Lipids Lipids are hydrophobic molecules Mostly CH nonpolar

Lipids • Lipids are hydrophobic molecules • Mostly C-H (non-polar) • are the one class of large biological molecules that do not consist of polymers • Uses: structure of cell membranes, energy source 1

Lipids • Fats • Phospholipids • Steroids 2
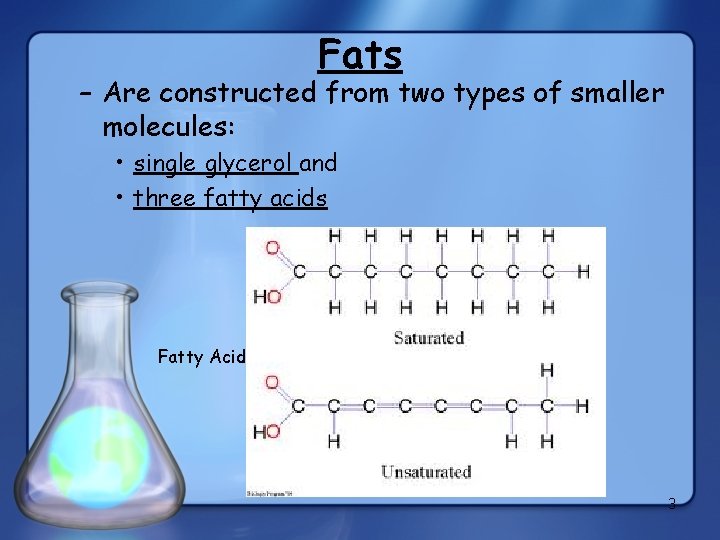
Fats – Are constructed from two types of smaller molecules: • single glycerol and • three fatty acids Fatty Acid 3
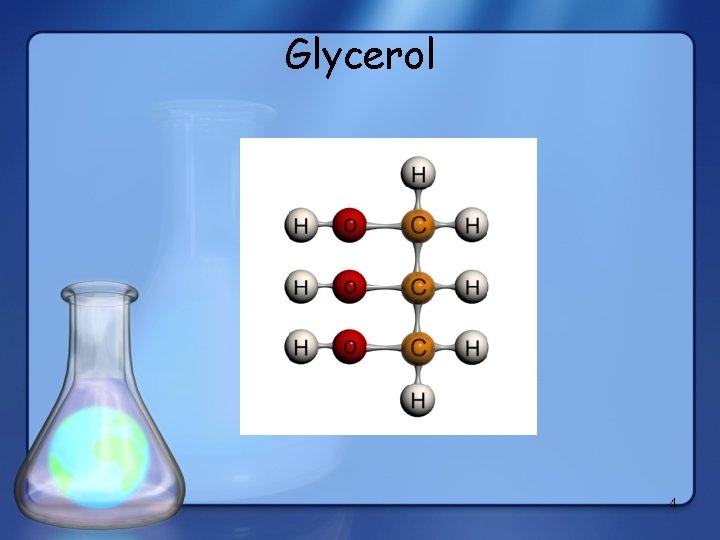
Glycerol 4
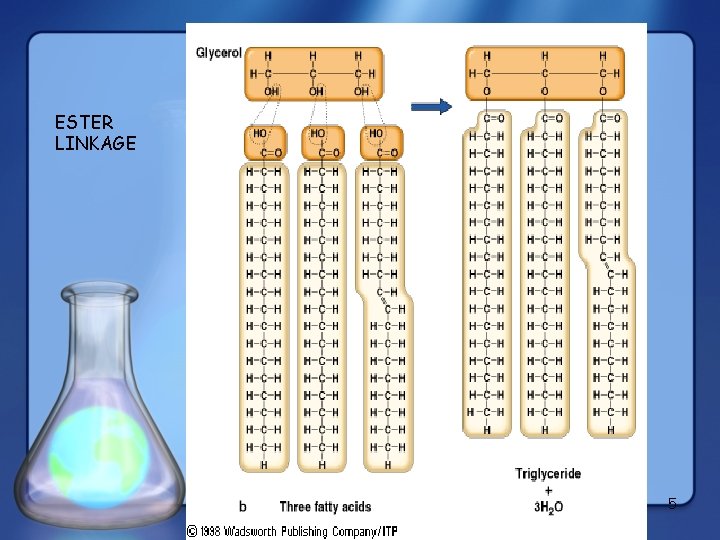
ESTER LINKAGE 5

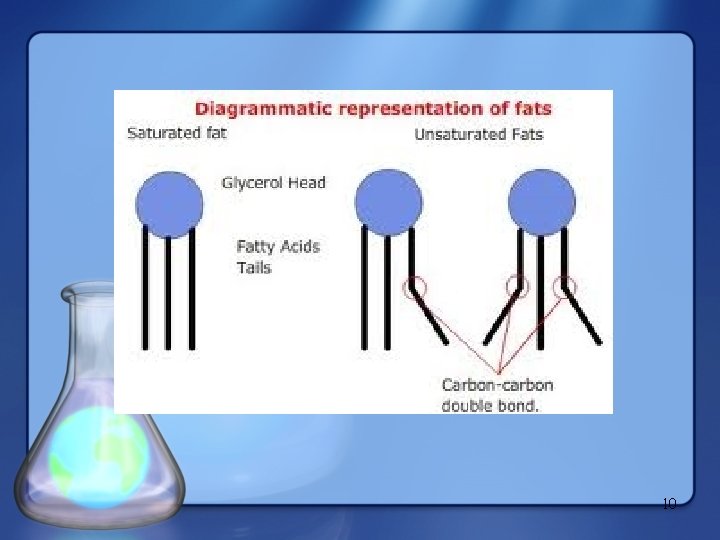
• Saturated fatty acids – Have the maximum number of hydrogen atoms possible – Have no double bonds – Are solid at room temperature (e. g. animal fats) Stearic acid 6 (a) Saturated fat and fatty acid Figure 5. 12
