Lipids OBJECTIVES 1 To understand terminology and classification
Lipids OBJECTIVES 1. To understand terminology and classification of lipids; 2. Important reactions of lipids; 3. Importance of lipids for food chemistry and food technology; 4. Hydrogenation of lipids.
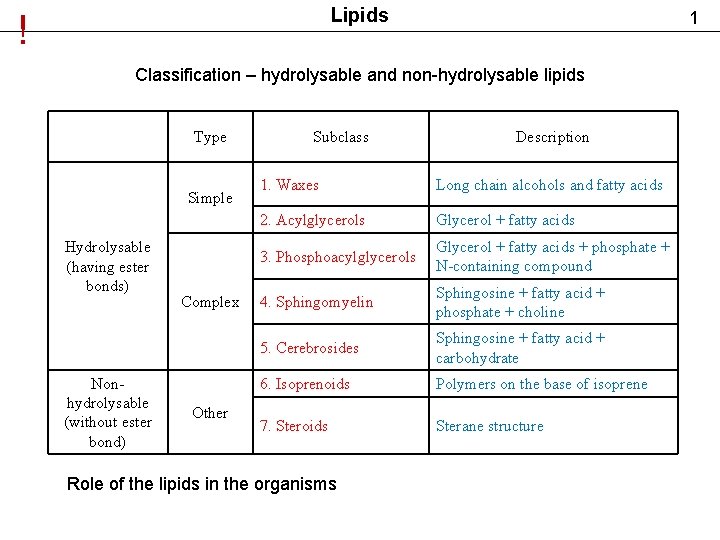
Lipids ! 1 Classification – hydrolysable and non-hydrolysable lipids Type Simple Hydrolysable (having ester bonds) Nonhydrolysable (without ester bond) Complex Other Subclass Description 1. Waxes Long chain alcohols and fatty acids 2. Acylglycerols Glycerol + fatty acids 3. Phosphoacylglycerols Glycerol + fatty acids + phosphate + N-containing compound 4. Sphingomyelin Sphingosine + fatty acid + phosphate + choline 5. Cerebrosides Sphingosine + fatty acid + carbohydrate 6. Isoprenoids Polymers on the base of isoprene 7. Steroids Sterane structure Role of the lipids in the organisms
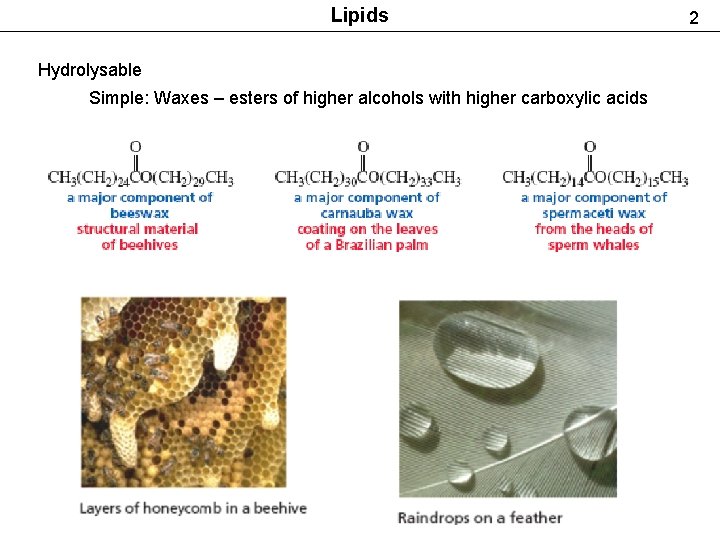
