Lipopheresis an New Approach to Resistant FSGS www









![Efficacy just after LIPOSORBER® treatment Non-effective 31. 9% [15/47] ≧ 3. 5 → < Efficacy just after LIPOSORBER® treatment Non-effective 31. 9% [15/47] ≧ 3. 5 → <](https://slidetodoc.com/presentation_image_h/b7288579f994bf2c48fe0c2a863d35cd/image-30.jpg)




- Slides: 47

Lipopheresis an New Approach to Resistant FSGS www. akronchildrens. org/giving

Lipopheresis an New Approach to Resistant FSGS Rupesh Raina, MD, FAAP, FACP, FASN and FNKF Consultant Nephrologist Adult-Pediatric Kidney Disease/Hypertension Associate Professor of Medicine Staff Medical Research, Rebecca D. Considine Research Staff at AGMC at Cleveland Clinic and Akron Children's Hospital, Akron, Ohio Council Member for Faculty of Internal Medicine - Northeast Ohio Medical University, Rootstown, Ohio Faculty Staff at Case Western Reserve University School of Medicine, Cleveland, Ohio. www. akronchildrens. org/giving

Focal segmental glomerulosclerosis (FSGS) • It is a histologic finding that may result from a variety of insults to the kidney • FSGS typically presents with proteinuria and has a high risk of progressive loss of renal function • Therapy of primary FSGS incorporates conservative management and immunosuppression regimens to control proteinuria and preserve kidney function. • In long-term cohort studies in adults and children with primary FSGS, renal survival has been directly associated with degree of proteinuria control www. akronchildrens. org/giving

Focal segmental glomerulosclerosis (FSGS) • Current strategies for control of FSGS use a stepwise approach with a goal of normalization of urinary protein excretion and the prevention of kidney failure. • Progress in this field remains a priority in order to prevent the trajectory toward renal failure for patients proven to be resistant to treatment and to identify therapeutic regimens with minimal toxicity www. akronchildrens. org/giving

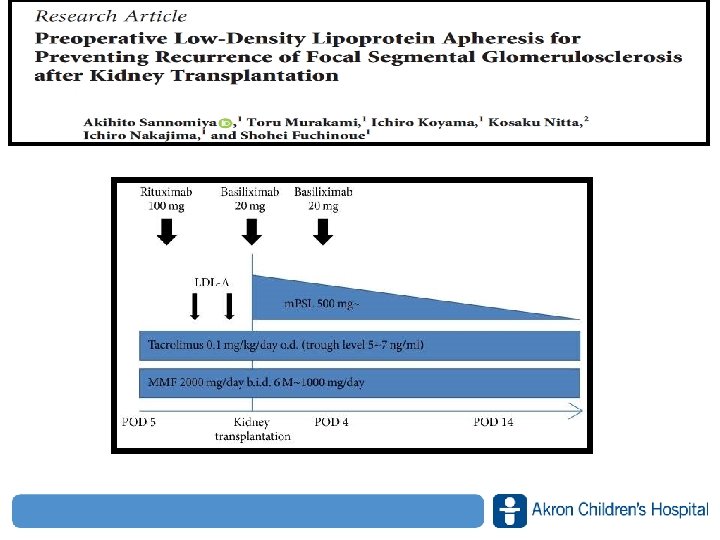
Recurrence of FSGS after Kidney Transplantation • FSGS can recur after kidney transplantation, and it was suggested that a humoral factor influencing glomerular permeability may be involved in its recurrence • The recurrence rate of FSGS is 20– 50% after transplantation and recurrence eventually leads to graft loss which occurs in about half of the patients • Steroid pulse therapy, plasma exchange, and administration of rituximab (an anti-CD 20 monoclonal antibody) has also been tried with minimal response J Transp Secur 2018: 1– 5 www. akronchildrens. org/giving

Recurrence of FSGS after Kidney Transplantation • FSGS after transplantation are recognized -Early recurrence characterized by a massive proteinuria within hours to days after transplantation -Late recurrence that develops insidiously several months or years after transplantation • The possibility of decreasing or absorbing the potent pathogenic permeability factor might be expected in both LDL apheresis and plasma exchange • At least super rapid recurrence of FSGS such as 1– 14 days after transplantation could be prevented with LDLapheresis J Transp Secur 2018: 1– 5 www. akronchildrens. org/giving

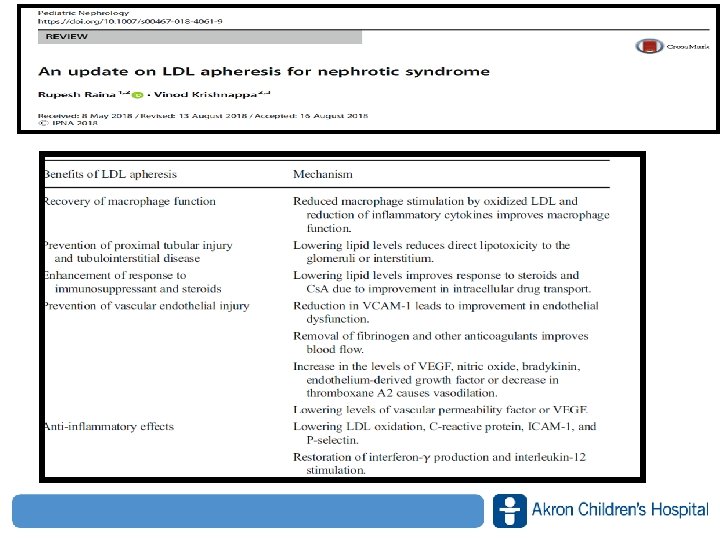
Liposorber Mechanism 1. Direct effect of lipid (LDL, VLDL, ox. LDL) adsorption a) Reduction of macrophage stimulation by ox-LDL b) Amelioration of macrophage dysfunction c) Reduction of inflammatory cytokines 2. Vasodilatory and anticoagulant effect by absorption of various pathogenic factors by dextran sulfate a) Reduction of fibrinogen and coagulatory factors b) Increase of VEGF/NO/bradykinin production, and decrease in thromboxane A 2 c) Absorption of vascular permeability factor 3. Enhancement of immunosuppressant response by improved intracellular drug transport a) Improved corticosteroid response b) Enhancement of transmembrane cyclosporine A transport via lipoprotein receptor c) Inhibitory effects upon MDR-1 gene expression www. akronchildrens. org/giving Kidney Int Suppl 71: S 6–S 9

