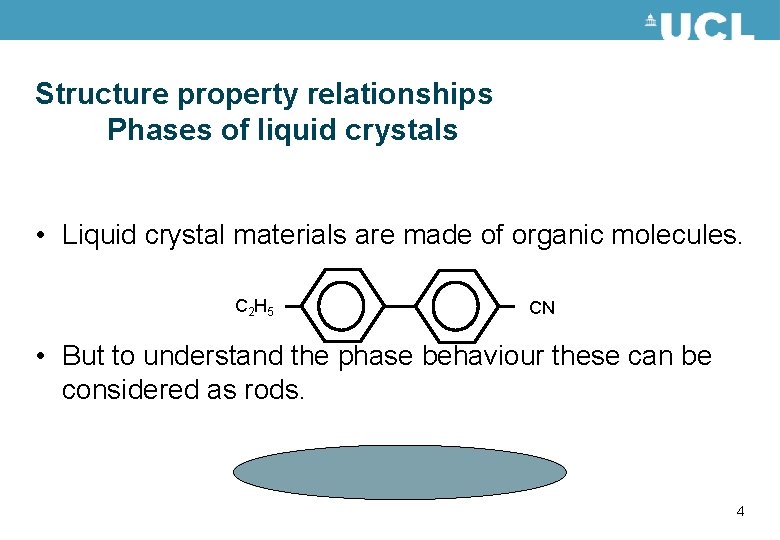
Structure property relationships Phases of liquid crystals • Liquid crystal materials are made of organic molecules. C 2 H 5 CN • But to understand the phase behaviour these can be considered as rods. 4

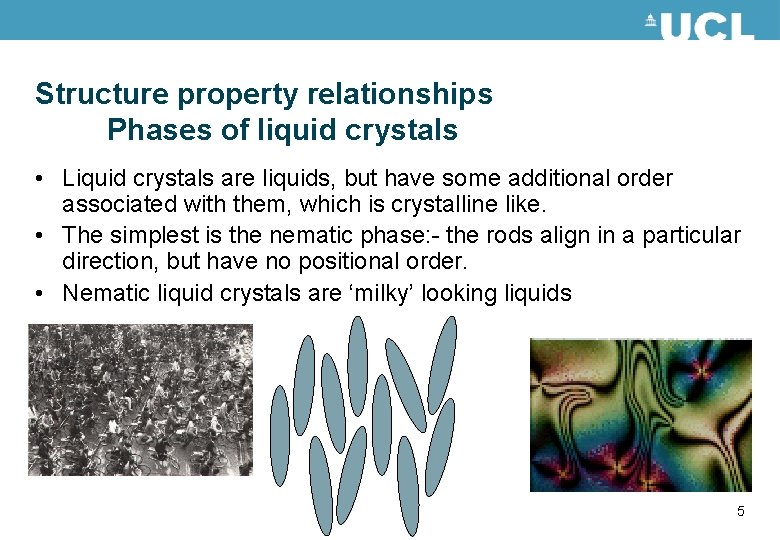
Structure property relationships Phases of liquid crystals • Liquid crystals are liquids, but have some additional order associated with them, which is crystalline like. • The simplest is the nematic phase: - the rods align in a particular direction, but have no positional order. • Nematic liquid crystals are ‘milky’ looking liquids 5

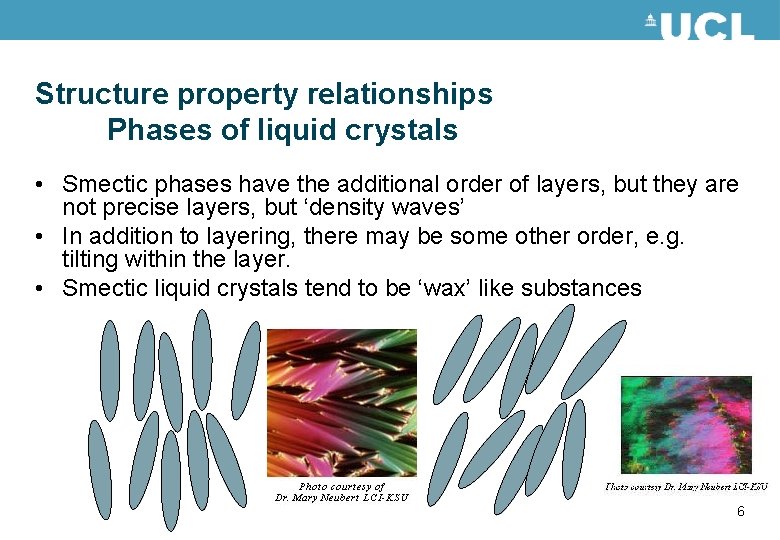
Structure property relationships Phases of liquid crystals • Smectic phases have the additional order of layers, but they are not precise layers, but ‘density waves’ • In addition to layering, there may be some other order, e. g. tilting within the layer. • Smectic liquid crystals tend to be ‘wax’ like substances 6

Structure property relationships Phases of liquid crystals • Other smectic phases have additional order within the layers • This order may be in the form of hexagonal packing • The phases can be identified by the patterns that form and can be seen using a polarising microscope, or by X-ray scattering. • The order between the molecules can also be seen by NMR • Some of the polarising microscope images can be seen at http: //reynolds. ph. man. ac. uk/~dierking/gallery 1. html 7

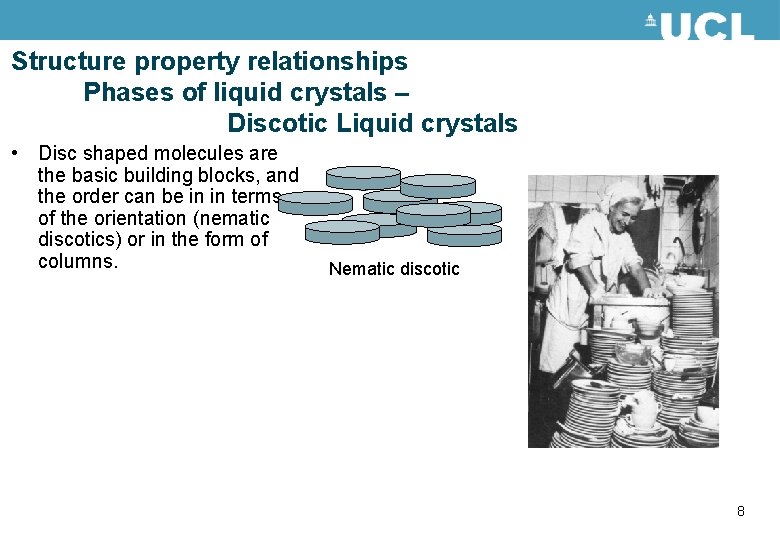
Structure property relationships Phases of liquid crystals – Discotic Liquid crystals • Disc shaped molecules are the basic building blocks, and the order can be in in terms of the orientation (nematic discotics) or in the form of columns. Nematic discotic 8

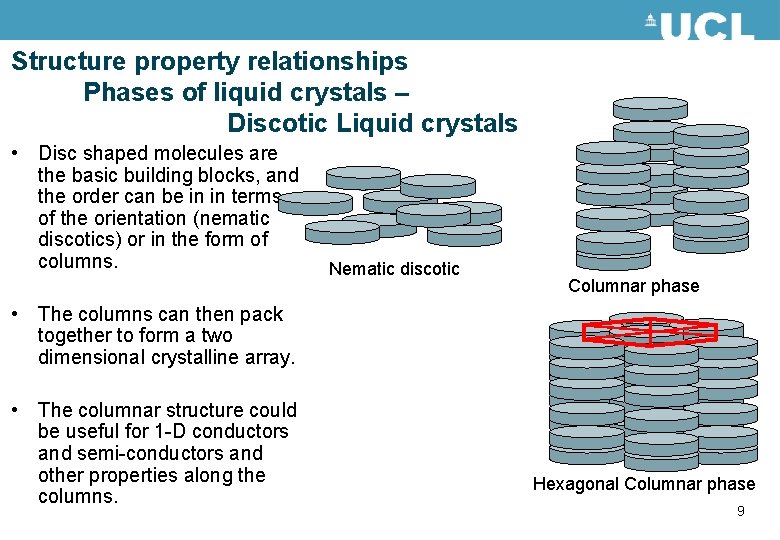
Structure property relationships Phases of liquid crystals – Discotic Liquid crystals • Disc shaped molecules are the basic building blocks, and the order can be in in terms of the orientation (nematic discotics) or in the form of columns. Nematic discotic Columnar phase • The columns can then pack together to form a two dimensional crystalline array. • The columnar structure could be useful for 1 -D conductors and semi-conductors and other properties along the columns. Hexagonal Columnar phase 9

Structure property relationships Phases of liquid crystals Thermotropic liquid crystals phase forms as a function of temperature Lyotropic liquid crystals Phase forms as a function of concentration in a solvent 10

Structure property relationships Phases of liquid crystals – Lyotropic liquid crystals. • Lyotropic phases occur for molecules dissolved in a solution • Different phases occur with concentration • Often the solvent is water and the molecules have an hydrophilic end and an hydrophobic end (e. g. detergents with polar (hydrophilic) and non-polar (hydrophobic) end groups). • The lyotropic liquid crystals form many different phases, as with thermotropic liquid crystals, but depending on concentration as well as temperature Hexagonal phase 11

Liquid crystal templates • Lyotropic liquid crystal structures can be converted to solid structures using the sol-gel process to give silicates with the same structure as the liquid crystal phase. • Other methods can be used to form metal nanoparticles 12

Structure property relationships Phases of liquid crystals – Lyotropic liquid crystals. • The lamellar phases are found in cell membranes • This allows a liquid environment to exist, so transporting material around, but with a layer which controls the transport of material across the layer • An example of the phase transition is from the lamellar liquid crystal phase to a gel phase, sometimes an undesirable transition. • This transition occurs at different temperatures and pressures depending on the environment that the organism lives in and what is required water Lamellar phase water gel phase 13

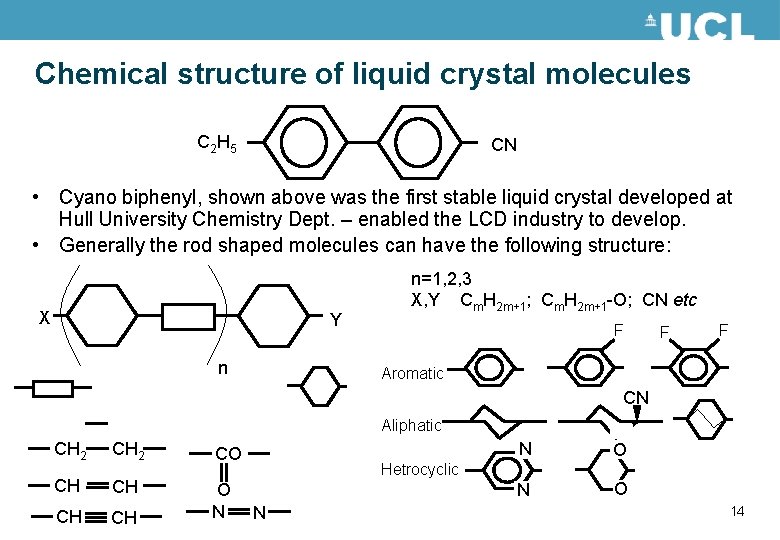
Chemical structure of liquid crystal molecules C 2 H 5 CN • Cyano biphenyl, shown above was the first stable liquid crystal developed at Hull University Chemistry Dept. – enabled the LCD industry to develop. • Generally the rod shaped molecules can have the following structure: X Y n n=1, 2, 3 X, Y Cm. H 2 m+1; Cm. H 2 m+1 -O; CN etc F F F Aromatic CN Aliphatic CH 2 CO CH CH O N N O Hetrocyclic N 14

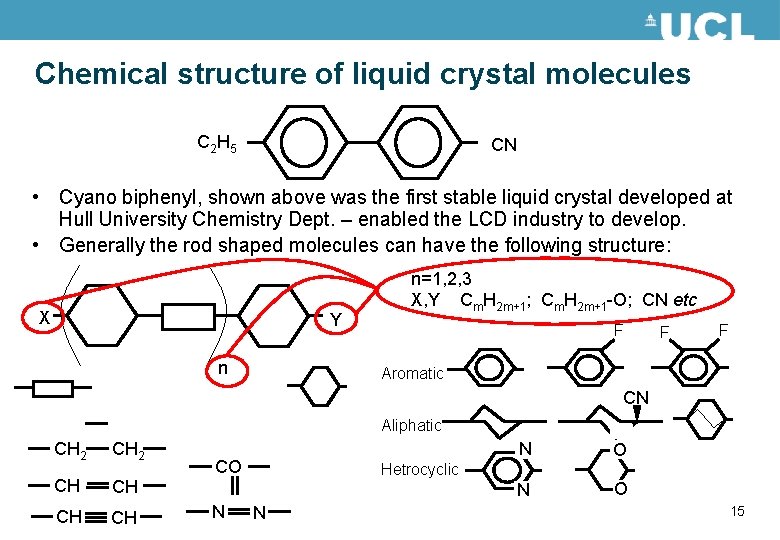
Chemical structure of liquid crystal molecules C 2 H 5 CN • Cyano biphenyl, shown above was the first stable liquid crystal developed at Hull University Chemistry Dept. – enabled the LCD industry to develop. • Generally the rod shaped molecules can have the following structure: X Y n n=1, 2, 3 X, Y Cm. H 2 m+1; Cm. H 2 m+1 -O; CN etc F F F Aromatic CN Aliphatic CH 2 CH CH CO N N O Hetrocyclic N 15