Lipids Hydrolysable Simple: Waxes – esters of higher alcohols with higher carboxylic acids 2
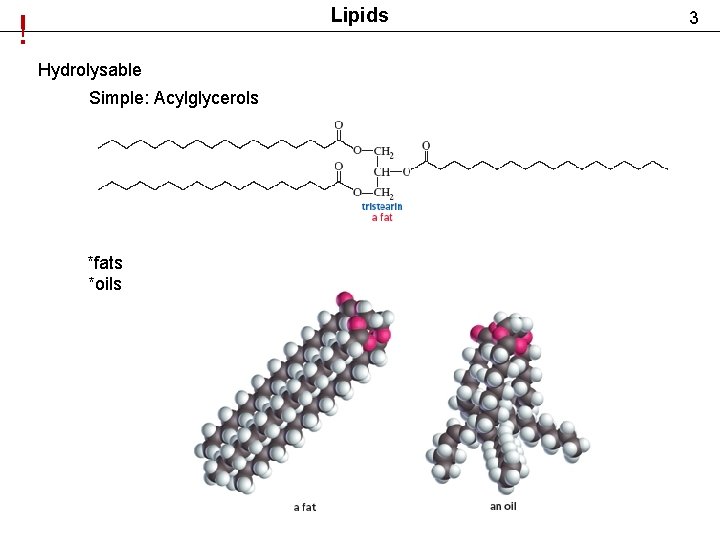
Lipids ! Hydrolysable Simple: Acylglycerols *fats *oils 3
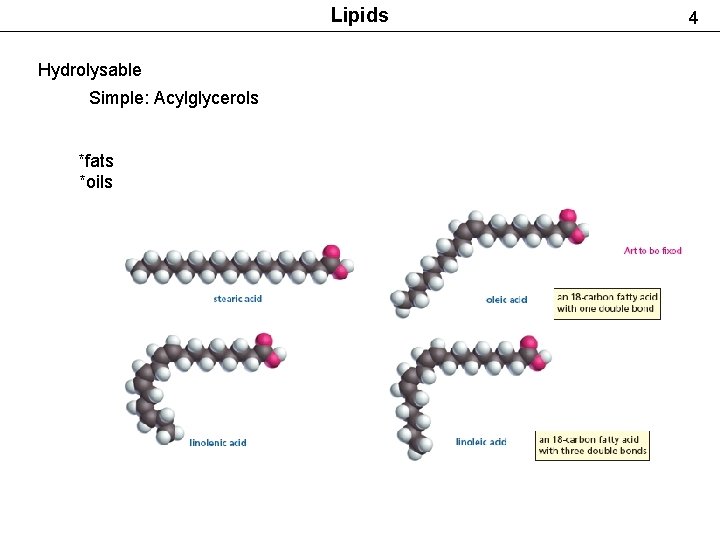
Lipids Hydrolysable Simple: Acylglycerols *fats *oils 4
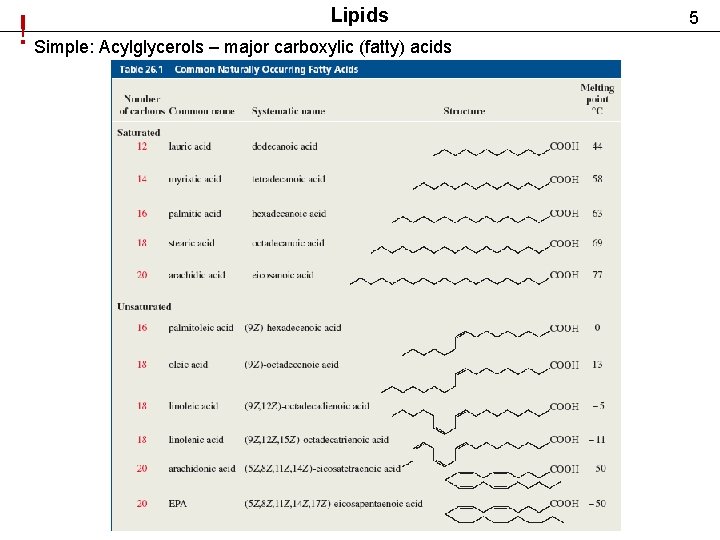
Lipids ! Simple: Acylglycerols – major carboxylic (fatty) acids 5
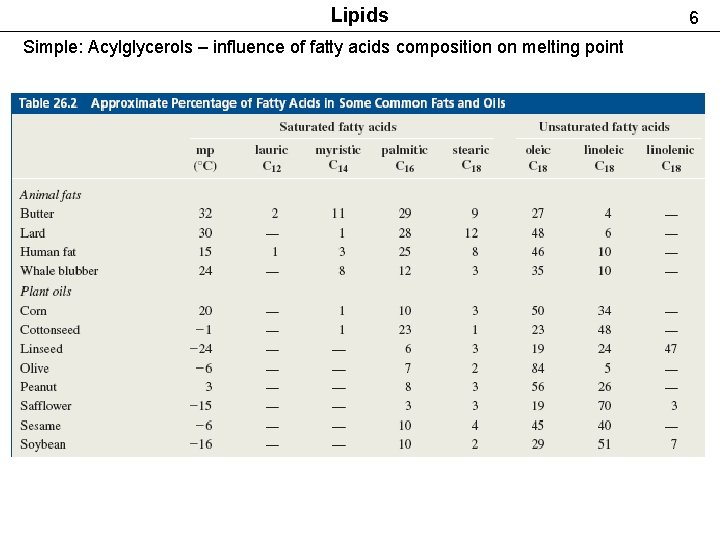
Lipids Simple: Acylglycerols – influence of fatty acids composition on melting point 6
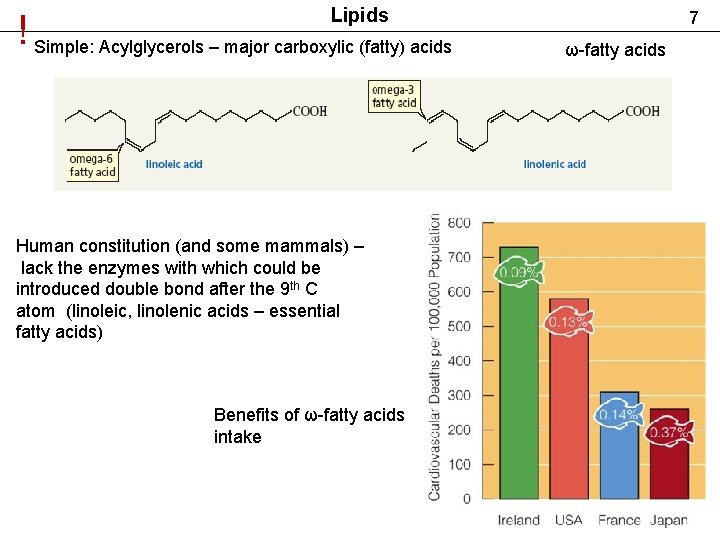
Lipids ! Simple: Acylglycerols – major carboxylic (fatty) acids Human constitution (and some mammals) – lack the enzymes with which could be introduced double bond after the 9 th C atom (linoleic, linolenic acids – essential fatty acids) Benefits of ω-fatty acids intake 7 ω-fatty acids
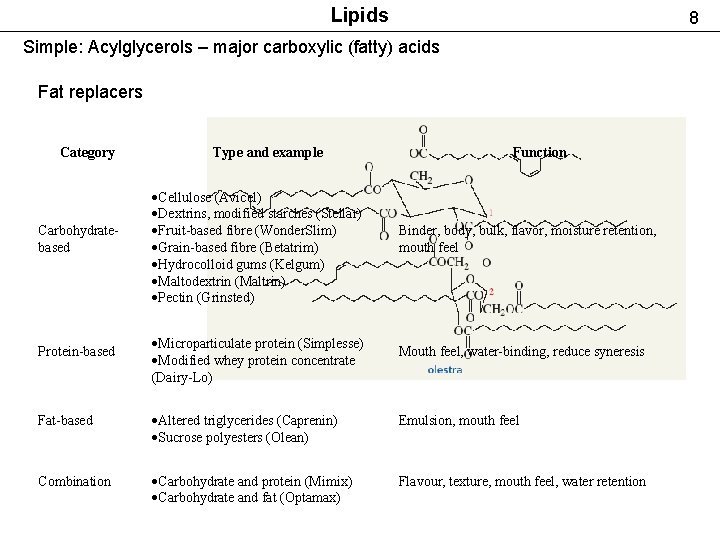