www. akronchildrens. org/giving

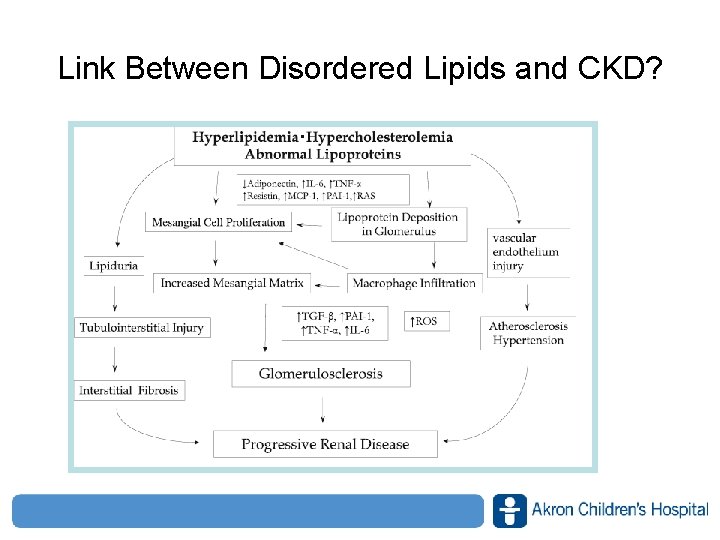
Link Between Disordered Lipids and CKD? www. akronchildrens. org/giving

www. akronchildrens. org/giving

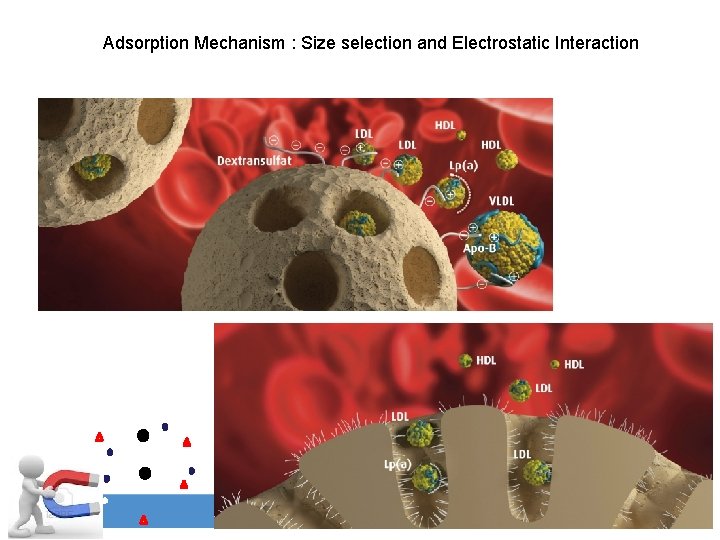
Adsorption Mechanism : Size selection and Electrostatic Interaction www. akronchildrens. org/giving

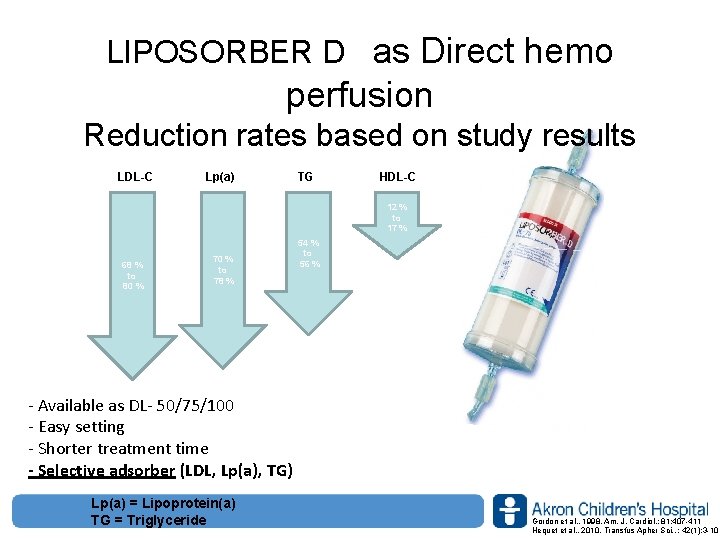
LIPOSORBER D as Direct hemo perfusion Reduction rates based on study results LDL-C Lp(a) TG HDL-C 12 % to 17 % 68 % to 80 % 54 % to 56 % 70 % to 78 % ‐ Available as DL‐ 50/75/100 ‐ Easy setting ‐ Shorter treatment time - Selective adsorber (LDL, Lp(a), TG) Lp(a) = Lipoprotein(a) TG = Triglyceride www. akronchildrens. org/giving Gordon et al. , 1998, Am. J. Cardiol. ; 81: 407 -411 Hequet et al. , 2010, Transfus Apher Sci. , ; 42(1): 3 -10

www. akronchildrens. org/giving

Liposorber Mechanism www. akronchildrens. org/giving

Liposorber Mechanism • Podocytes are capable of internalizing large amounts of extracellular fluid and molecules by macropinocytosis – nonspecific process that results in small vesicles of less than 0. 1 μm in diameter and involves both coated and noncoated vesicles • Active clearance mechanism to prevent the filter from clogging. • Podocytes play a specialized role in the uptake and removal of plasma proteins that cross the GBM. www. akronchildrens. org/giving

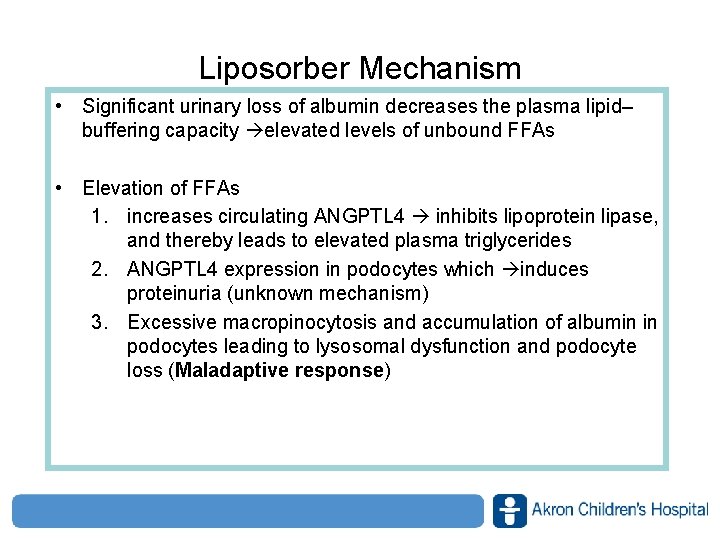
Liposorber Mechanism • Significant urinary loss of albumin decreases the plasma lipid– buffering capacity elevated levels of unbound FFAs • Elevation of FFAs 1. increases circulating ANGPTL 4 inhibits lipoprotein lipase, and thereby leads to elevated plasma triglycerides 2. ANGPTL 4 expression in podocytes which induces proteinuria (unknown mechanism) 3. Excessive macropinocytosis and accumulation of albumin in podocytes leading to lysosomal dysfunction and podocyte loss (Maladaptive response) www. akronchildrens. org/giving