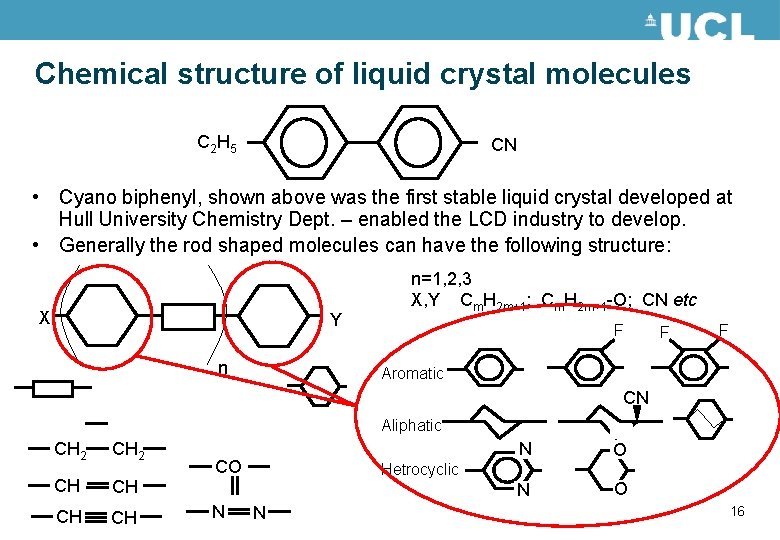
Chemical structure of liquid crystal molecules C 2 H 5 CN • Cyano biphenyl, shown above was the first stable liquid crystal developed at Hull University Chemistry Dept. – enabled the LCD industry to develop. • Generally the rod shaped molecules can have the following structure: X Y n n=1, 2, 3 X, Y Cm. H 2 m+1; Cm. H 2 m+1 -O; CN etc F F F Aromatic CN Aliphatic CH 2 CH CH CO N N O Hetrocyclic N 16

Chemical structure of liquid crystal molecules C 2 H 5 CN • Cyano biphenyl, shown above was the first stable liquid crystal developed at Hull University Chemistry Dept. – enabled the LCD industry to develop. • Generally the rod shaped molecules can have the following structure: X Y n n=1, 2, 3 X, Y Cm. H 2 m+1; Cm. H 2 m+1 -O; CN etc F F F Aromatic CN Aliphatic CH 2 CH CH CO N N O Hetrocyclic N 17

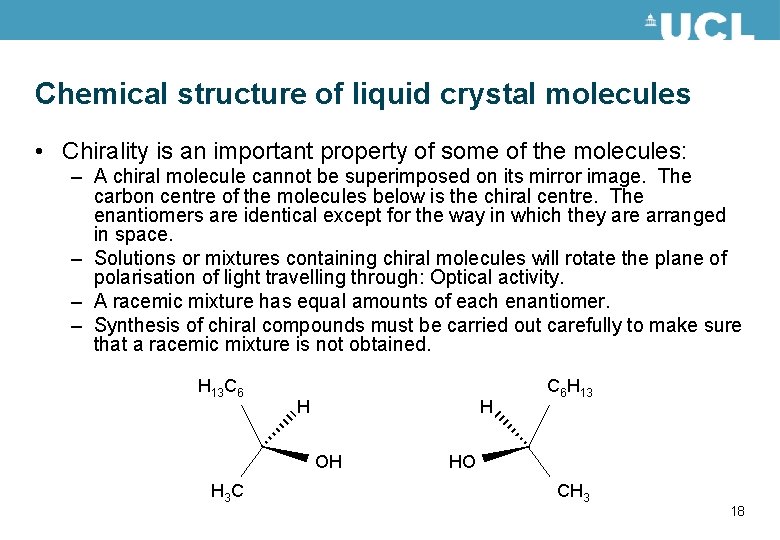
Chemical structure of liquid crystal molecules • Chirality is an important property of some of the molecules: – A chiral molecule cannot be superimposed on its mirror image. The carbon centre of the molecules below is the chiral centre. The enantiomers are identical except for the way in which they are arranged in space. – Solutions or mixtures containing chiral molecules will rotate the plane of polarisation of light travelling through: Optical activity. – A racemic mixture has equal amounts of each enantiomer. – Synthesis of chiral compounds must be carried out carefully to make sure that a racemic mixture is not obtained. H 13 C 6 H H OH H 3 C C 6 H 13 HO CH 3 18

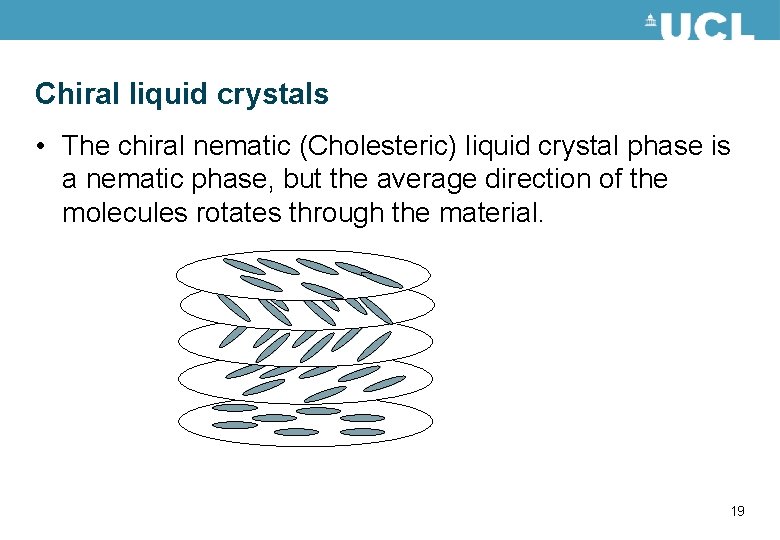
Chiral liquid crystals • The chiral nematic (Cholesteric) liquid crystal phase is a nematic phase, but the average direction of the molecules rotates through the material. 19

Chemical structure of liquid crystal molecules • The different chemical groups affect the physical properties in many ways, some important effects are as follows – – – Phase transition temperatures Dielectric properties Optical properties Visco-elastic properties Ferroelectric, flexoelectric coefficients Chirality • These physical properties in turn affect the performance of the displays and other devices that contain liquid crystals 20

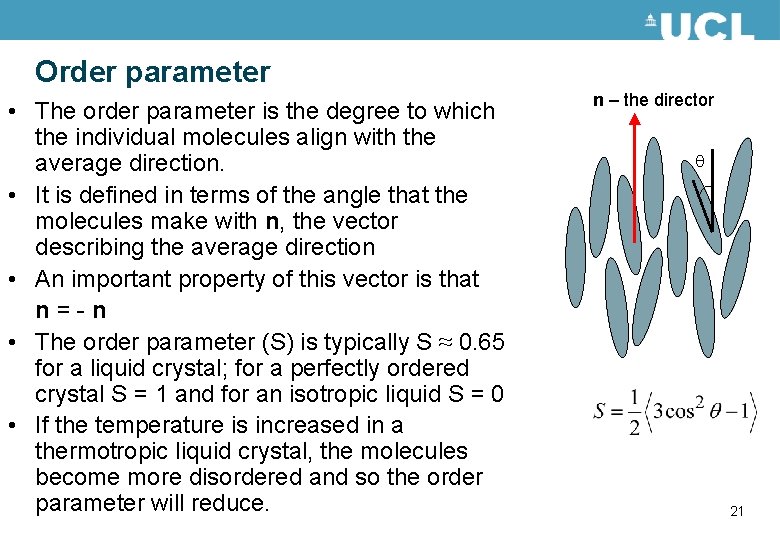
Order parameter • The order parameter is the degree to which the individual molecules align with the average direction. • It is defined in terms of the angle that the molecules make with n, the vector describing the average direction • An important property of this vector is that n = - n • The order parameter (S) is typically S ≈ 0. 65 for a liquid crystal; for a perfectly ordered crystal S = 1 and for an isotropic liquid S = 0 • If the temperature is increased in a thermotropic liquid crystal, the molecules become more disordered and so the order parameter will reduce. n – the director 21

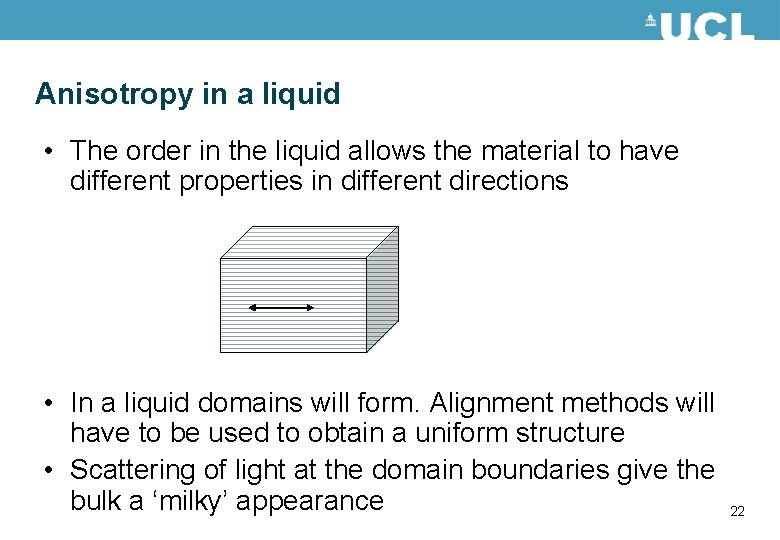
Anisotropy in a liquid • The order in the liquid allows the material to have different properties in different directions • In a liquid domains will form. Alignment methods will have to be used to obtain a uniform structure • Scattering of light at the domain boundaries give the bulk a ‘milky’ appearance 22

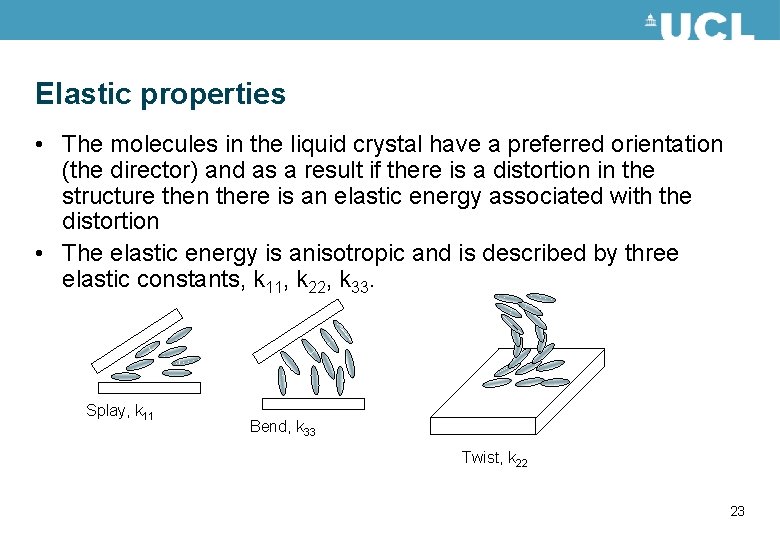
Elastic properties • The molecules in the liquid crystal have a preferred orientation (the director) and as a result if there is a distortion in the structure then there is an elastic energy associated with the distortion • The elastic energy is anisotropic and is described by three elastic constants, k 11, k 22, k 33. Splay, k 11 Bend, k 33 Twist, k 22 23

Dielectric properties • The electric permittivity of the liquid crystal is anisotropic • The permittivity is concerned with the polarisability of the material and the response of a material to an electric field. • D=eoer. E 24

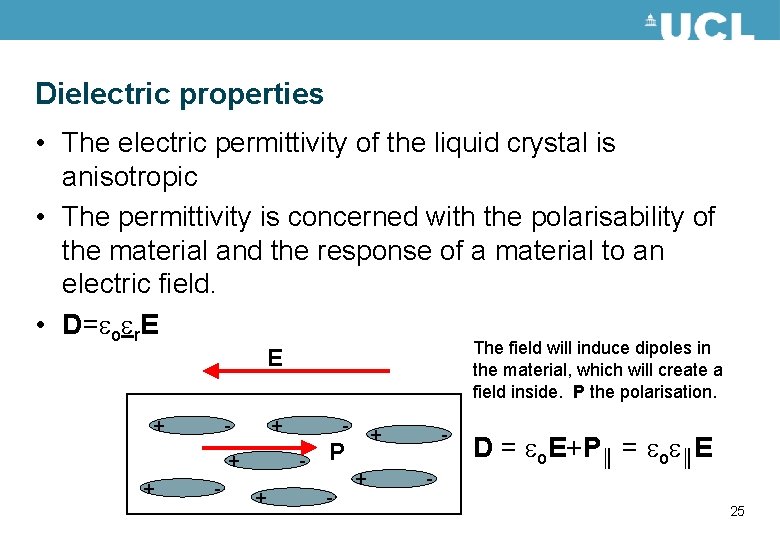
Dielectric properties • The electric permittivity of the liquid crystal is anisotropic • The permittivity is concerned with the polarisability of the material and the response of a material to an electric field. • D=eoer. E The field will induce dipoles in the material, which will create a field inside. P the polarisation. E + - + + + - - + + P - + - D = eo. E+P║ = eoe║E 25