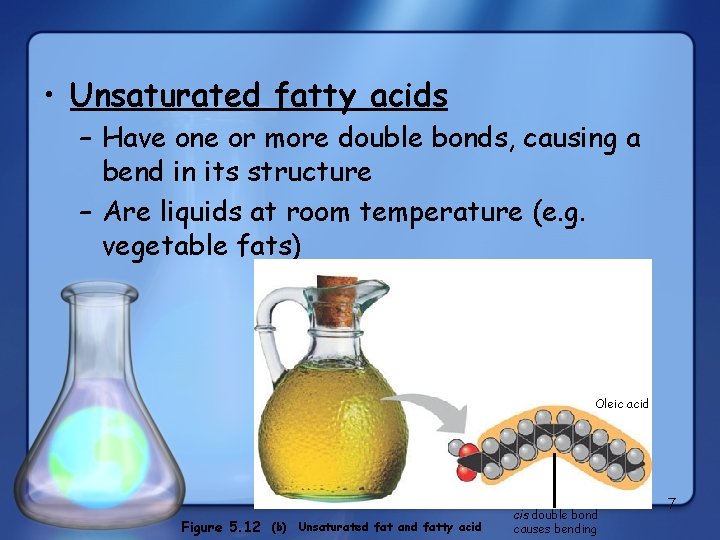
• Unsaturated fatty acids – Have one or more double bonds, causing a bend in its structure – Are liquids at room temperature (e. g. vegetable fats) Oleic acid Figure 5. 12 (b) Unsaturated fat and fatty acid cis double bond causes bending 7

Unsaturated Fats • Monounsaturated fats (MUFA) – Have one double bond in their fatty acids • Polyunsaturated fats (PUFA) Have more than one double bond in their fatty acid chains 8
10
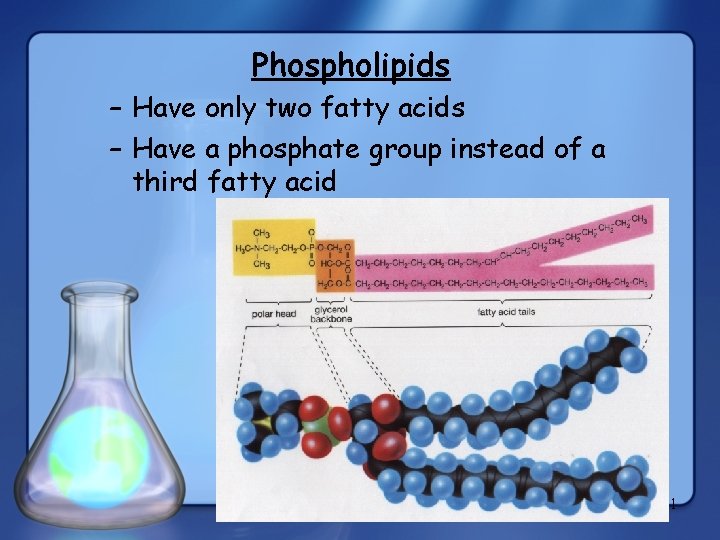
Phospholipids – Have only two fatty acids – Have a phosphate group instead of a third fatty acid 11
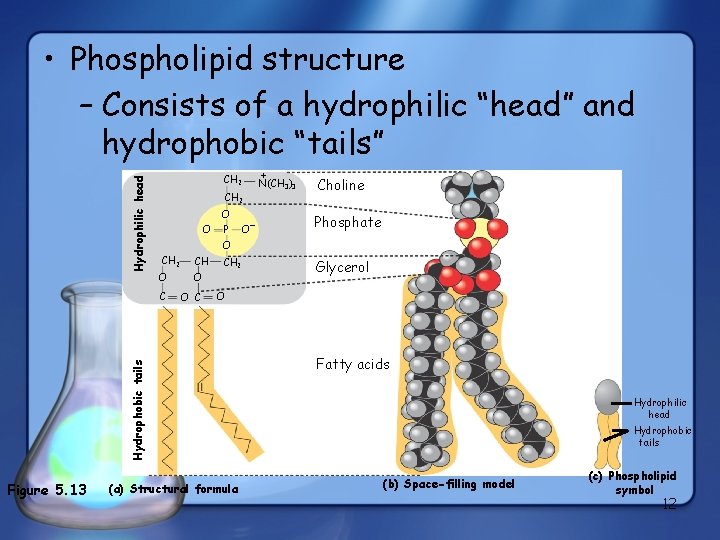
CH 2 O O P Figure 5. 13 O– + N(CH 3)3 Choline Phosphate O CH 2 CH O O C CH 2 Glycerol O Hydrophobic tails Hydrophilic head • Phospholipid structure – Consists of a hydrophilic “head” and