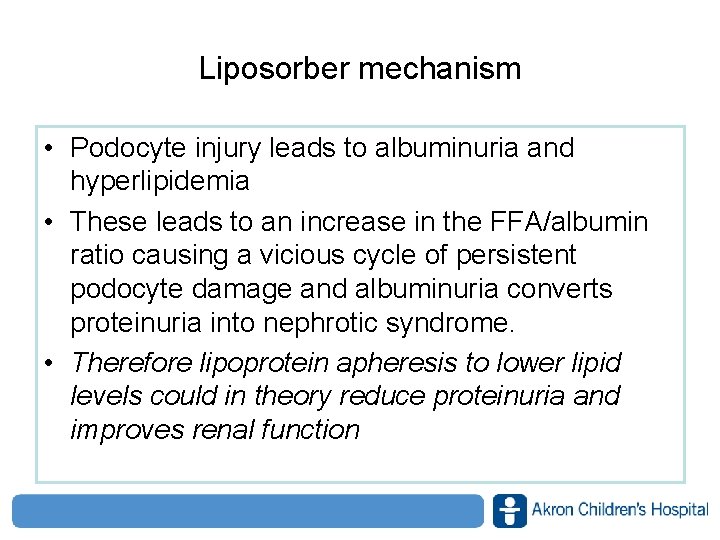
Liposorber mechanism • Podocyte injury leads to albuminuria and hyperlipidemia • These leads to an increase in the FFA/albumin ratio causing a vicious cycle of persistent podocyte damage and albuminuria converts proteinuria into nephrotic syndrome. • Therefore lipoprotein apheresis to lower lipid levels could in theory reduce proteinuria and improves renal function www. akronchildrens. org/giving

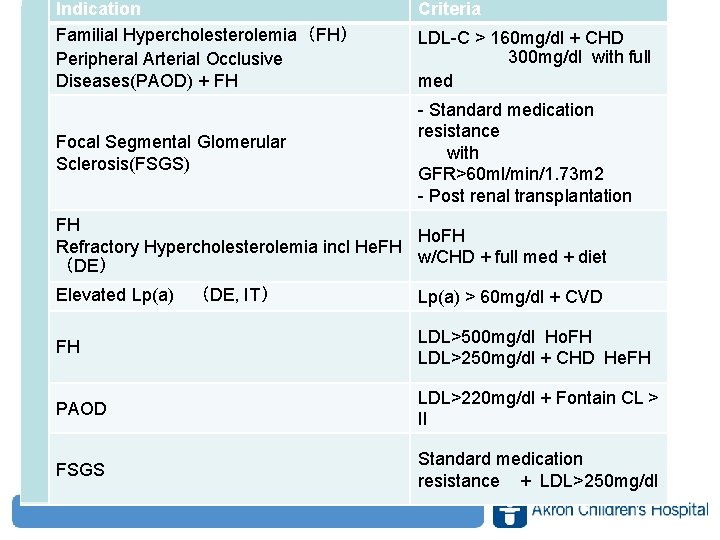
Indication Familial Hypercholesterolemia(FH) Peripheral Arterial Occlusive Diseases(PAOD) + FH U S Focal Segmental Glomerular Sclerosis(FSGS) Criteria LDL-C > 160 mg/dl + CHD 300 mg/dl with full med - Standard medication resistance with GFR>60 ml/min/1. 73 m 2 - Post renal transplantation FH Ho. FH Refractory Hypercholesterolemia incl He. FH E w/CHD + full med + diet (DE) U Elevated Lp(a) (DE, IT) Lp(a) > 60 mg/dl + CVD FH J PAOD P FSGS LDL>500 mg/dl Ho. FH LDL>250 mg/dl + CHD He. FH LDL>220 mg/dl + Fontain CL > II Standard medication resistance + LDL>250 mg/dl www. akronchildrens. org/giving

www. akronchildrens. org/giving

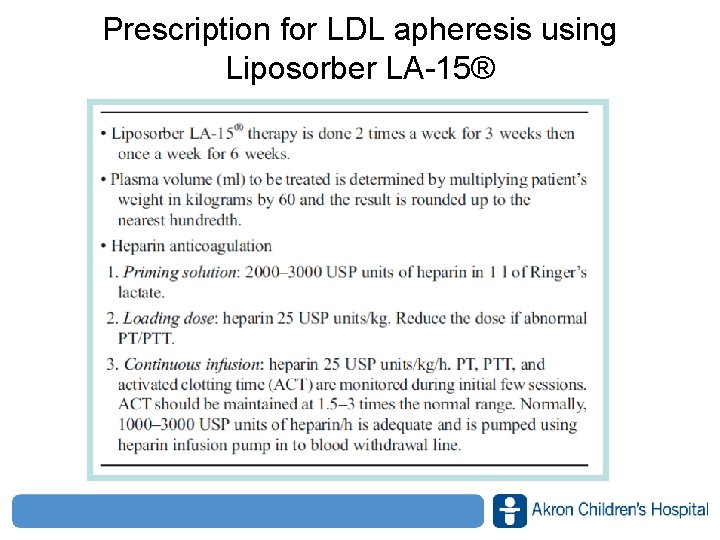
Prescription for LDL apheresis using Liposorber LA-15® www. akronchildrens. org/giving

Using the Liposorber to Treat Nephrotic Syndrome • In a sense nephrotic syndrome represents a common endpoint for podocyte injury • It doesn’t matter that there could be 1000 mechanism of podocyte injury • One example is the pt that would otherwise need nephrectomies, dialysis and then transplant – with the risk of recurrence – Could the liposorber allow this pt to keep their kidneys • Another example: congenital nephrotic syndrome www. akronchildrens. org/giving