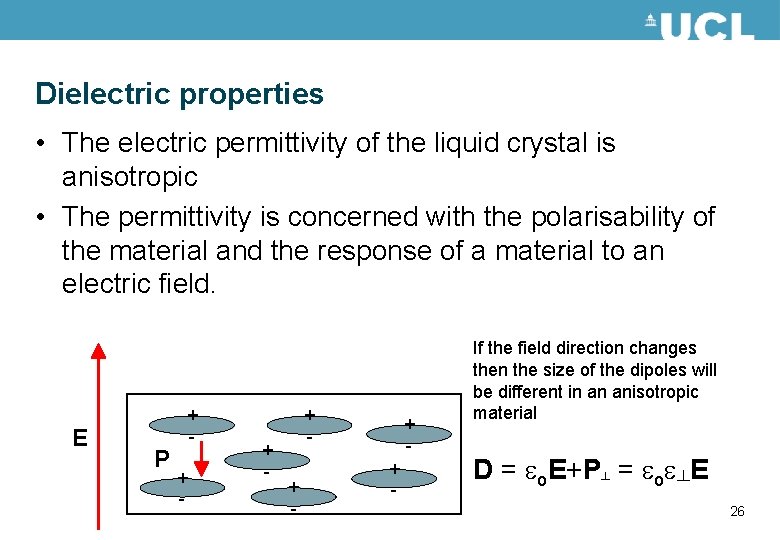
Dielectric properties • The electric permittivity of the liquid crystal is anisotropic • The permittivity is concerned with the polarisability of the material and the response of a material to an electric field. E P + + - If the field direction changes then the size of the dipoles will be different in an anisotropic material D = eo. E+P┴ = eoe┴E 26

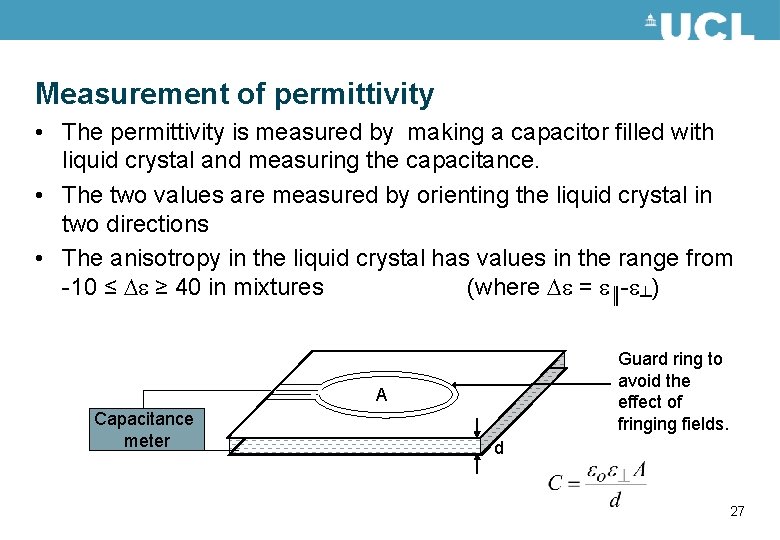
Measurement of permittivity • The permittivity is measured by making a capacitor filled with liquid crystal and measuring the capacitance. • The two values are measured by orienting the liquid crystal in two directions • The anisotropy in the liquid crystal has values in the range from -10 ≤ e ≥ 40 in mixtures (where e = e║-e┴) Guard ring to avoid the effect of fringing fields. A Capacitance meter d 27

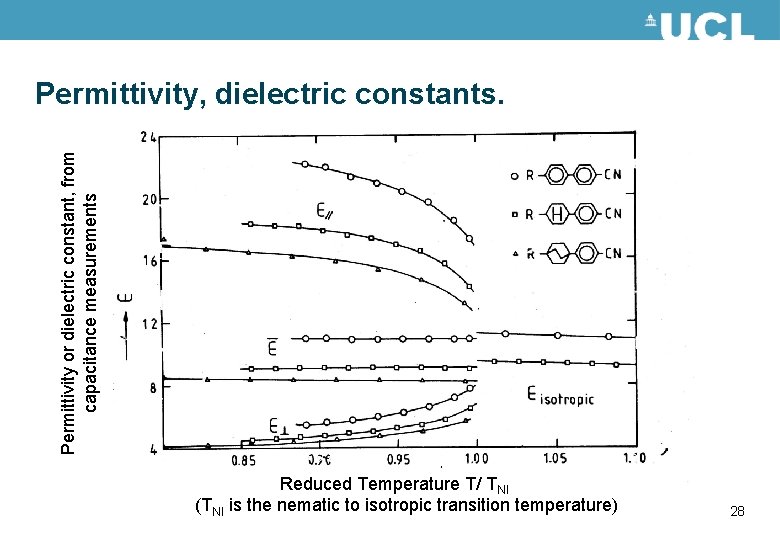
Permittivity or dielectric constant, from capacitance measurements Permittivity, dielectric constants. Reduced Temperature T/ TNI (TNI is the nematic to isotropic transition temperature) 28

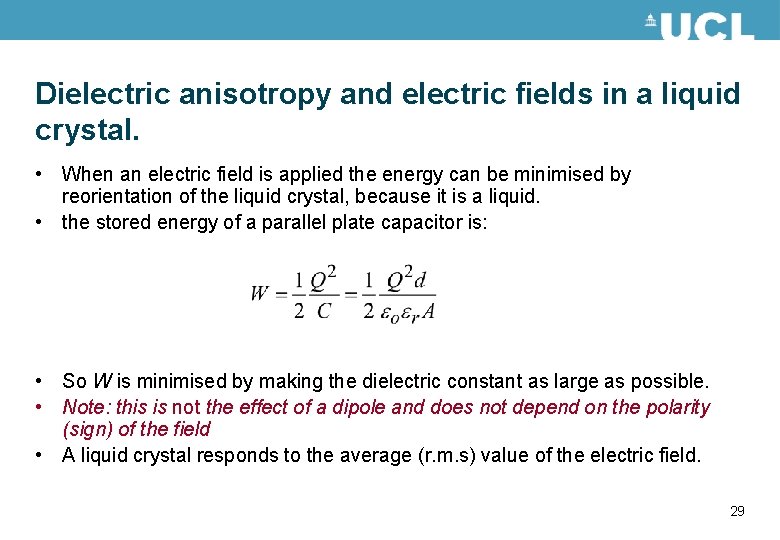
Dielectric anisotropy and electric fields in a liquid crystal. • When an electric field is applied the energy can be minimised by reorientation of the liquid crystal, because it is a liquid. • the stored energy of a parallel plate capacitor is: • So W is minimised by making the dielectric constant as large as possible. • Note: this is not the effect of a dipole and does not depend on the polarity (sign) of the field • A liquid crystal responds to the average (r. m. s) value of the electric field. 29

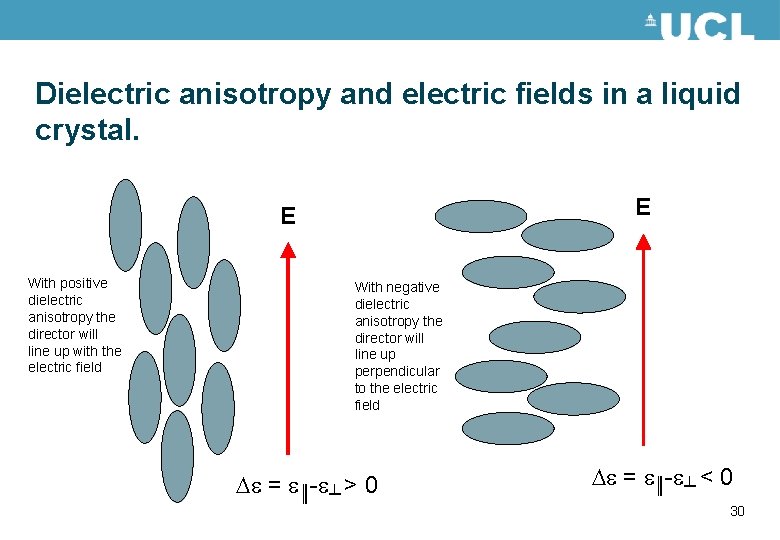
Dielectric anisotropy and electric fields in a liquid crystal. E E With positive dielectric anisotropy the director will line up with the electric field With negative dielectric anisotropy the director will line up perpendicular to the electric field e = e║-e┴ > 0 e = e║-e┴ < 0 30

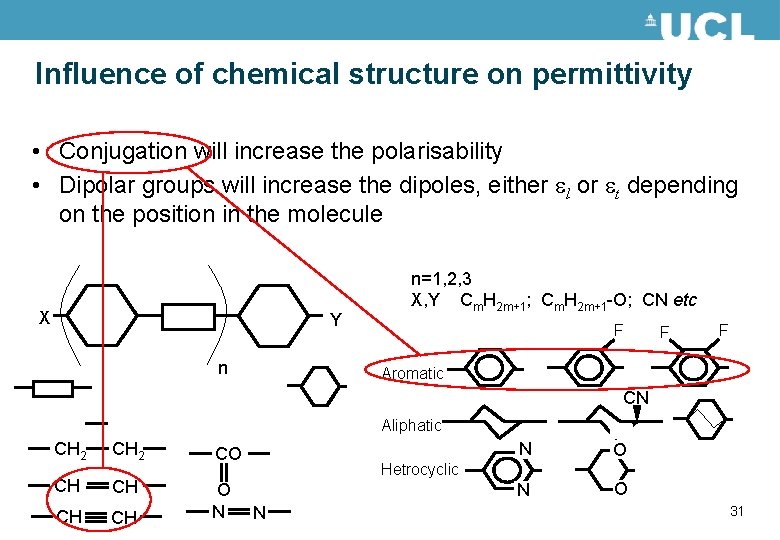
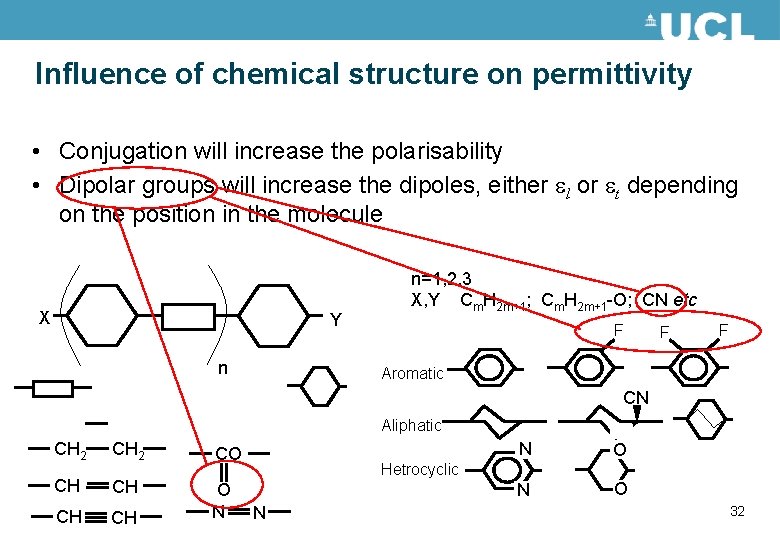
Influence of chemical structure on permittivity • Conjugation will increase the polarisability • Dipolar groups will increase the dipoles, either el or et depending on the position in the molecule X Y n n=1, 2, 3 X, Y Cm. H 2 m+1; Cm. H 2 m+1 -O; CN etc F F F Aromatic CN Aliphatic CH 2 CO CH CH O N N O Hetrocyclic N 31

Influence of chemical structure on permittivity • Conjugation will increase the polarisability • Dipolar groups will increase the dipoles, either el or et depending on the position in the molecule X Y n n=1, 2, 3 X, Y Cm. H 2 m+1; Cm. H 2 m+1 -O; CN etc F F F Aromatic CN Aliphatic CH 2 CO CH CH O N N O Hetrocyclic N 32

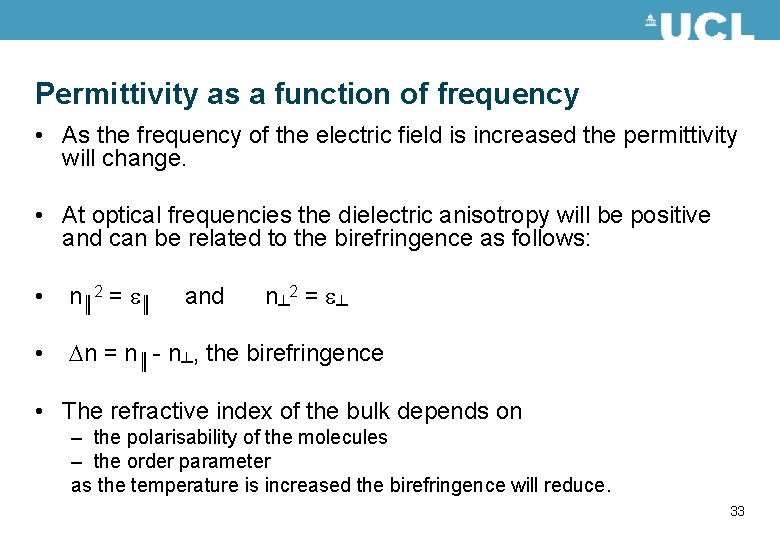
Permittivity as a function of frequency • As the frequency of the electric field is increased the permittivity will change. • At optical frequencies the dielectric anisotropy will be positive and can be related to the birefringence as follows: • n║ 2 = e║ and n┴ 2 = e┴ • n = n║ - n┴, the birefringence • The refractive index of the bulk depends on – the polarisability of the molecules – the order parameter as the temperature is increased the birefringence will reduce. 33

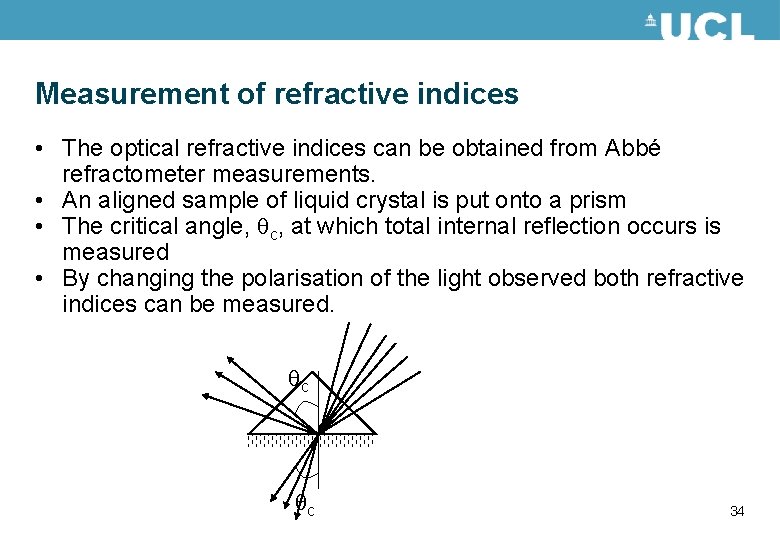
Measurement of refractive indices • The optical refractive indices can be obtained from Abbé refractometer measurements. • An aligned sample of liquid crystal is put onto a prism • The critical angle, c, at which total internal reflection occurs is measured • By changing the polarisation of the light observed both refractive indices can be measured. c c 34