Lipids 8 Simple: Acylglycerols – major carboxylic (fatty) acids Fat replacers Category Carbohydratebased Protein-based Type and example Cellulose (Avicel) Dextrins, modified starches (Stellar) Fruit-based fibre (Wonder. Slim) Grain-based fibre (Betatrim) Hydrocolloid gums (Kelgum) Maltodextrin (Maltrin) Pectin (Grinsted) Microparticulate protein (Simplesse) Modified whey protein concentrate (Dairy-Lo) Function Binder, body, bulk, flavor, moisture retention, mouth feel Mouth feel, water-binding, reduce syneresis Fat-based Altered triglycerides (Caprenin) Sucrose polyesters (Olean) Emulsion, mouth feel Combination Carbohydrate and protein (Mimix) Carbohydrate and fat (Optamax) Flavour, texture, mouth feel, water retention
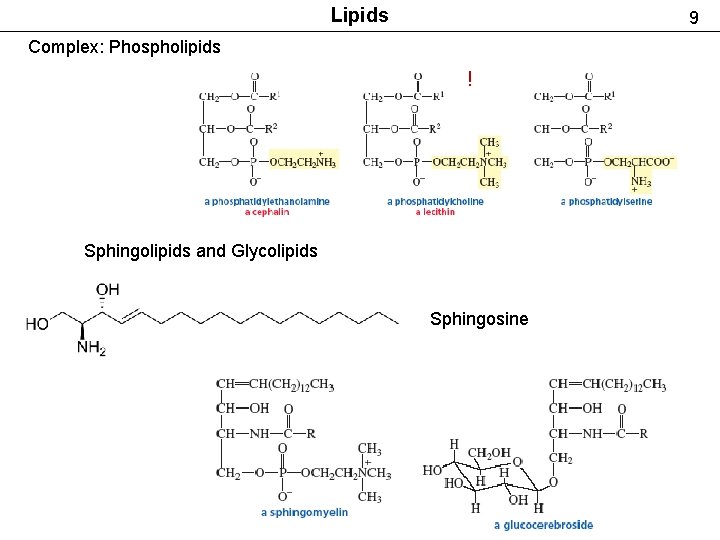
Lipids 9 Complex: Phospholipids ! Sphingolipids and Glycolipids Sphingosine
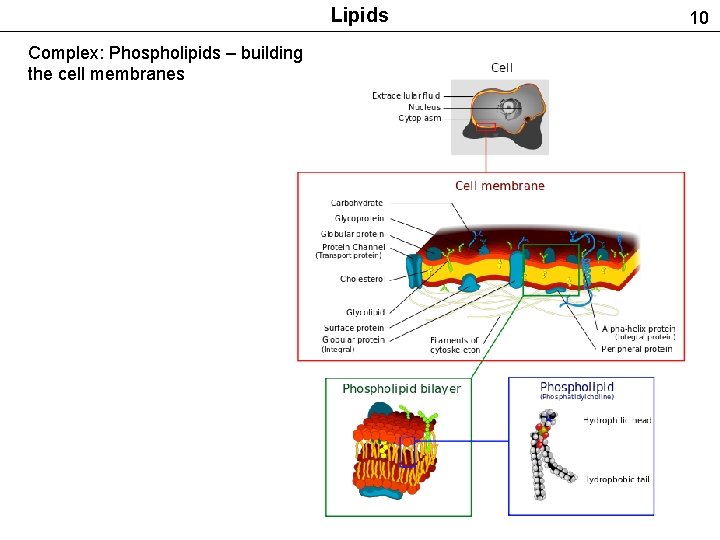
Lipids Complex: Phospholipids – building the cell membranes 10
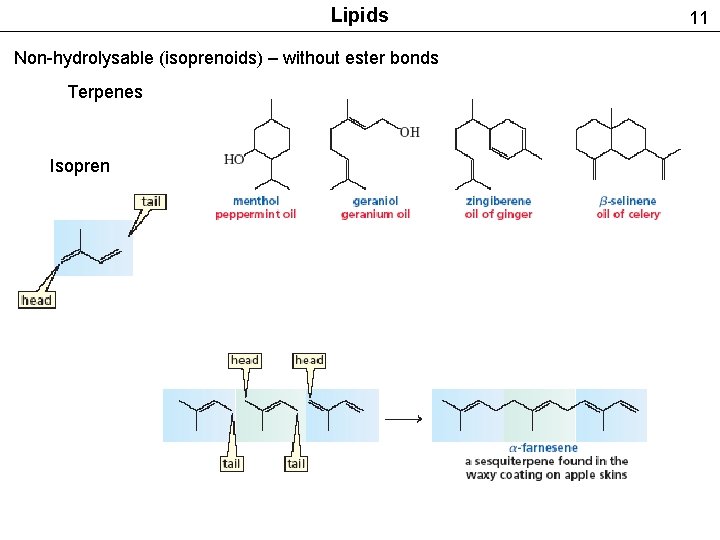