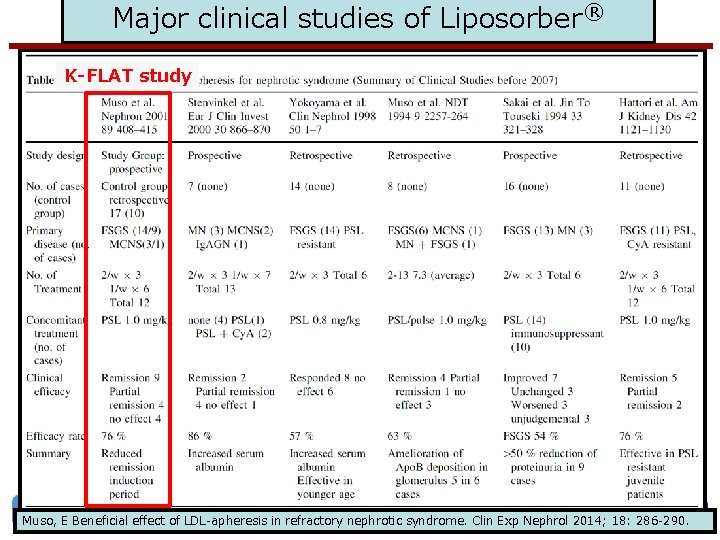
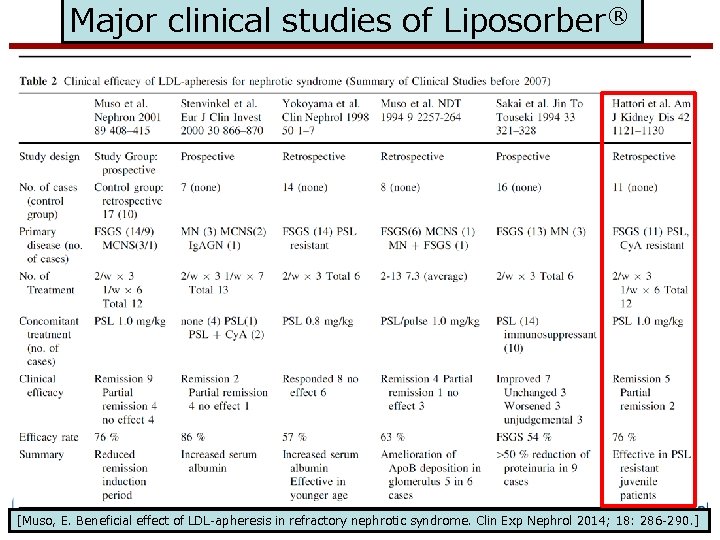
Major clinical studies of Liposorber® K-FLAT study www. akronchildrens. org/giving Muso, E Beneficial effect of LDL-apheresis in refractory nephrotic syndrome. Clin Exp Nephrol 2014; 18: 286 -290.

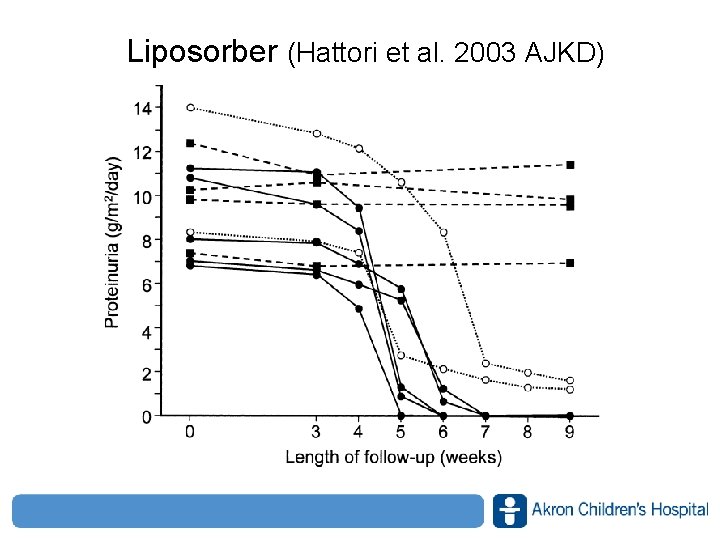
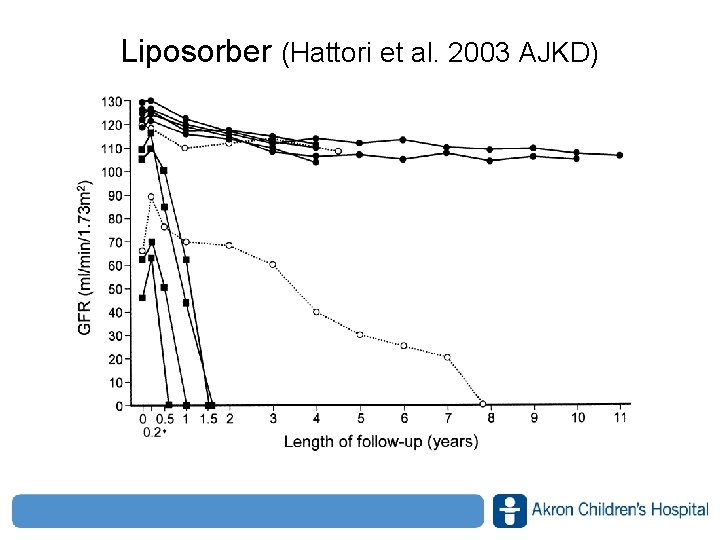
Liposorber (Hattori et al. 2003 AJKD) www. akronchildrens. org/giving

Liposorber (Hattori et al. 2003 AJKD) www. akronchildrens. org/giving

Liposorber (Hattori et al. 2003 AJKD) • What predicted a response? – Degree of tubulointerstitial lesions (lower better) – Selectivity of proteinuria (higher better) • Only complication was a line infection • Limitations – Small numbers, – ? Spontaneous remission – ? PDN response www. akronchildrens. org/giving

8. 7 ± 4. 0 6. 2 ± 3. 3 ↓ ↓ 8. 2 ± 7. 7 2. 7 ± 2. 7 Albumin [g/d. L] Urinary protein [g/day] Improvement of urinary protein (a) and serum protein (b) SM group LDL-A group 2. 8 ± 0. 4 2. 7 ± 0. 7 ↓ ↓ 2. 9 ± 0. 7 3. 1 ± 0. 7 SM group LDL-A : LDL-apheresis,SM : steroid monotherapy Muso, E et al. , Nephronwww. akronchildrens. org/giving 2001; 89: 408‐ 415 LDL-A group

Major clinical studies of Liposorber® www. akronchildrens. org/giving [Muso, E. Beneficial effect of LDL-apheresis in refractory nephrotic syndrome. Clin Exp Nephrol 2014; 18: 286 -290. ]