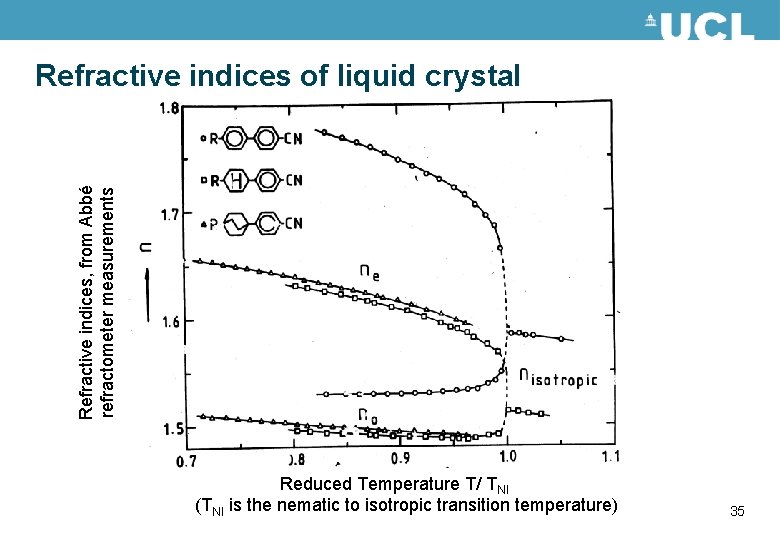
Refractive indices, from Abbé refractometer measurements Refractive indices of liquid crystal Reduced Temperature T/ TNI (TNI is the nematic to isotropic transition temperature) 35

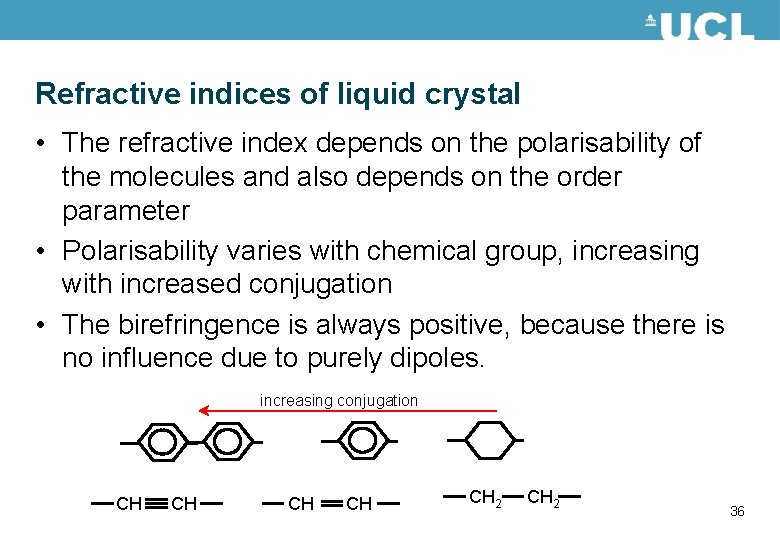
Refractive indices of liquid crystal • The refractive index depends on the polarisability of the molecules and also depends on the order parameter • Polarisability varies with chemical group, increasing with increased conjugation • The birefringence is always positive, because there is no influence due to purely dipoles. increasing conjugation CH CH CH 2 36

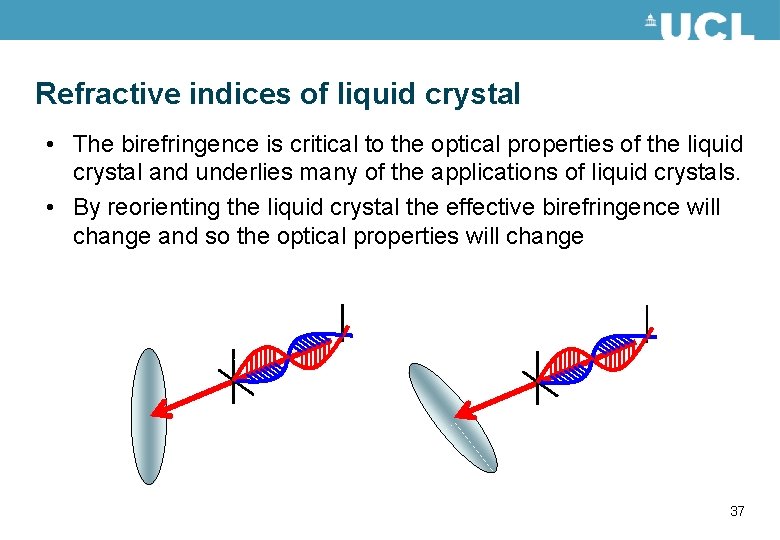
Refractive indices of liquid crystal • The birefringence is critical to the optical properties of the liquid crystal and underlies many of the applications of liquid crystals. • By reorienting the liquid crystal the effective birefringence will change and so the optical properties will change 37

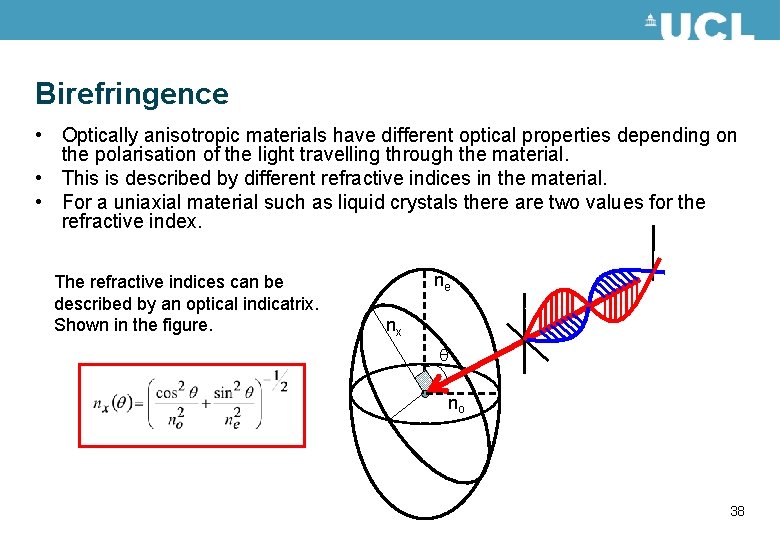
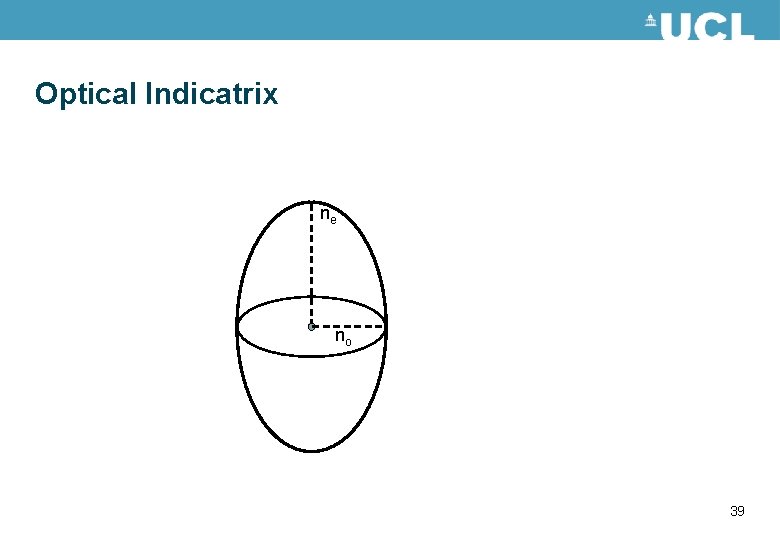
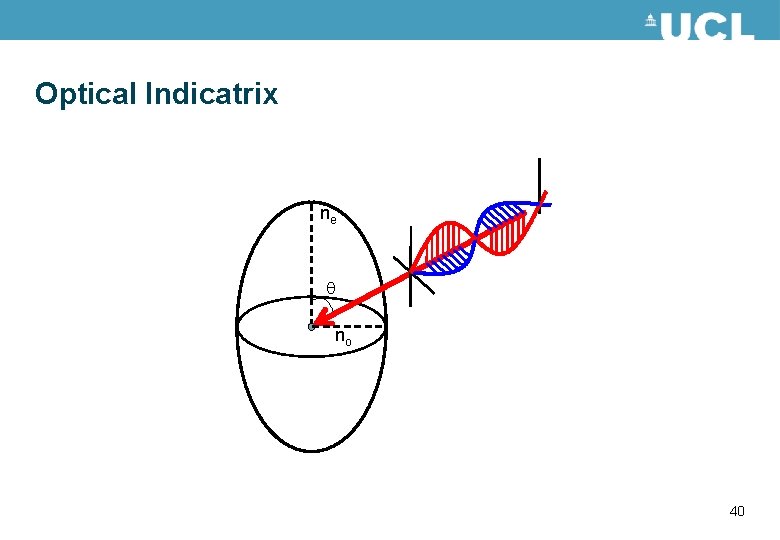
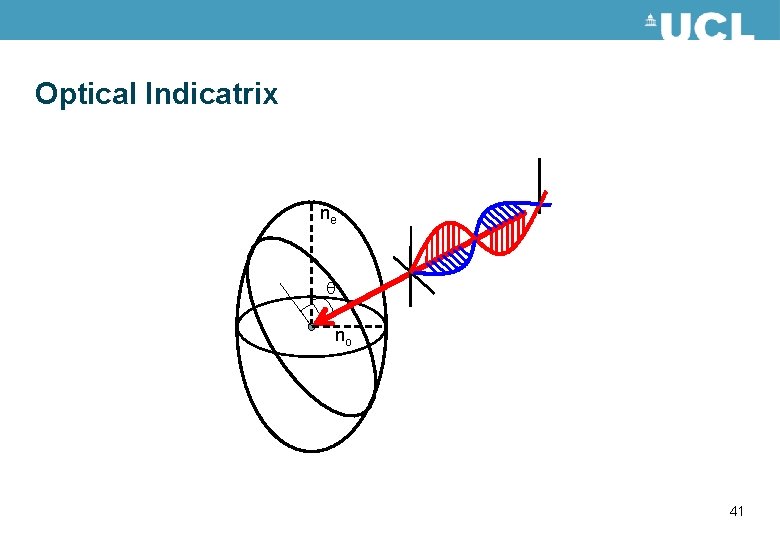
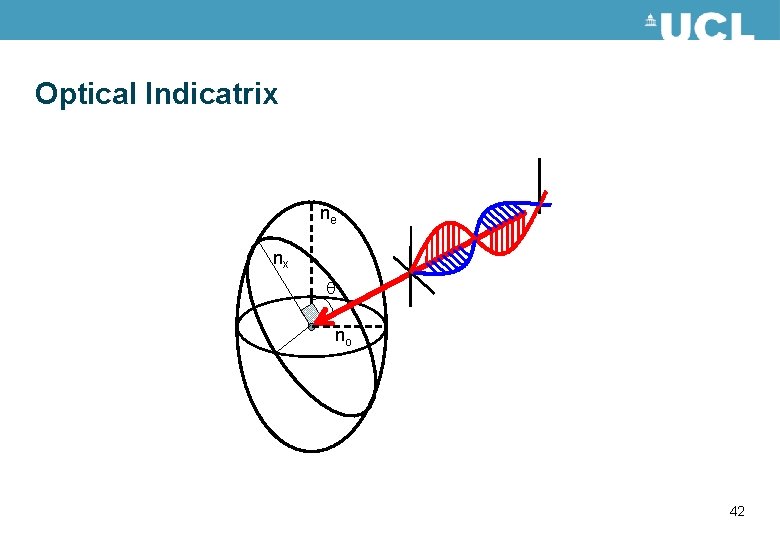
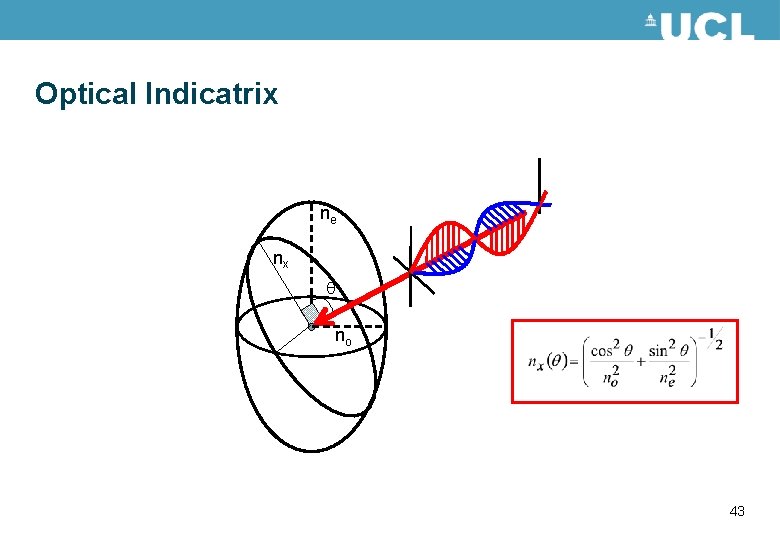
Birefringence • Optically anisotropic materials have different optical properties depending on the polarisation of the light travelling through the material. • This is described by different refractive indices in the material. • For a uniaxial material such as liquid crystals there are two values for the refractive index. The refractive indices can be described by an optical indicatrix. Shown in the figure. ne nx no 38