Lipids Non-hydrolysable (isoprenoids) – without ester bonds Terpenes Isopren 11
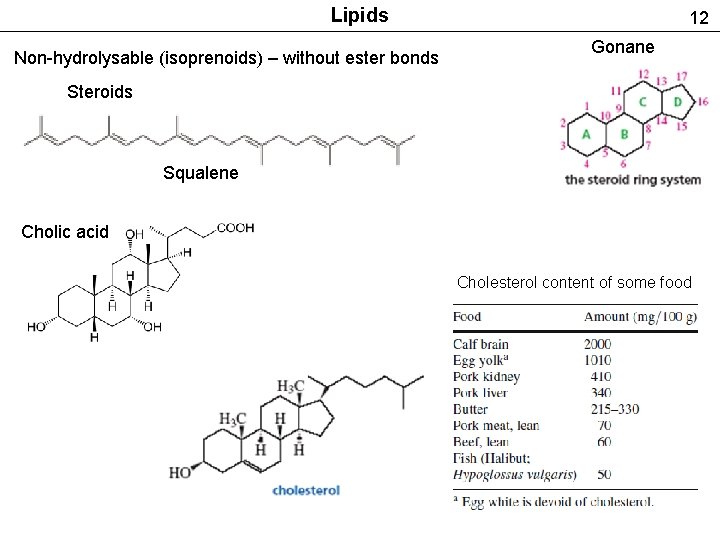
Lipids Non-hydrolysable (isoprenoids) – without ester bonds 12 Gonane Steroids Squalene Cholic acid Cholesterol content of some food
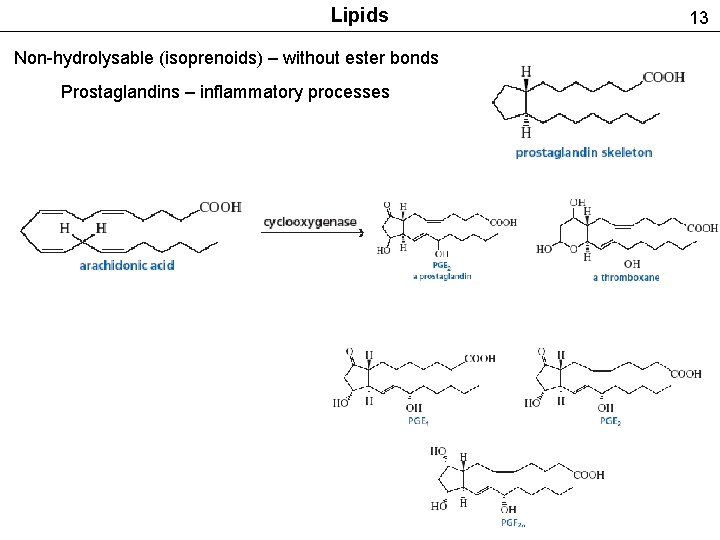
Lipids Non-hydrolysable (isoprenoids) – without ester bonds Prostaglandins – inflammatory processes 13
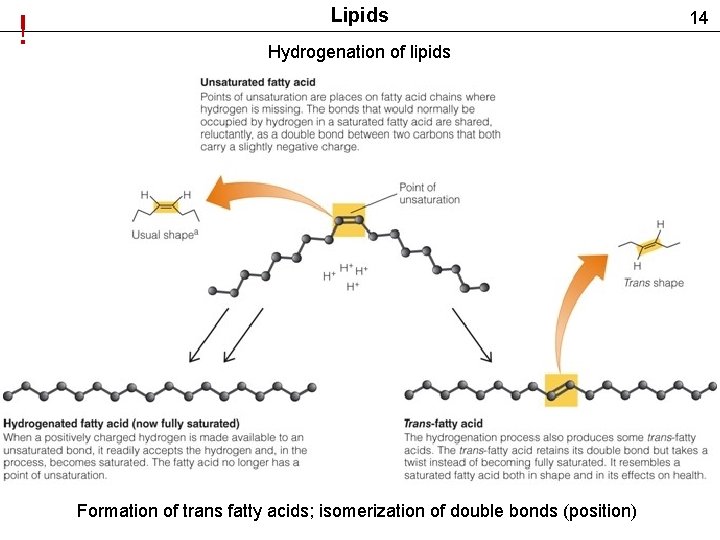
! Lipids Hydrogenation of lipids Formation of trans fatty acids; isomerization of double bonds (position) 14
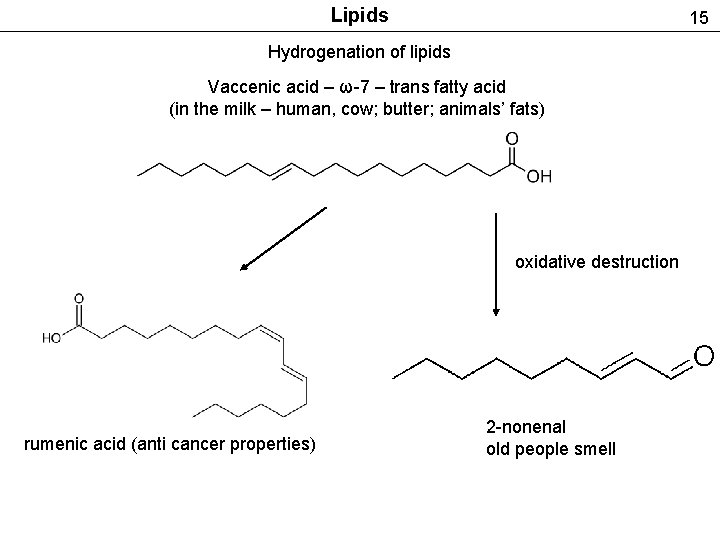
Lipids 15 Hydrogenation of lipids Vaccenic acid – ω-7 – trans fatty acid (in the milk – human, cow; butter; animals’ fats) oxidative destruction rumenic acid (anti cancer properties) 2 -nonenal old people smell
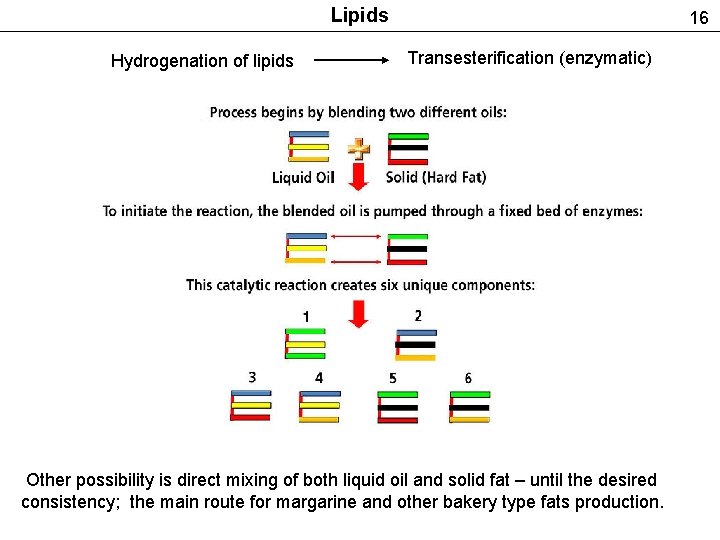