hydrophobic “tails” (a) Structural formula Fatty acids Hydrophilic head Hydrophobic tails (b) Space-filling model (c) Phospholipid symbol 12

Micelles • When phospholipids are added to water, they form micelles 13
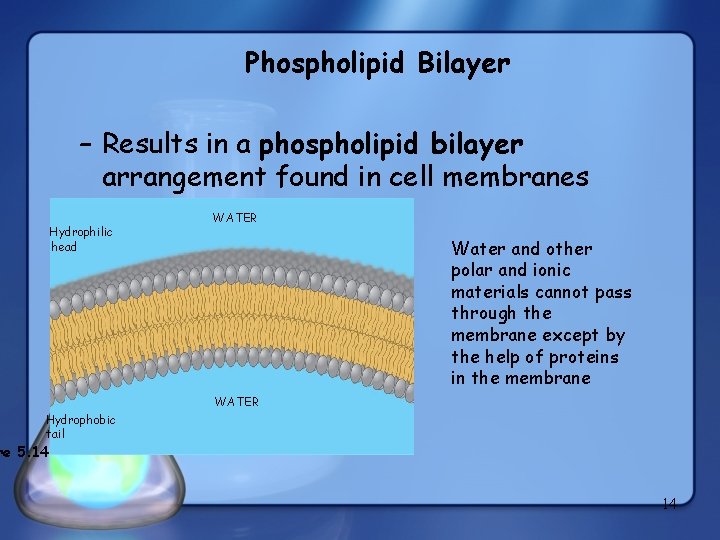
Phospholipid Bilayer – Results in a phospholipid bilayer arrangement found in cell membranes Hydrophilic head WATER Water and other polar and ionic materials cannot pass through the membrane except by the help of proteins in the membrane WATER Hydrophobic tail re 5. 14 14

Steroids • Steroids – Are lipids that have a carbon skeleton consisting of four fused rings – Contain many different functional groups 15
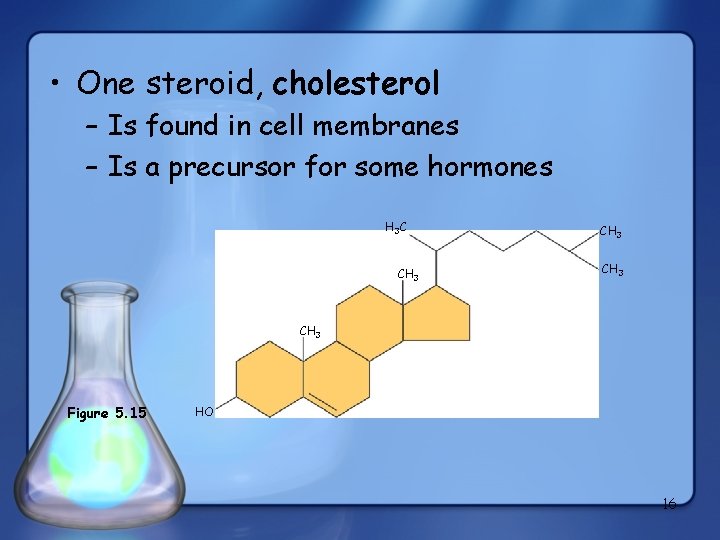
• One steroid, cholesterol – Is found in cell membranes – Is a precursor for some hormones H 3 C CH 3 Figure 5. 15 HO 16