Optical Indicatrix ne no 39

Optical Indicatrix ne no 40

Optical Indicatrix ne no 41

Optical Indicatrix ne nx no 42

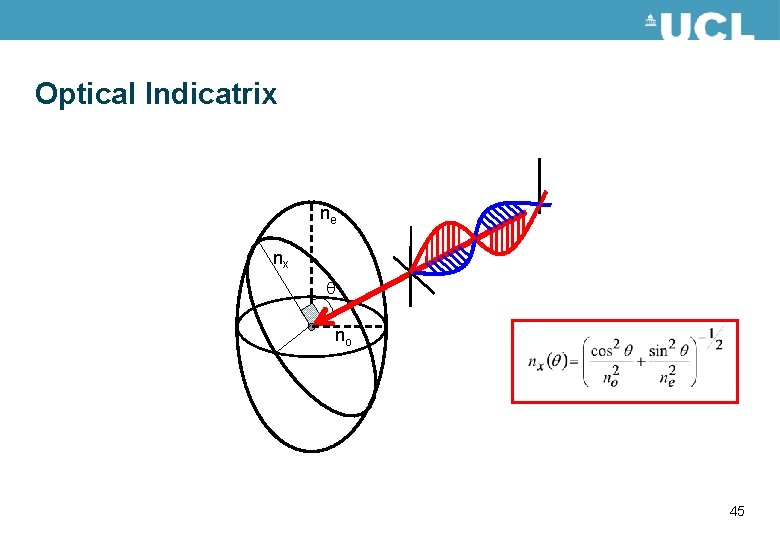
Optical Indicatrix ne nx no 43

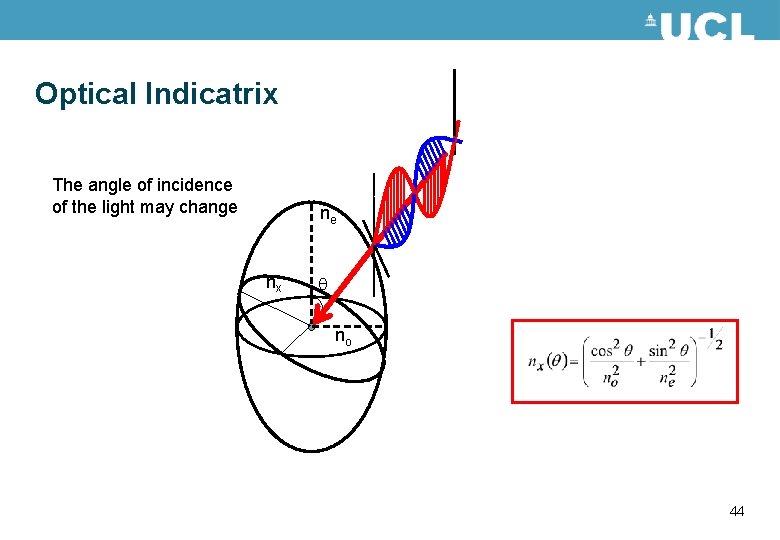
Optical Indicatrix The angle of incidence of the light may change ne nx no 44

Optical Indicatrix ne nx no 45

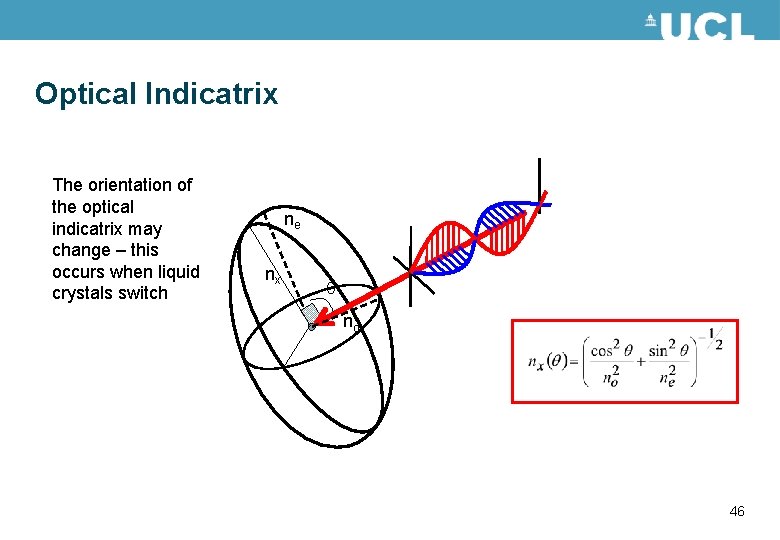
Optical Indicatrix The orientation of the optical indicatrix may change – this occurs when liquid crystals switch ne nx no 46

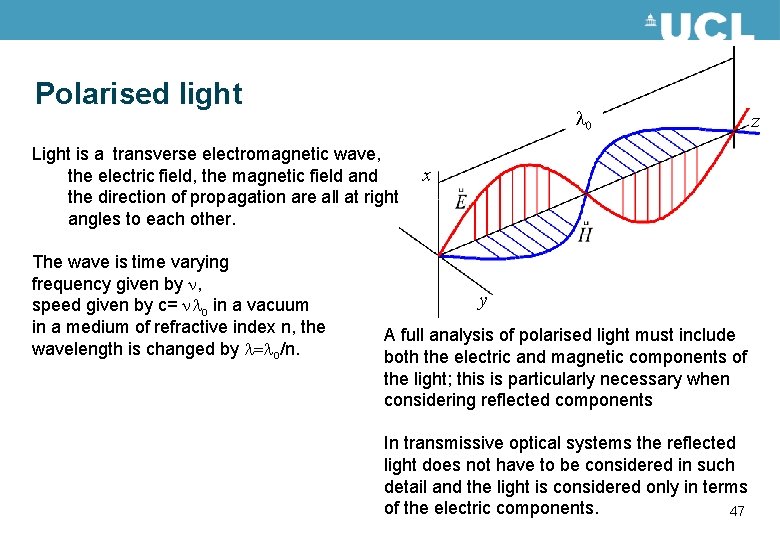
Polarised light 0 Light is a transverse electromagnetic wave, the electric field, the magnetic field and the direction of propagation are all at right angles to each other. The wave is time varying frequency given by n, speed given by c= n o in a vacuum in a medium of refractive index n, the wavelength is changed by = o/n. z x y A full analysis of polarised light must include both the electric and magnetic components of the light; this is particularly necessary when considering reflected components In transmissive optical systems the reflected light does not have to be considered in such detail and the light is considered only in terms of the electric components. 47

Polarised light Light can have two orthogonal states or polarisations. The waves can be written as follows: If f = 0 or 2 p or an integral multiple then the light is plane polarised with The direction of propagation is taken to be along z These are waves travelling in the z-direction with a relative phase between them of f. 48

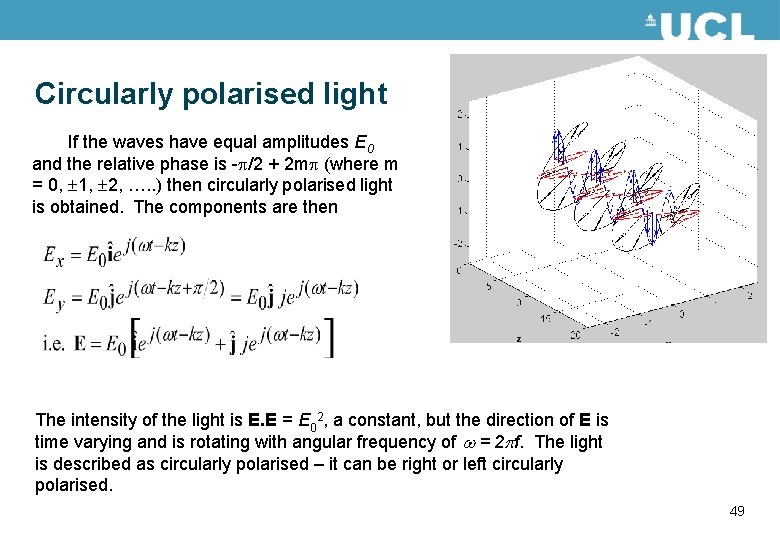
Circularly polarised light If the waves have equal amplitudes E 0 and the relative phase is -p/2 + 2 mp (where m = 0, 1, 2, …. . ) then circularly polarised light is obtained. The components are then The intensity of the light is E. E = E 02, a constant, but the direction of E is time varying and is rotating with angular frequency of w = 2 pf. The light is described as circularly polarised – it can be right or left circularly polarised. 49

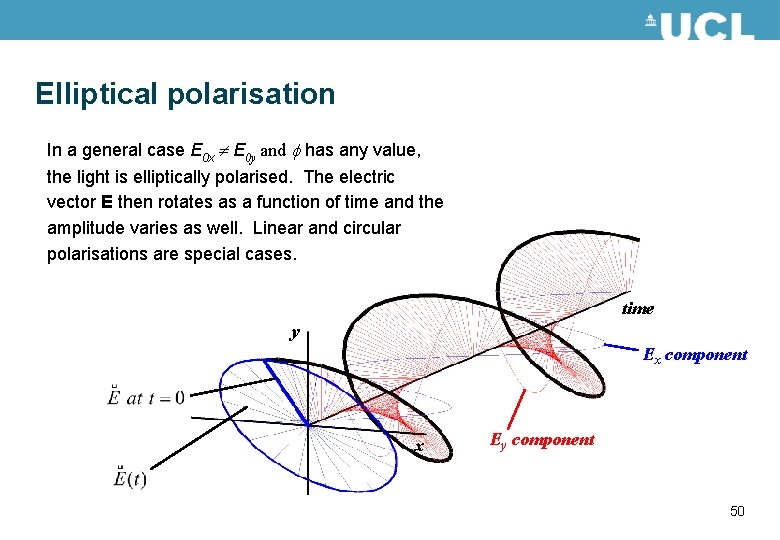
Elliptical polarisation In a general case E 0 x E 0 y and f has any value, the light is elliptically polarised. The electric vector E then rotates as a function of time and the amplitude varies as well. Linear and circular polarisations are special cases. time y Ex component x Ey component 50

Optical phase through a birefringent layer • As the two polarisations pass through the crystal the relative phase between them will change, because the values of k=2 pn/ will be different. • If a crystal has a thickness of d then one polarisation will have phase relative to z=0 of f=2 pnod/ , and the other of f=2 pnxd/. • The phase difference is f=2 p(nx-no)d/ 51

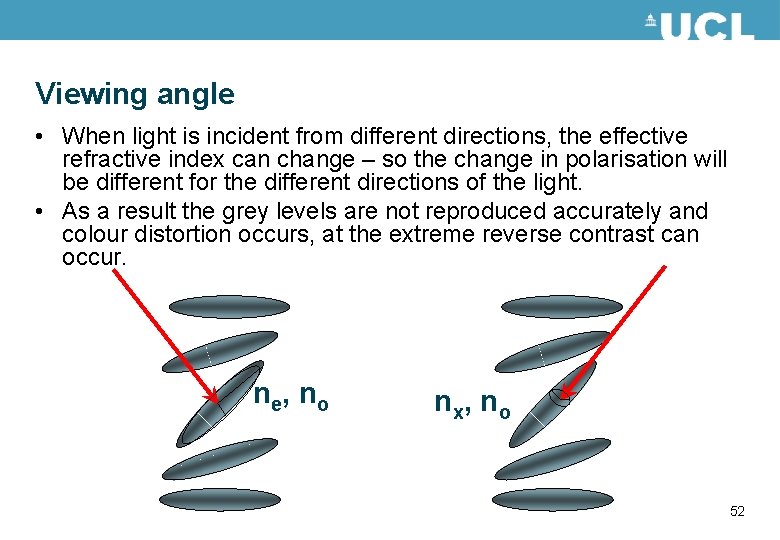
Viewing angle • When light is incident from different directions, the effective refractive index can change – so the change in polarisation will be different for the different directions of the light. • As a result the grey levels are not reproduced accurately and colour distortion occurs, at the extreme reverse contrast can occur. n e, n o n x, n o 52

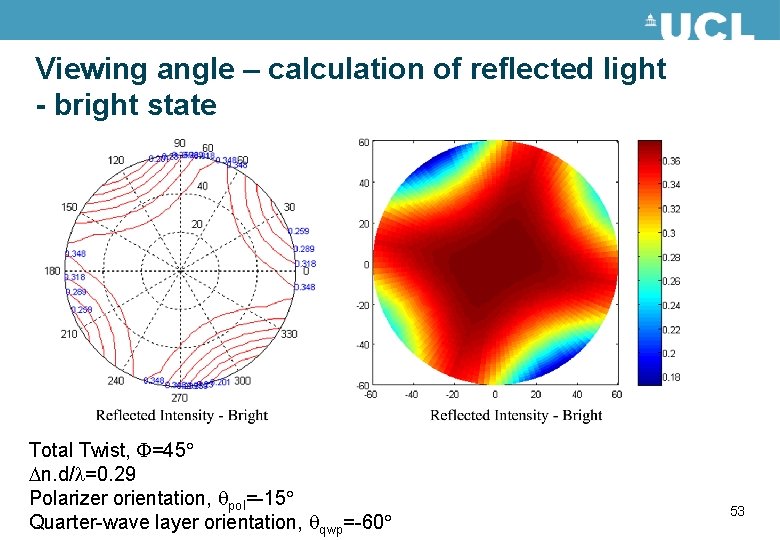
Viewing angle – calculation of reflected light - bright state Total Twist, =45 n. d/ =0. 29 Polarizer orientation, pol=-15 Quarter-wave layer orientation, qwp=-60 53