Lipids Hydrogenation of lipids 16 Transesterification (enzymatic) Other possibility is direct mixing of both liquid oil and solid fat – until the desired consistency; the main route for margarine and other bakery type fats production.
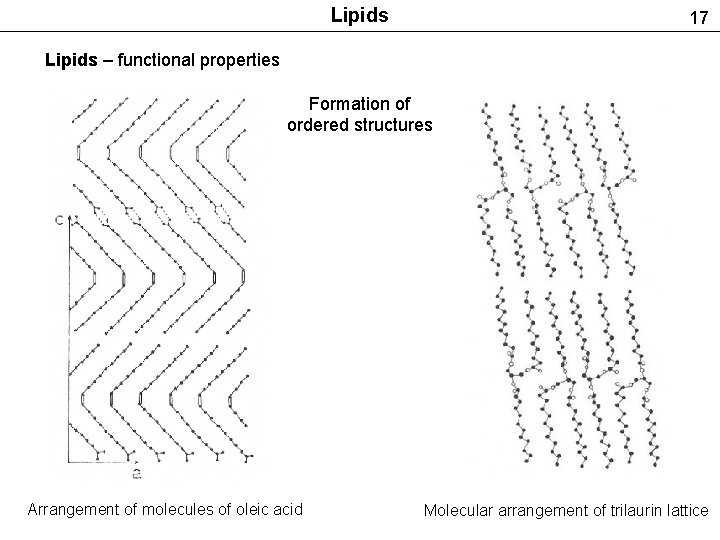
Lipids 17 Lipids – functional properties Formation of ordered structures Arrangement of molecules of oleic acid Molecular arrangement of trilaurin lattice
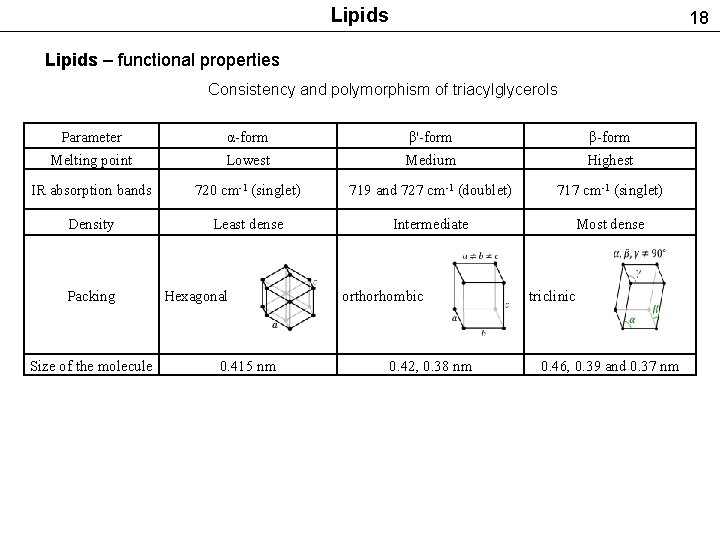
Lipids 18 Lipids – functional properties Consistency and polymorphism of triacylglycerols Parameter α-form β'-form β-form Melting point Lowest Medium Highest IR absorption bands 720 cm-1 (singlet) 719 and 727 cm-1 (doublet) 717 cm-1 (singlet) Density Least dense Intermediate Most dense Packing Size of the molecule Hexagonal 0. 415 nm orthorhombic 0. 42, 0. 38 nm triclinic 0. 46, 0. 39 and 0. 37 nm
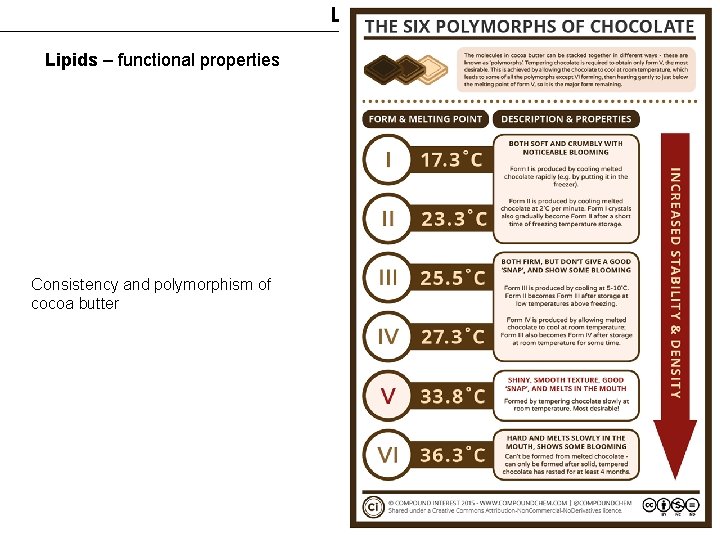
Lipids – functional properties Consistency and polymorphism of cocoa butter 18