Nucleic Acids • Nucleic acids store and transmit hereditary information • There are two types of nucleic acids – Deoxyribonucleic acid (DNA) – Ribonucleic acid (RNA) 17
Function of DNA and RNA • DNA – Stores information for the synthesis of specific proteins – Found in the nucleus of cells • RNA – Reads information in DNA – Transports information to protein building structures within cell 18
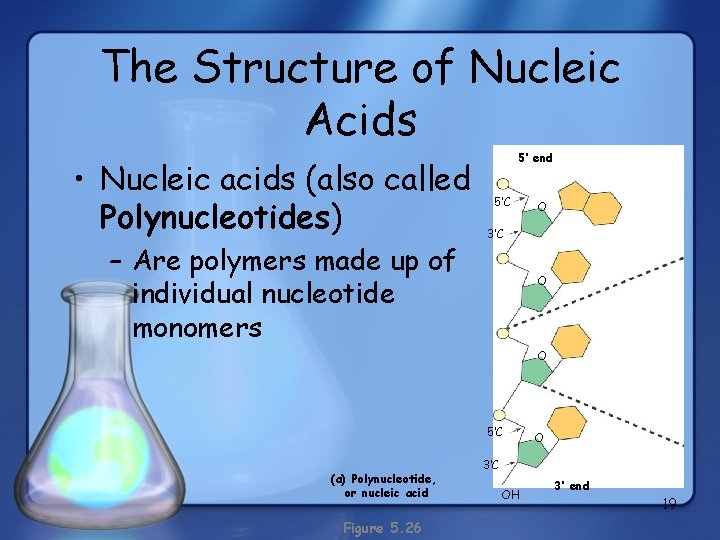
The Structure of Nucleic Acids • Nucleic acids (also called Polynucleotides) – Are polymers made up of individual nucleotide monomers 5’ end 5’C O 3’C O O 5’C (a) Polynucleotide, or nucleic acid Figure 5. 26 O 3’C OH 3’ end 19
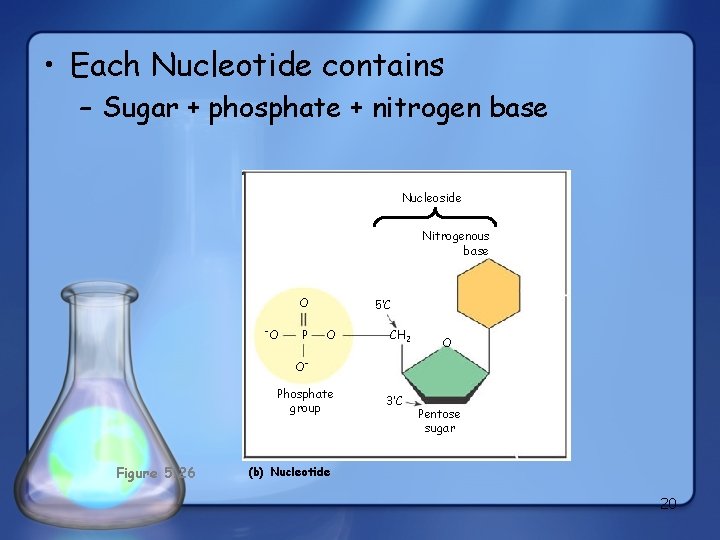
• Each Nucleotide contains – Sugar + phosphate + nitrogen base Nucleoside Nitrogenous base O O P 5’C O CH 2 O O Phosphate group Figure 5. 26 3’C Pentose sugar (b) Nucleotide 20