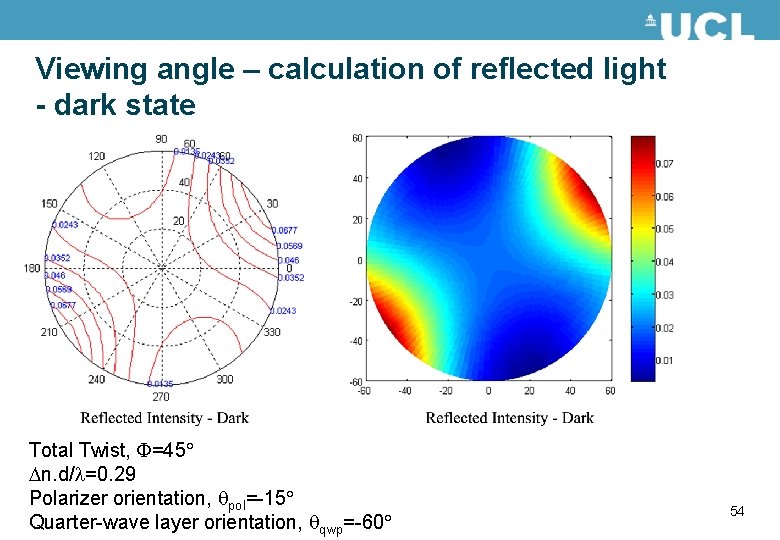
Viewing angle – calculation of reflected light - dark state Total Twist, =45 n. d/ =0. 29 Polarizer orientation, pol=-15 Quarter-wave layer orientation, qwp=-60 54

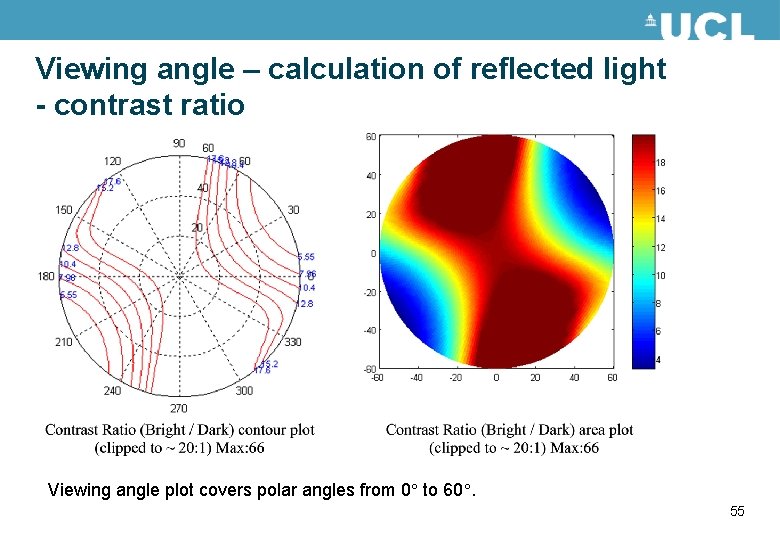
Viewing angle – calculation of reflected light - contrast ratio Viewing angle plot covers polar angles from 0 to 60. 55

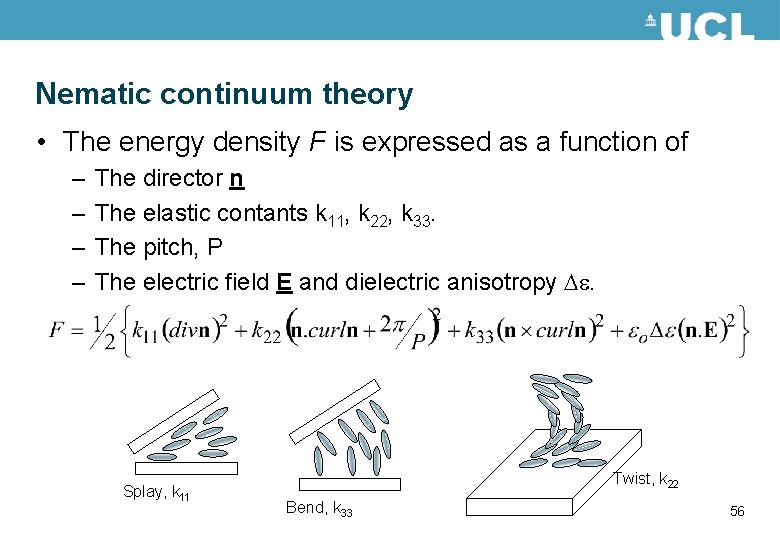
Nematic continuum theory • The energy density F is expressed as a function of – – The director n The elastic contants k 11, k 22, k 33. The pitch, P The electric field E and dielectric anisotropy e. Splay, k 11 Twist, k 22 Bend, k 33 56

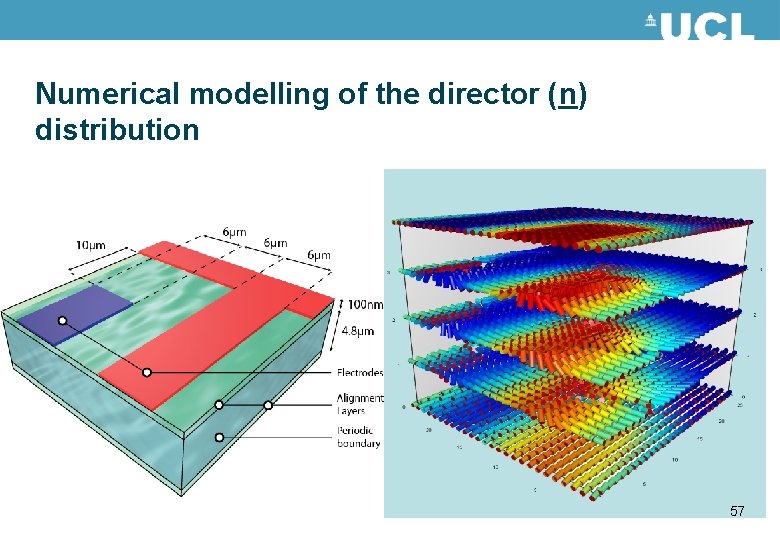
Numerical modelling of the director (n) distribution 57

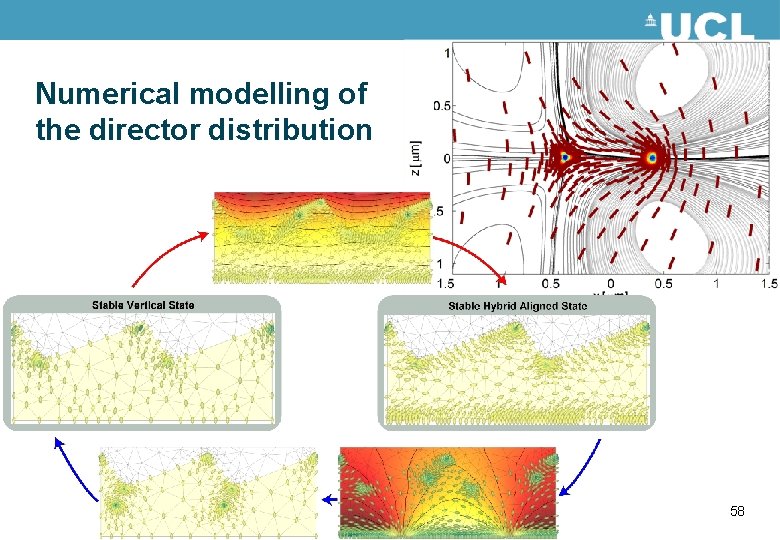
Numerical modelling of the director distribution 58

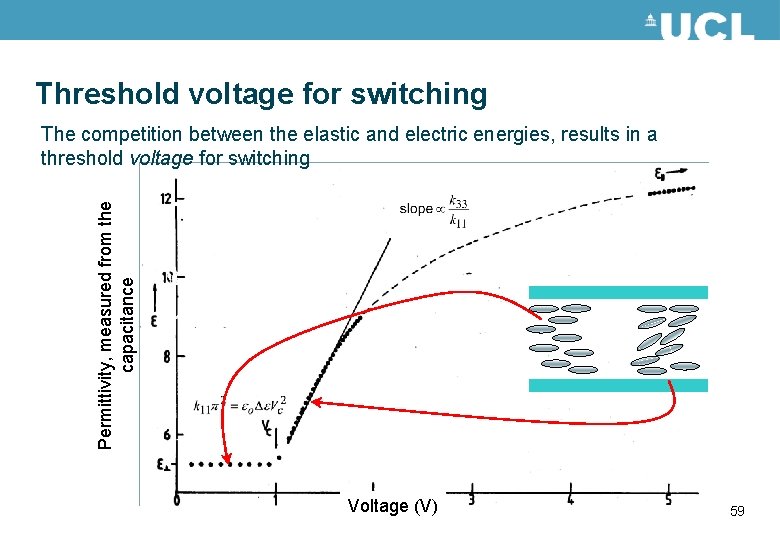
Threshold voltage for switching Permittivity, measured from the capacitance The competition between the elastic and electric energies, results in a threshold voltage for switching Voltage (V) 59

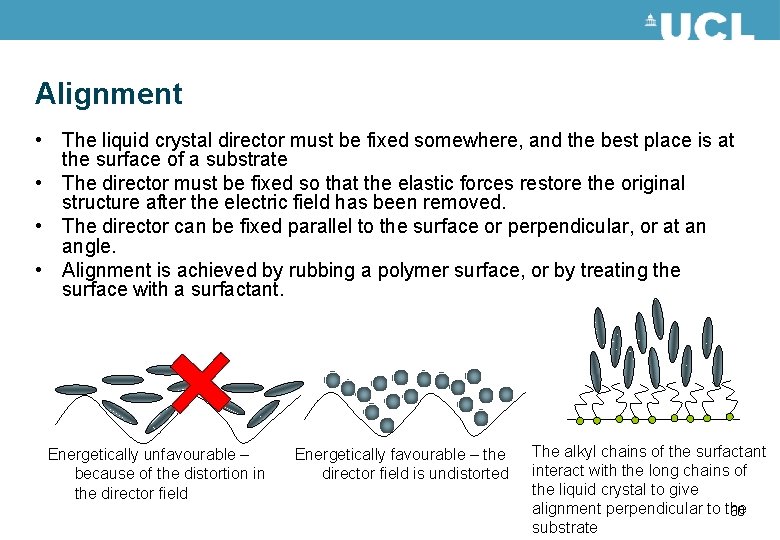
Alignment • The liquid crystal director must be fixed somewhere, and the best place is at the surface of a substrate • The director must be fixed so that the elastic forces restore the original structure after the electric field has been removed. • The director can be fixed parallel to the surface or perpendicular, or at an angle. • Alignment is achieved by rubbing a polymer surface, or by treating the surface with a surfactant. Energetically unfavourable – because of the distortion in the director field Energetically favourable – the director field is undistorted The alkyl chains of the surfactant interact with the long chains of the liquid crystal to give alignment perpendicular to the 60 substrate

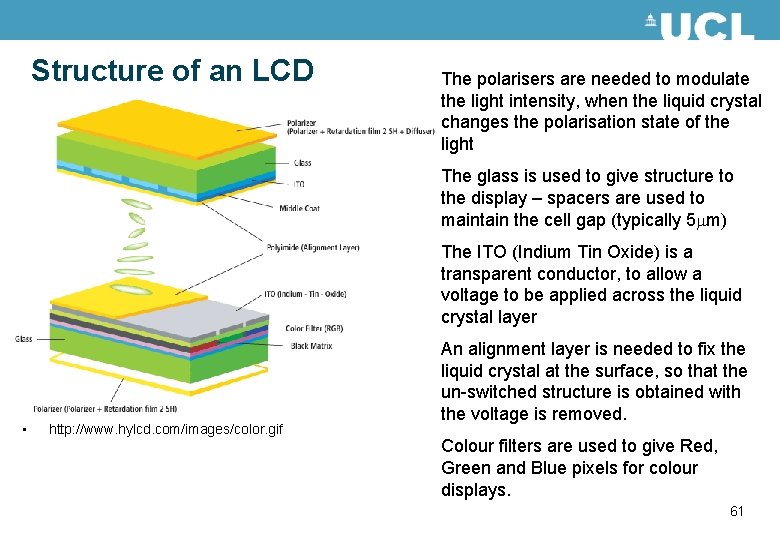
Structure of an LCD The polarisers are needed to modulate the light intensity, when the liquid crystal changes the polarisation state of the light The glass is used to give structure to the display – spacers are used to maintain the cell gap (typically 5 mm) The ITO (Indium Tin Oxide) is a transparent conductor, to allow a voltage to be applied across the liquid crystal layer • http: //www. hylcd. com/images/color. gif An alignment layer is needed to fix the liquid crystal at the surface, so that the un-switched structure is obtained with the voltage is removed. Colour filters are used to give Red, Green and Blue pixels for colour displays. 61