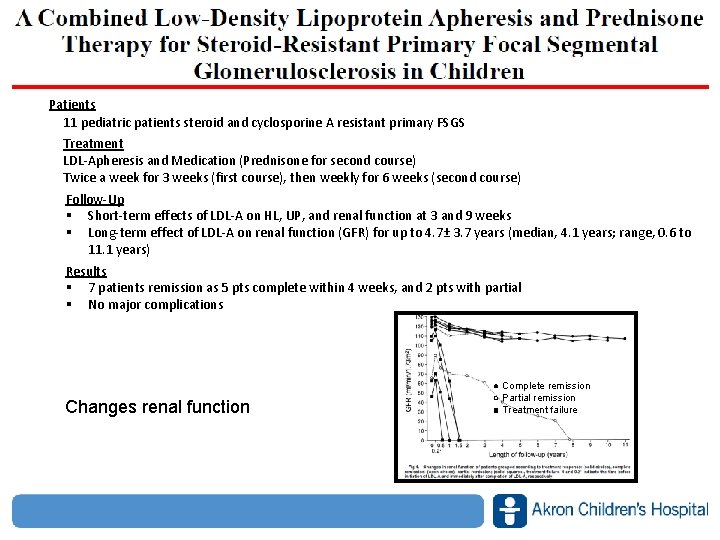
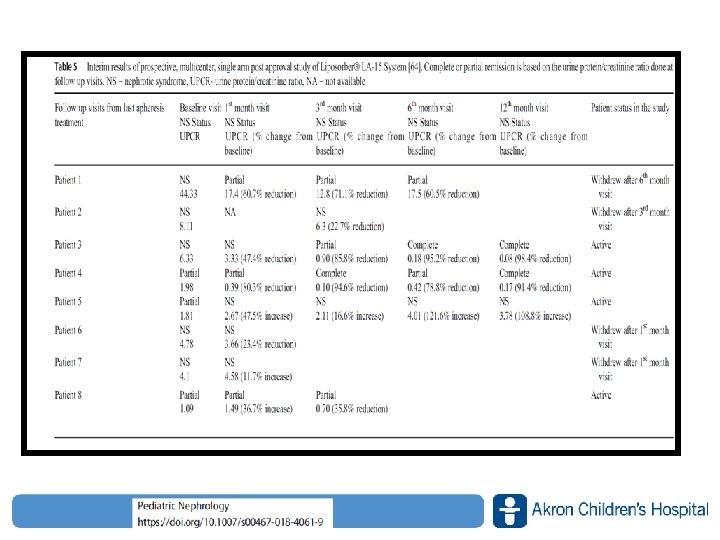
Patients 11 pediatric patients steroid and cyclosporine A resistant primary FSGS Treatment LDL‐Apheresis and Medication (Prednisone for second course) Twice a week for 3 weeks (first course), then weekly for 6 weeks (second course) Follow‐Up § Short‐term effects of LDL‐A on HL, UP, and renal function at 3 and 9 weeks § Long‐term effect of LDL‐A on renal function (GFR) for up to 4. 7± 3. 7 years (median, 4. 1 years; range, 0. 6 to 11. 1 years) Results § 7 patients remission as 5 pts complete within 4 weeks, and 2 pts with partial § No major complications ● Complete remission ○ Partial remission ■ Treatment failure Changes renal function www. akronchildrens. org/giving

POLARIS study Prospective Observational Survey on the Long-term Effects of LDL Apheresis on Drug-Resistant Nephrotic Syndrome - Multi centric prospective study, 47 pts enrolled - Observational study in ordinary clinical practice. No study protocol, physician's discretion oriented - Evaluate Short term clinical effectiveness --- 1 month Long term outcome --- 2 years www. akronchildrens. org/giving
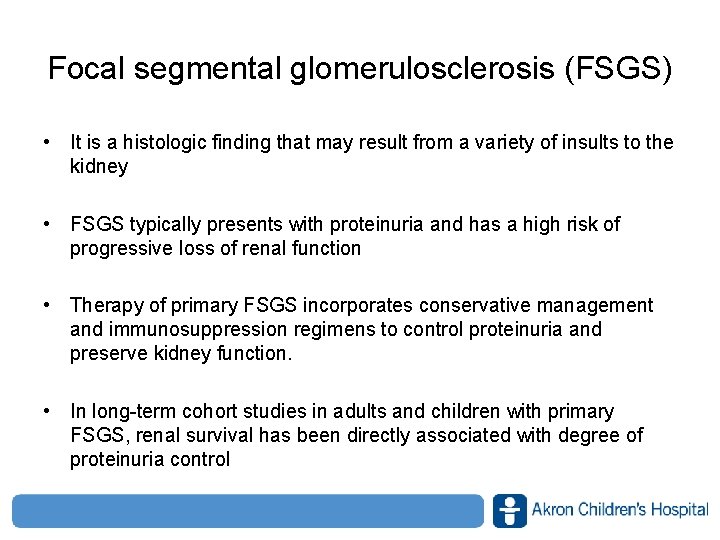
![Efficacy just after LIPOSORBER treatment Noneffective 31 9 1547 3 5 Efficacy just after LIPOSORBER® treatment Non-effective 31. 9% [15/47] ≧ 3. 5 → <](https://slidetodoc.com/presentation_image_h/b7288579f994bf2c48fe0c2a863d35cd/image-30.jpg)
Efficacy just after LIPOSORBER® treatment Non-effective 31. 9% [15/47] ≧ 3. 5 → < 1. 5 g 21. 3% [10/47] ≧ 3. 5 1. 0 ‐ 3. 5 g/day 31. 9% [15/47] Efficacy rate : 53. 2 % [25/47] www. akronchildrens. org/giving

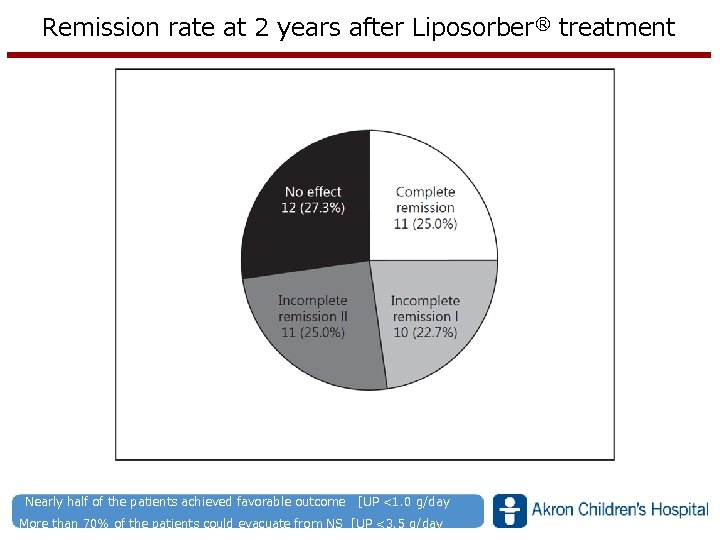
Remission rate at 2 years after Liposorber® treatment Nearly half of the patients achieved favorable outcome [UP <1. 0 g/day www. akronchildrens. org/giving More than 70% of the patients could evacuate from NS [UP <3. 5 g/day