Lipids 20 Lipids – functional properties Fat consistency – melting and crystallization properties of fats Several factors have important influences on the consistency of commercial fats: Proportion of solids in the fat. In general, the greater the solids content, the firmer the fat. It has been estimated that plastic commercial fats, at workable temperatures, increase in either firmness or viscosity by about 10% with each increment of crystals. Number, size, and kind of crystals. At a given solids content, a large number of small crystals produces a harder fat than does a small number of large crystals. Larger, soft crystals are typically produced by slow cooling. Crystals composed of highmelting acylglycerols provide greater stiffening power than do those of lower melting acylglycerols.

Lipids 21 Lipids – functional properties Fat consistency Viscosity of the liquid. Oils differ in viscosity at a given temperature and this will influence viscosity of the melt, as well as consistency of a solidliquid lipid mixture. Temperature treatment. If a fat tends to supercool excessively, this can be overcome by melting the crystalline fat at the lowest possible temperature, holding it for an extended period of time at a temperature just above its melting point, and then cooling it. This facilitates formation of numerous crystal nuclei, numerous small crystals, and a firm consistency. Mechanical working. Crystallized fats are generally thixotropic; that is, they become reversibly softer after vigorous agitation and only gradually regain their original firmness. If a melted fat is mechanically agitated during solidification, it will be much softer than if allowed to solidify in a static condition. In the static state the growing crystals form structures of relatively great strength. These structures can be deformed by mechanical working.
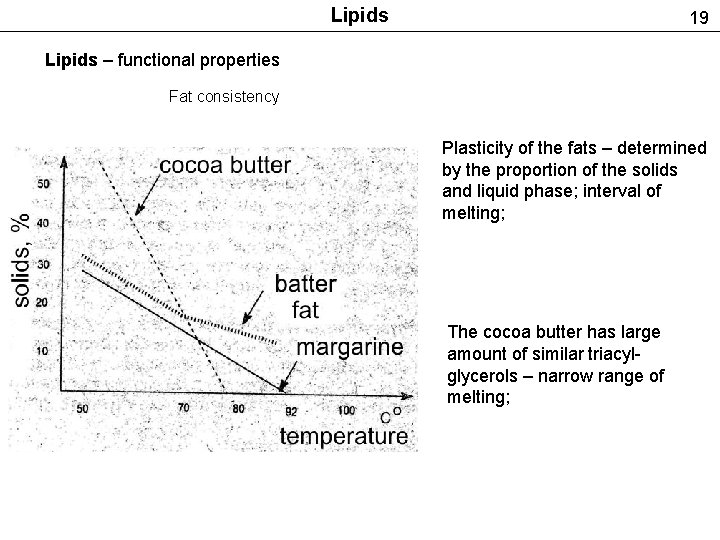