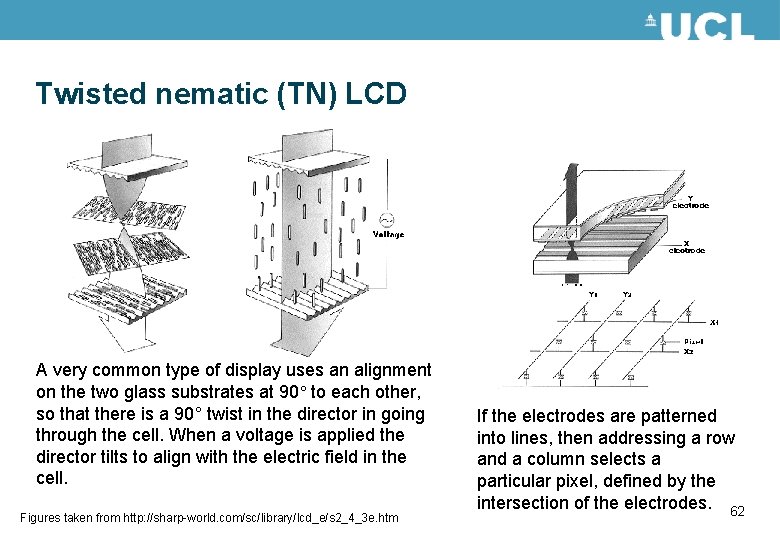
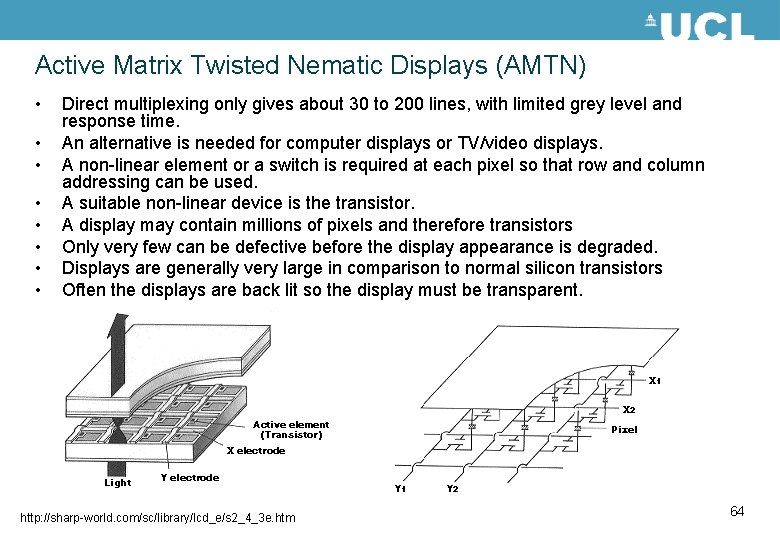
Twisted nematic (TN) LCD A very common type of display uses an alignment on the two glass substrates at 90° to each other, so that there is a 90° twist in the director in going through the cell. When a voltage is applied the director tilts to align with the electric field in the cell. Figures taken from http: //sharp-world. com/sc/library/lcd_e/s 2_4_3 e. htm If the electrodes are patterned into lines, then addressing a row and a column selects a particular pixel, defined by the intersection of the electrodes. 62

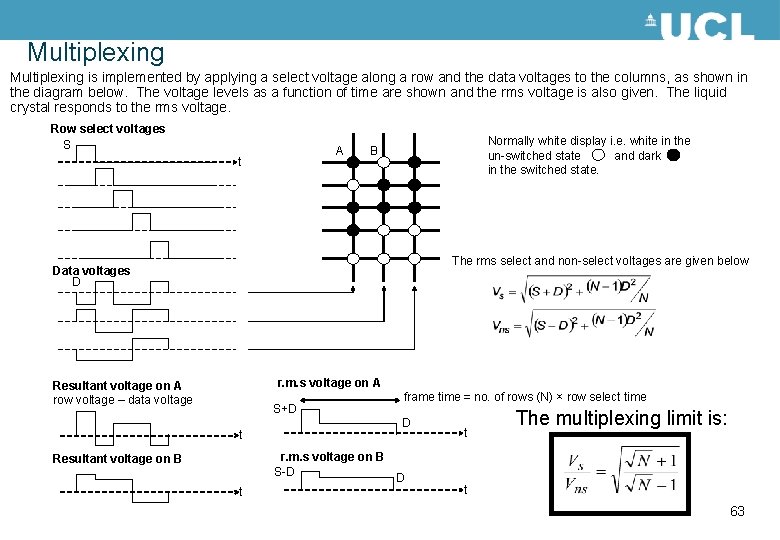
Multiplexing is implemented by applying a select voltage along a row and the data voltages to the columns, as shown in the diagram below. The voltage levels as a function of time are shown and the rms voltage is also given. The liquid crystal responds to the rms voltage. Row select voltages S A t Normally white display i. e. white in the un-switched state and dark in the switched state. B The rms select and non-select voltages are given below Data voltages D r. m. s voltage on A Resultant voltage on A row voltage – data voltage S+D t frame time = no. of rows (N) × row select time D r. m. s voltage on B S-D D Resultant voltage on B t t The multiplexing limit is: t 63

Active Matrix Twisted Nematic Displays (AMTN) • • Direct multiplexing only gives about 30 to 200 lines, with limited grey level and response time. An alternative is needed for computer displays or TV/video displays. A non-linear element or a switch is required at each pixel so that row and column addressing can be used. A suitable non-linear device is the transistor. A display may contain millions of pixels and therefore transistors Only very few can be defective before the display appearance is degraded. Displays are generally very large in comparison to normal silicon transistors Often the displays are back lit so the display must be transparent. http: //sharp-world. com/sc/library/lcd_e/s 2_4_3 e. htm 64

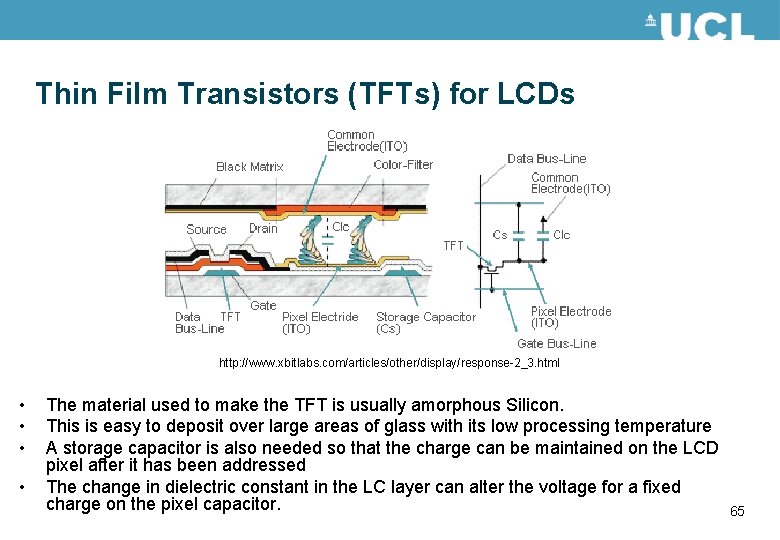
Thin Film Transistors (TFTs) for LCDs http: //www. xbitlabs. com/articles/other/display/response-2_3. html • • The material used to make the TFT is usually amorphous Silicon. This is easy to deposit over large areas of glass with its low processing temperature A storage capacitor is also needed so that the charge can be maintained on the LCD pixel after it has been addressed The change in dielectric constant in the LC layer can alter the voltage for a fixed charge on the pixel capacitor. 65

Materials for TFTs Amorphous Silicon + easy to deposit over large areas and at low temperature - compatible with glass substrates - But it has relatively low mobility - Larger areas are required to overcome the low mobility, so that the pixel can be addressed in the line address time - The transistor must be shielded so that no photoconduction occurs - Large shielding reduces the light throughput - Large transistors mean that the leakage current can be a problem Poly-silicon - Amorphous Silicon can be re-crystallised by local heating to form poly-crystalline Silicon + The higher mobility means that transistors are fast enough to be used for control and signal processing as well as the pixel switches + Inclusion of some electronics onto the glass means fewer connections to the glass, improving performance and simplifying fabrication. + Smaller transistors mean lower leakage currents and so the pixels hold the charge better 66

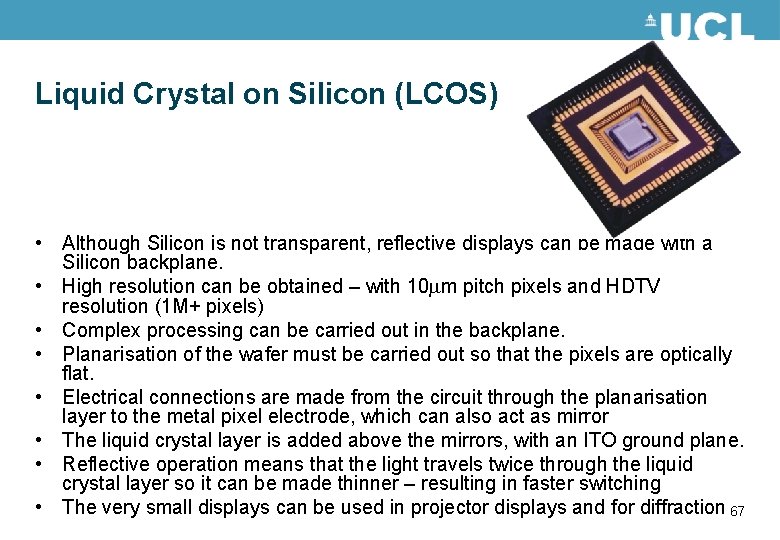
Liquid Crystal on Silicon (LCOS) • Although Silicon is not transparent, reflective displays can be made with a Silicon backplane. • High resolution can be obtained – with 10 mm pitch pixels and HDTV resolution (1 M+ pixels) • Complex processing can be carried out in the backplane. • Planarisation of the wafer must be carried out so that the pixels are optically flat. • Electrical connections are made from the circuit through the planarisation layer to the metal pixel electrode, which can also act as mirror • The liquid crystal layer is added above the mirrors, with an ITO ground plane. • Reflective operation means that the light travels twice through the liquid crystal layer so it can be made thinner – resulting in faster switching • The very small displays can be used in projector displays and for diffraction 67

LC modes for Active Matrix Liquid Crystal Displays (AMTN) • Conventionally the 90° twisted nematic liquid crystal is used but: Twisted nematics have a problem with the viewing angle characteristics and the dark state. • Other LC modes to improve the properties are - Vertically Aligned Nematic (VAN) - In-Plane Switching • Compensation films, which are birefringent films, can be added to the front surface of the device to compensate for the off-axis birefringence variations. 68

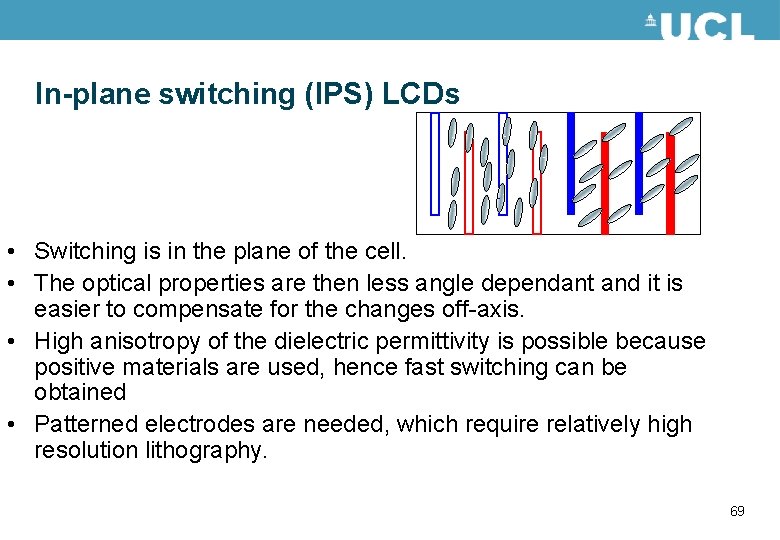
In-plane switching (IPS) LCDs • Switching is in the plane of the cell. • The optical properties are then less angle dependant and it is easier to compensate for the changes off-axis. • High anisotropy of the dielectric permittivity is possible because positive materials are used, hence fast switching can be obtained • Patterned electrodes are needed, which require relatively high resolution lithography. 69

In-plane switching (IPS) LCDs LC • Alternate voltages are applied to the electrodes • The modelled results show the director distribution around the end of an IPS electrode structure 70

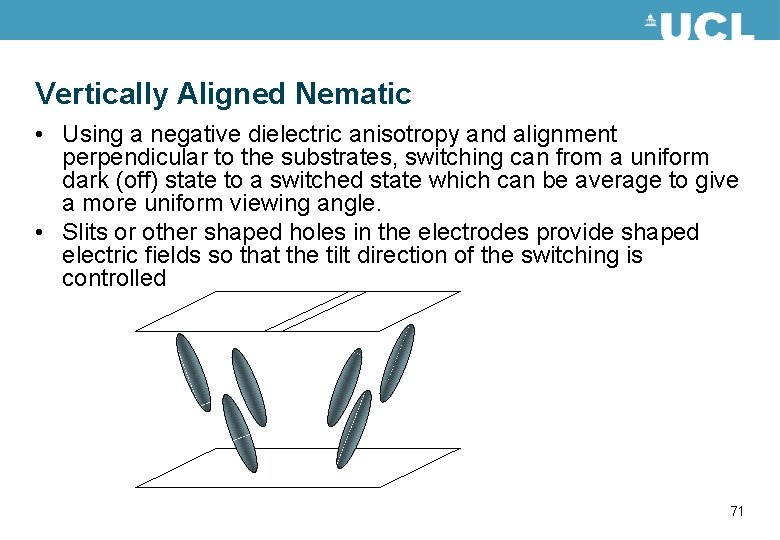
Vertically Aligned Nematic • Using a negative dielectric anisotropy and alignment perpendicular to the substrates, switching can from a uniform dark (off) state to a switched state which can be average to give a more uniform viewing angle. • Slits or other shaped holes in the electrodes provide shaped electric fields so that the tilt direction of the switching is controlled 71