LDL apheresis improves corticosteroids action by restoration of cellular uptake and inhibition of drug efflux LDL apheresis LDL Apheresis Repress MDR-1 gene Upregulate GC receptor S. Uchida at al Y. Chikamoto at al Mechanism of glucocorticoid action and factors engaged in development of steroid resistance www. akronchildrens. org/giving [Świerczewska, M et al. Acta Biochimica 60: 339 -344, 2013 ]

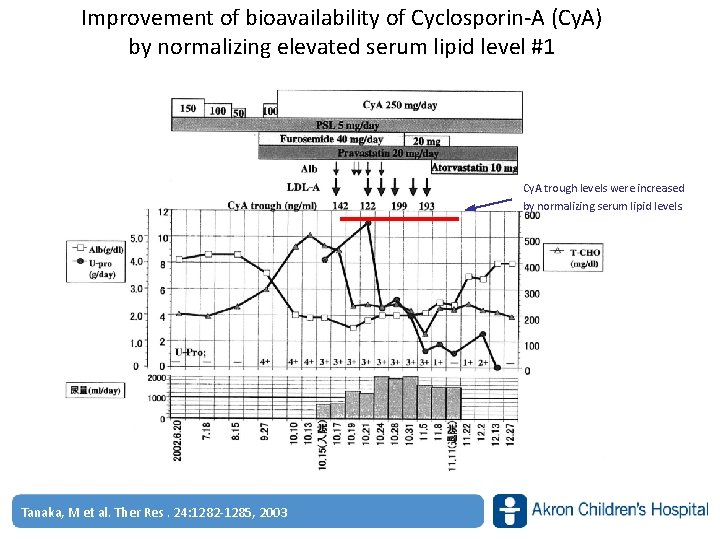
Improvement of bioavailability of Cyclosporin‐A (Cy. A) by normalizing elevated serum lipid level #1 Cy. A trough levels were increased by normalizing serum lipid levels. Tanaka, M et al. Ther Res. 24: 1282‐ 1285, www. akronchildrens. org/giving 2003

Summary Liposorber in FSGS ■ Long history of the treatment in adults and ped’s. ■ Rapid and long term therapeutic effects & improve nephrotic syndrome with de-lipidation. ■ Recommended several guidelines in Japan. www. akronchildrens. org/giving

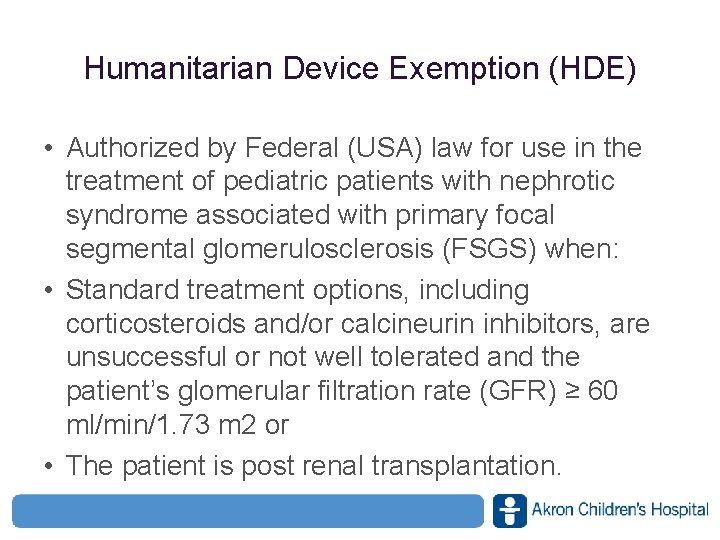
Humanitarian Device Exemption (HDE) • Authorized by Federal (USA) law for use in the treatment of pediatric patients with nephrotic syndrome associated with primary focal segmental glomerulosclerosis (FSGS) when: • Standard treatment options, including corticosteroids and/or calcineurin inhibitors, are unsuccessful or not well tolerated and the patient’s glomerular filtration rate (GFR) ≥ 60 ml/min/1. 73 m 2 or • The patient is post renal transplantation. www. akronchildrens. org/giving

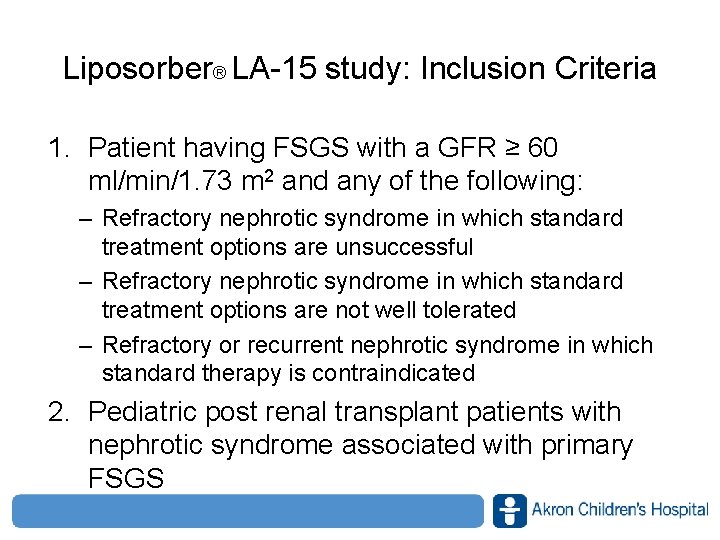
Liposorber® LA-15 study: Inclusion Criteria 1. Patient having FSGS with a GFR ≥ 60 ml/min/1. 73 m 2 and any of the following: – Refractory nephrotic syndrome in which standard treatment options are unsuccessful – Refractory nephrotic syndrome in which standard treatment options are not well tolerated – Refractory or recurrent nephrotic syndrome in which standard therapy is contraindicated 2. Pediatric post renal transplant patients with nephrotic syndrome associated with primary FSGS www. akronchildrens. org/giving