Lipids 19 Lipids – functional properties Fat consistency Plasticity of the fats – determined by the proportion of the solids and liquid phase; interval of melting; The cocoa butter has large amount of similar triacylglycerols – narrow range of melting;
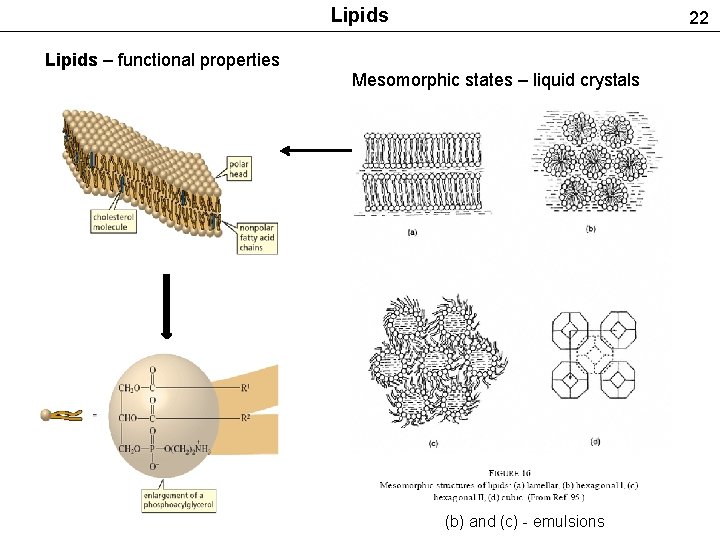
Lipids 22 Lipids – functional properties Mesomorphic states – liquid crystals (b) and (c) - emulsions
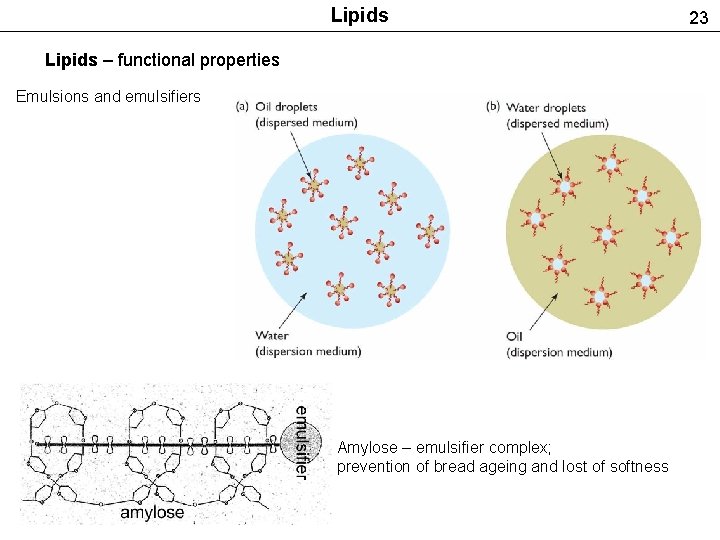
Lipids – functional properties Emulsions and emulsifiers Amylose – emulsifier complex; prevention of bread ageing and lost of softness 23
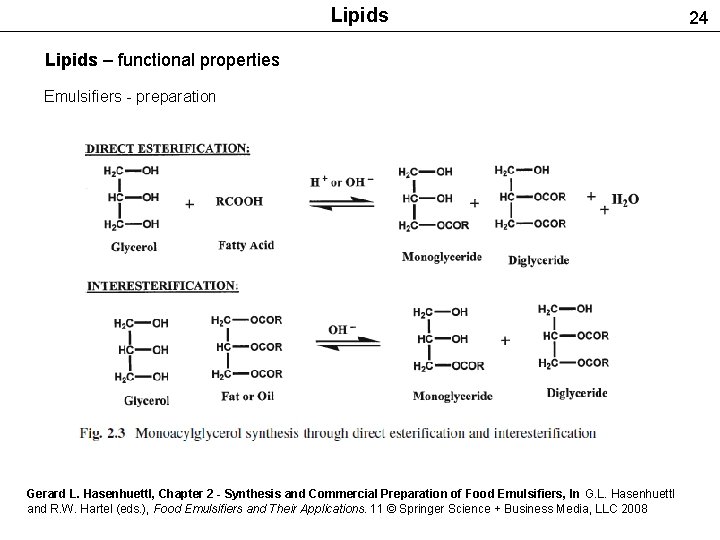
Lipids – functional properties Emulsifiers - preparation Gerard L. Hasenhuettl, Chapter 2 - Synthesis and Commercial Preparation of Food Emulsifiers, In G. L. Hasenhuettl and R. W. Hartel (eds. ), Food Emulsifiers and Their Applications. 11 © Springer Science + Business Media, LLC 2008 24
- Slides: 26