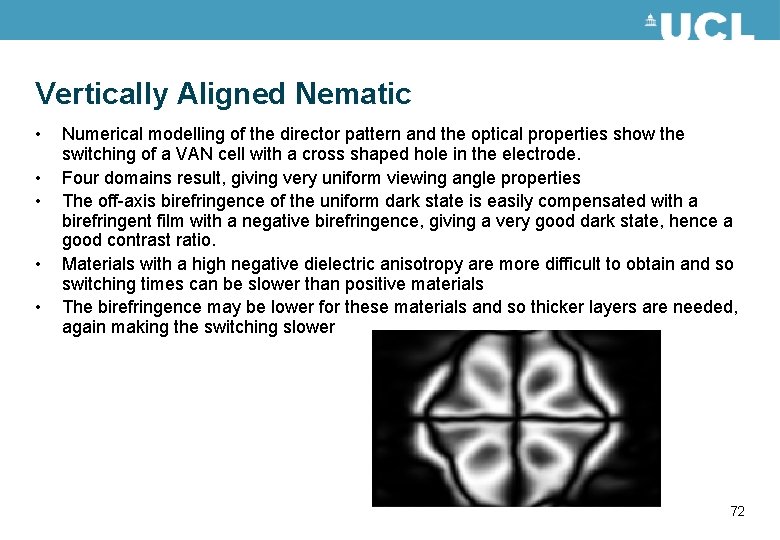
Vertically Aligned Nematic • • • Numerical modelling of the director pattern and the optical properties show the switching of a VAN cell with a cross shaped hole in the electrode. Four domains result, giving very uniform viewing angle properties The off-axis birefringence of the uniform dark state is easily compensated with a birefringent film with a negative birefringence, giving a very good dark state, hence a good contrast ratio. Materials with a high negative dielectric anisotropy are more difficult to obtain and so switching times can be slower than positive materials The birefringence may be lower for these materials and so thicker layers are needed, again making the switching slower 72

Cholesteric liquid crystals • Essentially the nematic phase, but with a chiral group which gives a twist to the structure. • The twist is often temperature dependant, but only if there is a Sm. A phase below the nematic and then the pitch increases in going closer the Sm. A phase, i. e. as the temperature decreases • The pitch of the twist can match the wavelength of light, , in which case selective reflection occurs across a band when = n. P, with n, the refractive index, going from ne to no. (Sometimes called Bragg scattering) • Only the circular polarisation matching the twist sense is selectively reflected • In droplet form, encapsulated into a polymer the cholesteric can be used as a thermometer • In an aligned form it can be used as a circular polariser, or as the reflector for a laser system. 73

Liquid crystals for laser systems and photonics • • • Laser dye molecules can be added to the cholesteric liquid crystal and then optically pumped and the liquid crystal structure forms the laser cavity. Changing the temperature slightly would then tune the wavelength of the laser Some cholesteric systems have been shown to have electrically tuned pitch, which allows electrical tuning of the laser wavelength The cavity can be made very small, which has advantages in laser operation Improvements will be to synthesis liquid crystal laser dye materials, which would allow very high concentrations of the dye and to find a method of electrically pumping the laser Blue phases are a three dimensional twisted structure, which is very interesting as a 3 D distributed Bragg system. Recent developments using dimers have allowed blue phases to exist over a wide temperature range. These have potential as self assembled photonic band gap structures A defect formed in the 3 D structure, or a line of defects give localised transmission of light in the band gap and control of photon movement through the structure. 74

Diffractive displays • Conventional LC displays rely on transmission/reflection or absorption of the light • This is inefficient since light is absorbed rather than redirected • Diffraction offers a way of steering the light into the region where it is required • Requires coherent illumination (i. e. laser light) and very high resolution structures (sub-wavelength) in the diffracting medium • The Fourier transform of the image is obtained and this is displayed on a Spatial Light Modulator (SLM) – or display! • Diffraction from the Fourier transform reconstructs the original image • Often image compression relies on taking the transform, so the processing could be simplified, so reducing the data rates required to the display • Only LCOS systems provide the resolution required • Both binary Ferroelectric liquid crystal and nematic LC can be used. • Intensity modulation of the diffraction pattern can be used, but phase modulation is more efficient 75

Diffractive Display demonstrated at Cambridge University Using Binary FLC LCOS Using analogue nematic LCOS 76

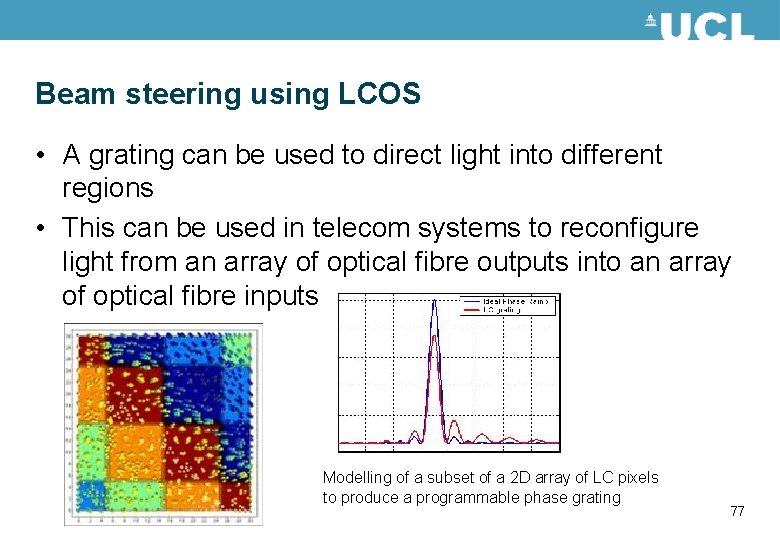
Beam steering using LCOS • A grating can be used to direct light into different regions • This can be used in telecom systems to reconfigure light from an array of optical fibre outputs into an array of optical fibre inputs Modelling of a subset of a 2 D array of LC pixels to produce a programmable phase grating 77

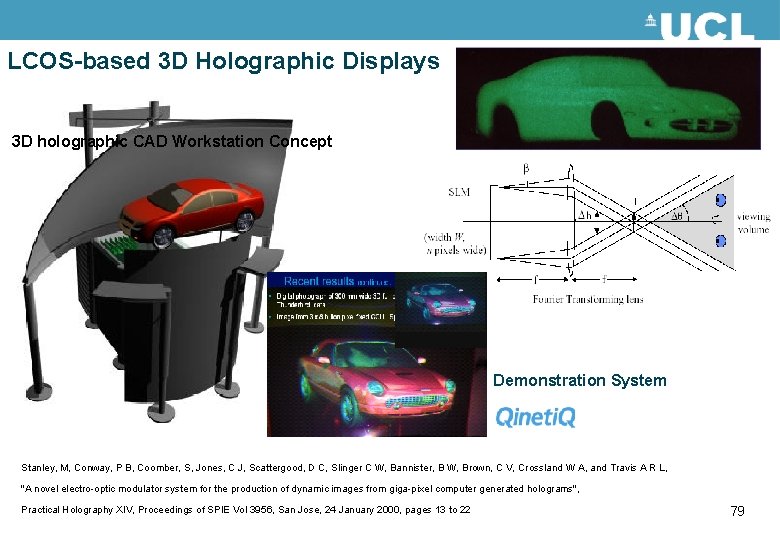
Holograms in LC devices • As well as providing a 2 D image by diffraction, at a higher resolution a hologram can be generated, which is a 3 D ‘image’. • The phase front of the light reflected from an object is reconstructed and this then appears like the object itself. • This has been demonstrated by Qinetiq, but is not in production 78

LCOS-based 3 D Holographic Displays 3 D holographic CAD Workstation Concept Demonstration System Stanley, M, Conway, P B, Coomber, S, Jones, C J, Scattergood, D C, Slinger C W, Bannister, B W, Brown, C V, Crossland W A, and Travis A R L, "A novel electro-optic modulator system for the production of dynamic images from giga-pixel computer generated holograms", Practical Holography XIV, Proceedings of SPIE Vol 3956, San Jose, 24 January 2000, pages 13 to 22 79

Bistable displays • • For portable displays the energy consumption is critical Bistability allows the display to be addressed and then it can be left in that state. The power can be removed once the display has been addressed, giving very low power operation for displays which do not have rapidly varying information Applications are for e-books, supermarket shelf labels, some mobile phone applications 80
![Post Aligned Bistable Device PABN 3 D Modelling Window 1 2 Device under Post Aligned Bistable Device (PABN) 3 -D Modelling Window [1], [2] • Device under](https://slidetodoc.com/presentation_image_h/a5fe9cdcaadc86dd3b9c7c866a77e12b/image-81.jpg)
Post Aligned Bistable Device (PABN) 3 -D Modelling Window [1], [2] • Device under development at Hewlett-Packard. • Periodic array of microscopic square posts. • Two stable states of operation, planar and tilted. 2. 6 – 3. 0 µm Top: Homeotropic Sides: Periodic 0. 6 µm 1. 2 µm Bottom and Post: Planar Degenerate [1] Kitson S. Geisow A. , “Bistable Alignment of Nematic Liquid Crystals Around Microscopic Posts”, Mol. Cryst. Liq. Cryst. , vol. 412, pp. 153 -161, 2004 [2] Kitson S. Geisow A. , ”Controllable Alignment of Liquid Crystals Around Microscopic Posts: Stabilisation of Multiple States”, Appl. Phys. Lett. , vol. 80, no. 19, pp. 3635 -3637, May 2002 81

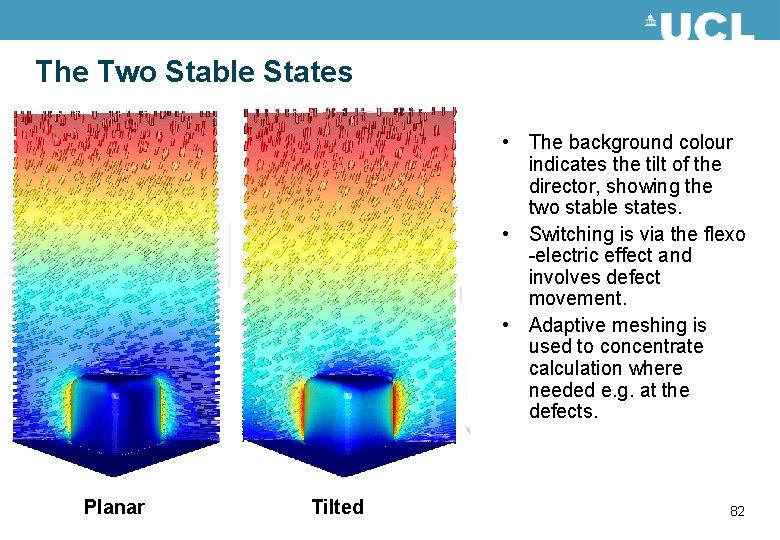
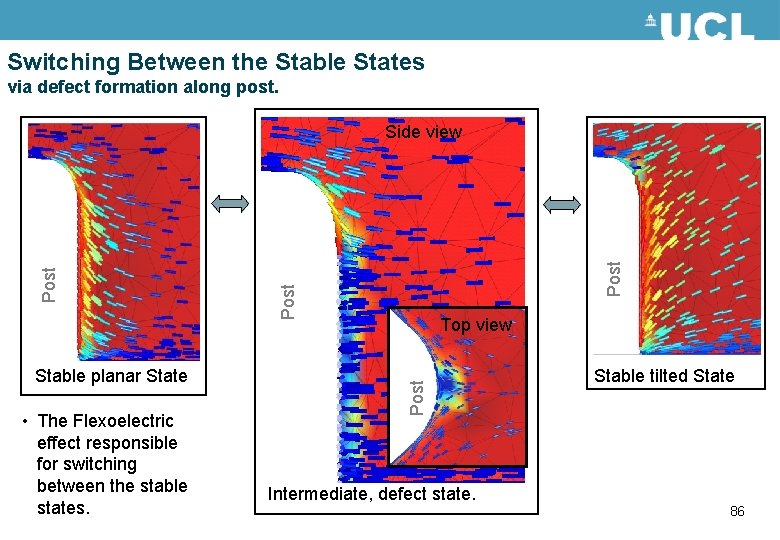
The Two Stable States • The background colour indicates the tilt of the director, showing the two stable states. • Switching is via the flexo -electric effect and involves defect movement. • Adaptive meshing is used to concentrate calculation where needed e. g. at the defects. Planar Tilted 82

Doping liquid crystals • Adding anisotropic dyes to liquid crystals has been done for a long time for simple displays. • The alignment in the liquid crystal aligns the dye molecules which can then be switched • Absorption occurs only along one direction of the dye and so the light absorption can be switched • Other additives are being considered – carbon nano-tubes, very small particles of ferroelectric crystal, biological molecules • This could be to enhance the anisotropy of the liquid crystal properties or to control the orientation of the dopant • Control of the position of defects in the liquid crystal can be used to move particles in the liquid crystal and so could be used to position very small particles A Pair of Point Defects http: //www. lci. kent. edu/polmicpic. html 83

Colloidal crystals in liquid crystal “Two-Dimensional Nematic Colloidal Crystals Self-Assembled by Topological Defects” Muševič Igor, Škarabot Miha, Tkalec Uroš, Miha Ravnik, Slobodan Žumer, Science (vol. 313 p. 954) 84

Summary • Liquid crystals give electrically switchable anisotropic properties • The organic molecules can be synthesized to have many different properties and mixture formulation allows greater flexibility and control • Optical anisotropy can be switched so as to modulate the polarisation of light, or the phase of light passing through the layer • Displays are the most common application, but there are many different configurations depending on the application requirements • High resolution structures in the liquid crystal allow them to be used as diffraction or holographic displays • Future applications in photonics may be based on the use of particles or dopants in the liquid crystal 85

Switching Between the Stable States via defect formation along post. • The Flexoelectric effect responsible for switching between the stable states. Post Top view Post Stable planar State Post Side view Stable tilted State Intermediate, defect state. 86