Liposorber® LA-15 system for LDL apheresis in drug resistant primary focal segmental glomerulosclerosis patients: Interim results from a prospective, multicenter, single-arm intervention study 1, 2* Rupesh Raina MD , Vinod Krishnappa 2*, Cheryl Sanchez-Kazi 3, Alejandro Quiroga 4, Katherine E. Twombley 5, Robert Mathias 6, Megan Lo 7, Shefali Mahesh 8, Joshua Zaritsky 9 • Objectives: To examine the safety and probable benefit at 1, 3, 6, 12 and 24 months following completion of apheresis treatment using Liposorber® LA-15 system in patients with nephrotic syndrome due to refractory primary FSGS, or primary FSGS associated NS in post renal transplant children. • Material and methods: Prospective, multicenter, single-arm intervention study using Liposorber® LA-15 system. Study was initiated in July 2014 with expected enrollment completion in August 2018. Patients ≤ 21 years old with drug resistant or drug intolerant NS secondary to primary FSGS with glomerular filtration rate (GFR) ≥ 60 ml/min/1. 73 m 2 or post renal transplant patients ≤ 21 years old with primary FSGS associated NS were included in the study. Each patient will have a total of 12 apheresis sessions with Liposorber® LA-15 system over a period of 9 weeks. All the patients will be followed up at 1, 3, 6, 12 and 24 months following completion of apheresis treatment. Presentation at WCN congress ISN April 2019 www. akronchildrens. org/giving

Liposorber® LA-15 system for LDL apheresis in drug resistant primary focal segmental glomerulosclerosis patients: Interim results from a prospective, multicenter, single-arm intervention study Rupesh Raina MD 1, 2*, Vinod Krishnappa 2*, Cheryl Sanchez-Kazi 3, Alejandro Quiroga 4, Katherine E. Twombley 5, Robert Mathias 6, Megan Lo 7, Shefali Mahesh 8, Joshua Zaritsky 9 • As of October 2017, 14 patients from 6 sites have been enrolled in to the study. • A total of 11 patients (including 5 protocol deviations) completed 12 apheresis treatments over a period of 9 weeks. • At one-month follow-up, 1 of 4 patients (25%) attained partial remission of NS. • Furthermore, 2 of 3 subjects (33. 3%) and 2 of 2 subjects (100%) had partial/complete remission at 3 and 6 months follow-up respectively. • Only one patient who was followed up for 12 months had complete remission of NS. • Improvement or stable e. GFR was noted in all the patients (100%) over the follow-up period. www. akronchildrens. org/giving

www. akronchildrens. org/giving

www. akronchildrens. org/giving

www. akronchildrens. org/giving

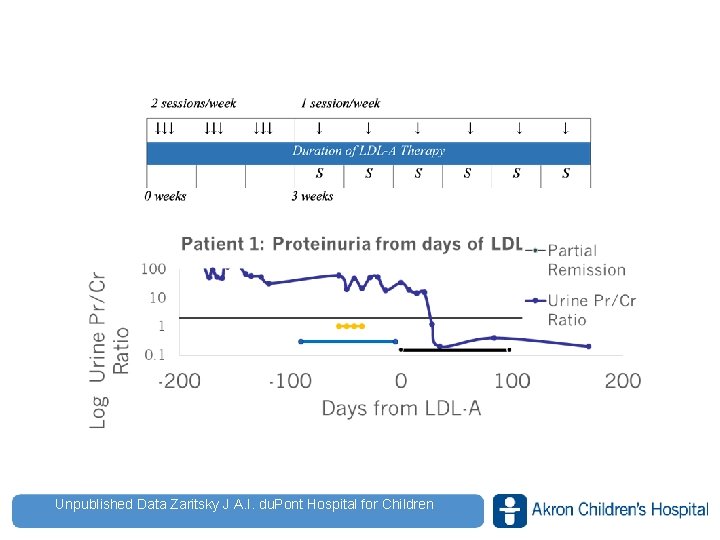
Unpublished Data Zaritsky J A. I. du. Pont Hospital for Children www. akronchildrens. org/giving

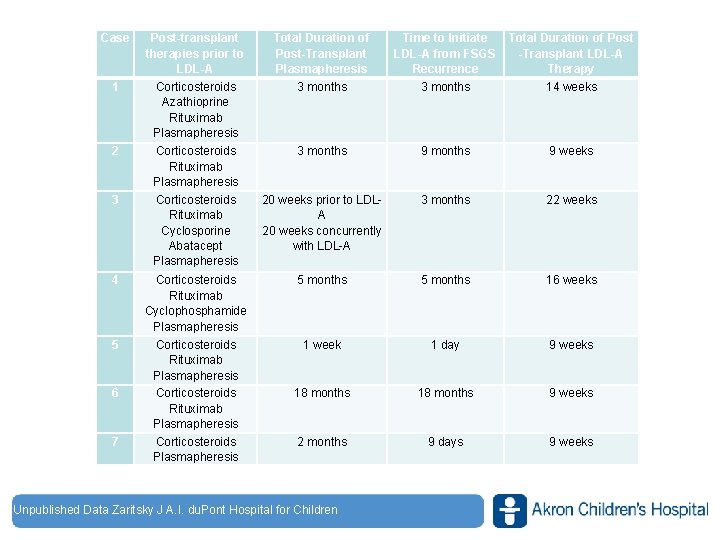
Case 1 2 3 Post-transplant therapies prior to LDL-A Corticosteroids Azathioprine Rituximab Plasmapheresis Total Duration of Post-Transplant Plasmapheresis 3 months Time to Initiate LDL-A from FSGS Recurrence 3 months Total Duration of Post -Transplant LDL-A Therapy 14 weeks Corticosteroids Rituximab Plasmapheresis Corticosteroids Rituximab Cyclosporine Abatacept Plasmapheresis 3 months 9 weeks 20 weeks prior to LDLA 20 weeks concurrently with LDL-A 3 months 22 weeks 4 Corticosteroids Rituximab Cyclophosphamide Plasmapheresis 5 months 16 weeks 5 Corticosteroids Rituximab Plasmapheresis Corticosteroids Plasmapheresis 1 week 1 day 9 weeks 18 months 9 weeks 2 months 9 days 9 weeks 6 7 Unpublished Data Zaritsky J A. I. du. Pont Hospital for Children www. akronchildrens. org/giving

Unpublished Data Zaritsky J A. I. du. Pont Hospital for Children www. akronchildrens. org/giving