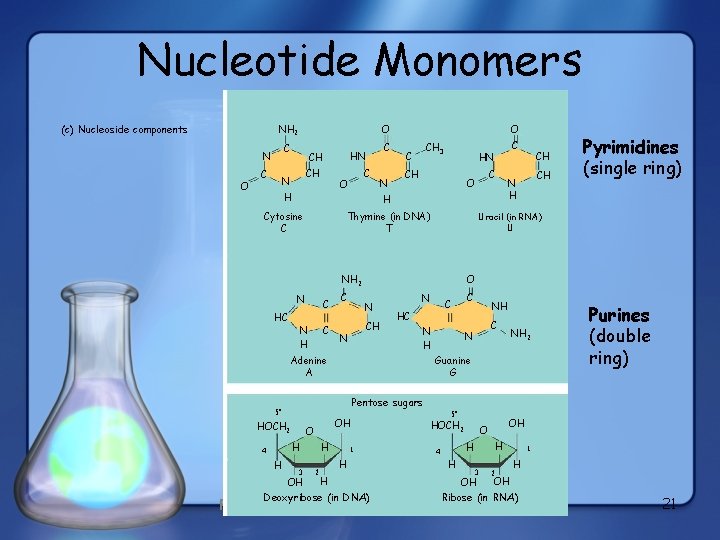
Nucleotide Monomers Nitrogenous bases Pyrimidines NH 2 (c) Nucleoside components C N O O C HN CH CH N C Cytosine C N CH 3 C CH HC C C N H CH CH H Thymine (in DNA) T Uracil (in. RNA) Uracil (in UU C N CH N Pyrimidines (single ring) O N C C HC N H Adenine A NH C N NH 2 Guanine G Purines (double ring) Pentose sugars 5” HOCH 2 OH O H H 4’ C O NH 2 N C HN N H O H C O H 3’ OH 2’ 1’ H H Deoxyribose (in DNA) Figure 5. 26 5” HOCH 2 H H 4’ H OH O 3’ OH 2’ 1’ H OH Ribose (in RNA) 21
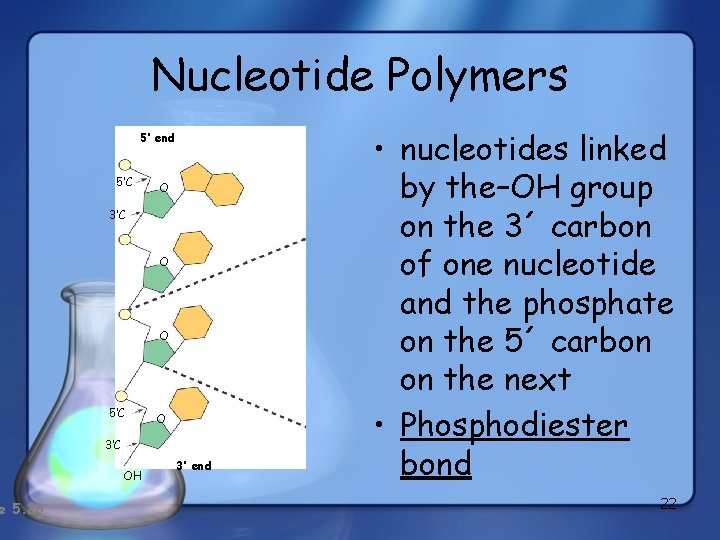
e 5. 26 Nucleotide Polymers 5’ end 5’C O 3’C O O 5’C O 3’C OH 3’ end • nucleotides linked by the–OH group on the 3´ carbon of one nucleotide and the phosphate on the 5´ carbon on the next • Phosphodiester bond 22