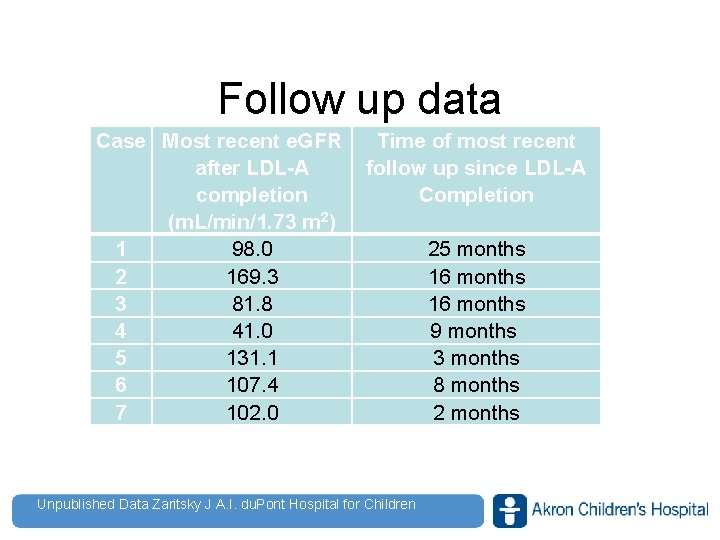
Follow up data Case Most recent e. GFR after LDL-A completion (m. L/min/1. 73 m 2) 1 98. 0 2 169. 3 3 81. 8 4 41. 0 5 131. 1 6 107. 4 7 102. 0 Time of most recent follow up since LDL-A Completion 25 months 16 months 9 months 3 months 8 months 2 months Unpublished Data Zaritsky J A. I. du. Pont Hospital for Children www. akronchildrens. org/giving

Conclusion • LDL apheresis is a novel therapy for drug resistant (corticosteroids and/or calcineurin inhibitors) primary FSGS and post-renal transplant primary FSGS reoccurrence • However, long-term evaluation of the outcome in a larger study is required to confirm these finding • Partial/complete remission rates at 3 and 6 months following LDL apheresis were 33. 3% and 100% respectively. All patients showed stable or improved e. GFR during the follow-up period. • Given these interim results, full analysis after completion of the study will shed more light on the outcome of LDL apheresis using Liposorber® LA-15 system www. akronchildrens. org/giving

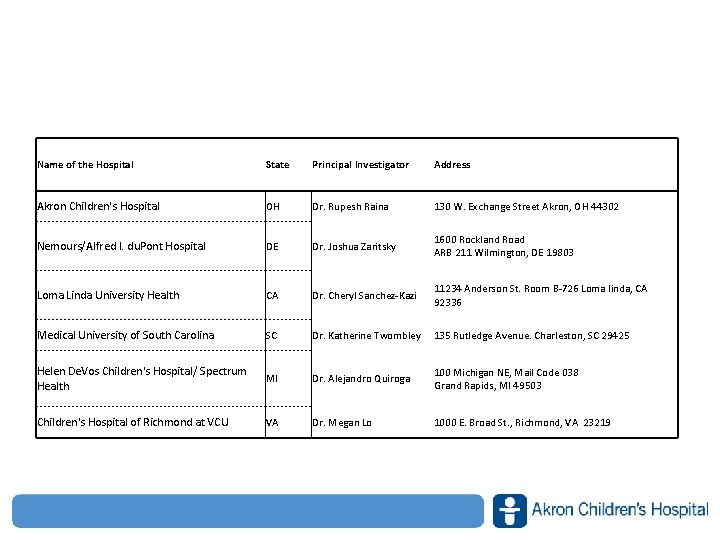
Name of the Hospital State Principal Investigator Address Akron Children's Hospital OH Dr. Rupesh Raina 130 W. Exchange Street Akron, OH 44302 Nemours/Alfred I. du. Pont Hospital DE Dr. Joshua Zaritsky 1600 Rockland Road ARB 211 Wilmington, DE 19803 Loma Linda University Health CA Dr. Cheryl Sanchez‐Kazi 11234 Anderson St. Room B‐ 726 Loma linda, CA 92336 Medical University of South Carolina SC Dr. Katherine Twombley 135 Rutledge Avenue. Charleston, SC 29425 Helen De. Vos Children's Hospital/ Spectrum Health MI Dr. Alejandro Quiroga 100 Michigan NE, Mail Code 038 Grand Rapids, MI 49503 Children's Hospital of Richmond at VCU VA Dr. Megan Lo 1000 E. Broad St. , Richmond, VA 23219 www. akronchildrens. org/giving
 Lipopheresis
Lipopheresis New-old approach to creating new ventures
New-old approach to creating new ventures Spill resistant vacuum breaker
Spill resistant vacuum breaker Cyanide-resistant respiration slideshare
Cyanide-resistant respiration slideshare Resistant materials tools
Resistant materials tools Collision resistant hash function
Collision resistant hash function Cryptographic
Cryptographic Rodent resistant fiber optic cable
Rodent resistant fiber optic cable Kyloc usmc
Kyloc usmc Scbf design example
Scbf design example Design of seismic-resistant steel building structures
Design of seismic-resistant steel building structures Penicilloic acid
Penicilloic acid Tamper resistant packaging examples
Tamper resistant packaging examples Packaging
Packaging Fire resistant cable thai yazaki
Fire resistant cable thai yazaki Flame resistant lab coat amazon
Flame resistant lab coat amazon Resistant ovary syndrome definition
Resistant ovary syndrome definition Skyscraper pendulum
Skyscraper pendulum Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Arc duct switchgear
Arc duct switchgear Datagram networks and virtual circuit networks
Datagram networks and virtual circuit networks Theoretical models of counseling
Theoretical models of counseling Waterfall and sprinkler strategy
Waterfall and sprinkler strategy Multiple approach avoidance
Multiple approach avoidance Bandura's reciprocal determinism
Bandura's reciprocal determinism What is research approach
What is research approach Traditional approach to systems implementation
Traditional approach to systems implementation Tony wagner's seven survival skills
Tony wagner's seven survival skills Word power: a new approach for content analysis
Word power: a new approach for content analysis A new approach to cross-modal multimedia retrieval
A new approach to cross-modal multimedia retrieval Paradoxical theory of change
Paradoxical theory of change New approach directives
New approach directives Rules for speech punctuation
Rules for speech punctuation New york, new jersey, pennsylvania, and delaware
New york, new jersey, pennsylvania, and delaware Fresh oil, new wine scripture
Fresh oil, new wine scripture Marquee orchard 14
Marquee orchard 14 Characteristics of the articles of confederation
Characteristics of the articles of confederation Marketing management
Marketing management Njbta
Njbta New classical macroeconomics
New classical macroeconomics Chapter 16 toward a new heaven and a new earth
Chapter 16 toward a new heaven and a new earth Both new hampshire and new york desire more territory
Both new hampshire and new york desire more territory New classical and new keynesian macroeconomics
New classical and new keynesian macroeconomics Comparing progressive presidents
Comparing progressive presidents Enophthalmous
Enophthalmous Hand approach general paper
Hand approach general paper Aice general paper rubric
Aice general paper rubric Word aware song
Word aware song