Gene • The sequence of bases along a nucleotide polymer – Is unique for each gene 23
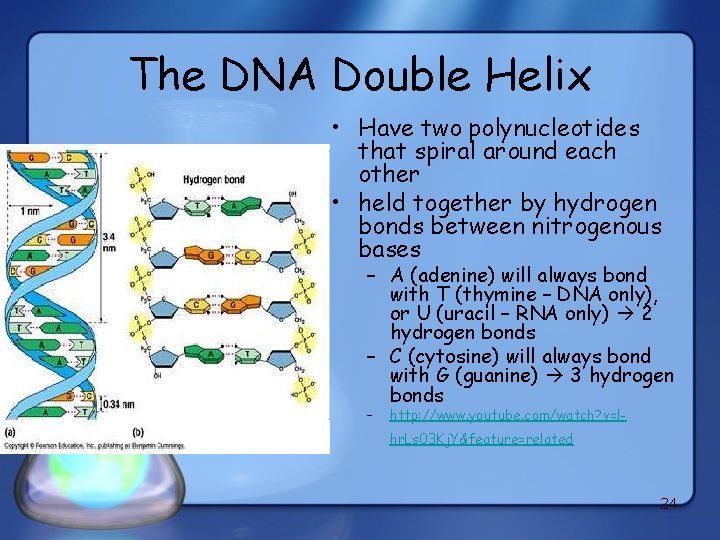
The DNA Double Helix • Have two polynucleotides that spiral around each other • held together by hydrogen bonds between nitrogenous bases – A (adenine) will always bond with T (thymine – DNA only), or U (uracil – RNA only) 2 hydrogen bonds – C (cytosine) will always bond with G (guanine) 3 hydrogen bonds – http: //www. youtube. com/watch? v=lhr. Ls 03 Kj. Y&feature=related 24
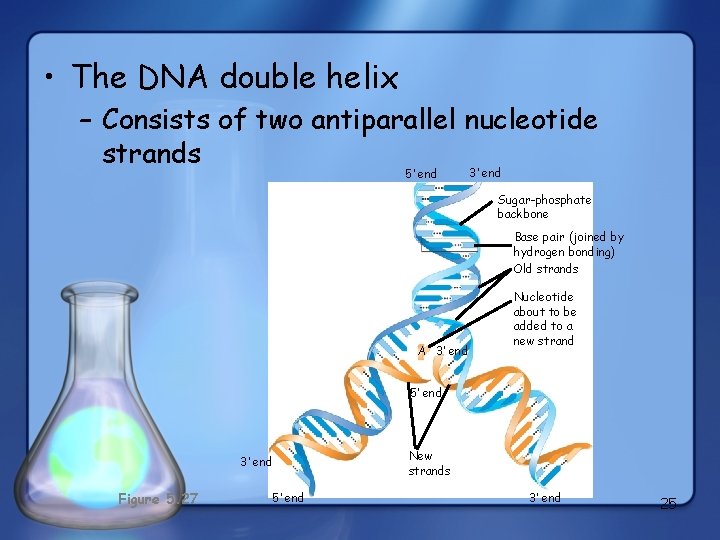
• The DNA double helix – Consists of two antiparallel nucleotide strands 3’ end 5’ end Sugar-phosphate backbone Base pair (joined by hydrogen bonding) Old strands A 3’ end Nucleotide about to be added to a new strand 5’ end 3’ end Figure 5. 27 5’ end New strands 3’ end 25
- Slides: 25