LIPIDS LIPIDS A water insoluble compounds but soluble
LIPIDS

LIPIDS A water insoluble compounds, but soluble in ether, benzena, acetone and chloroform Consists of glicerol & 3 fatty acids. Forms: solid lipid liquid fat Energy contents 2. 25 times higher than carbohydrate……. WHY? ?
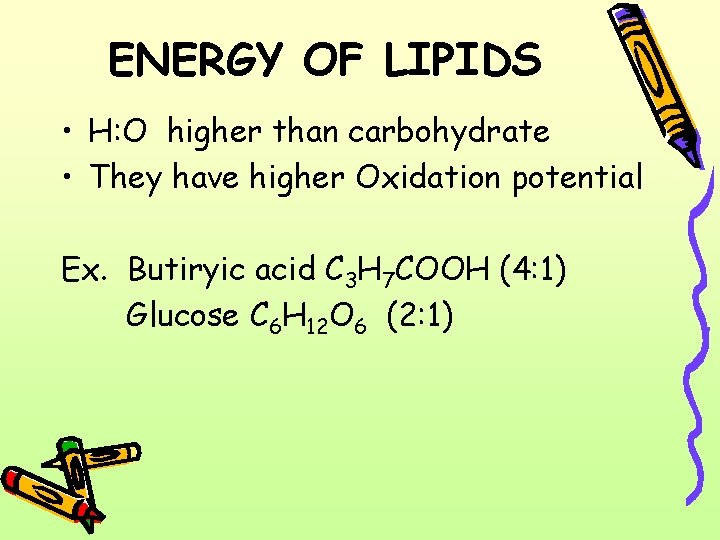
ENERGY OF LIPIDS • H: O higher than carbohydrate • They have higher Oxidation potential Ex. Butiryic acid C 3 H 7 COOH (4: 1) Glucose C 6 H 12 O 6 (2: 1)

Classifition: 1. Simple lipids: esther, fatty acids and glicerol 2. Mixed lipids: simplelipid + other molecules such as N, S, P etc 3. Lipids obtained from hydrolysis
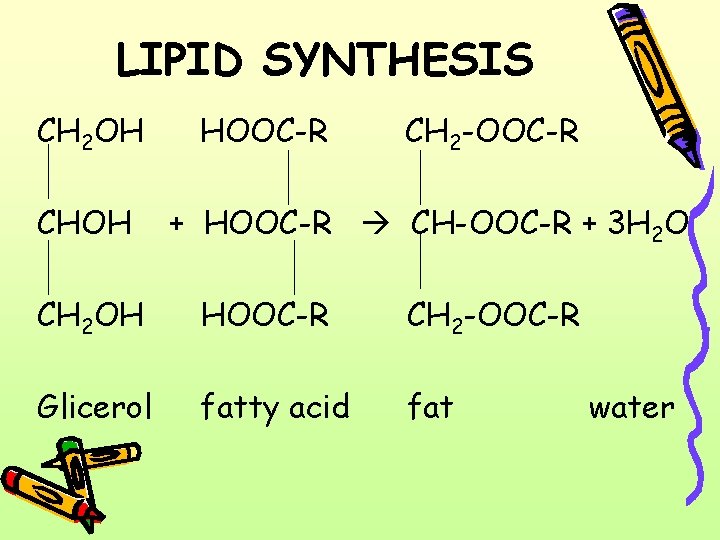
LIPID SYNTHESIS CH 2 OH CHOH HOOC-R CH 2 -OOC-R + HOOC-R CH-OOC-R + 3 H 2 O CH 2 OH HOOC-R CH 2 -OOC-R Glicerol fatty acid fat water
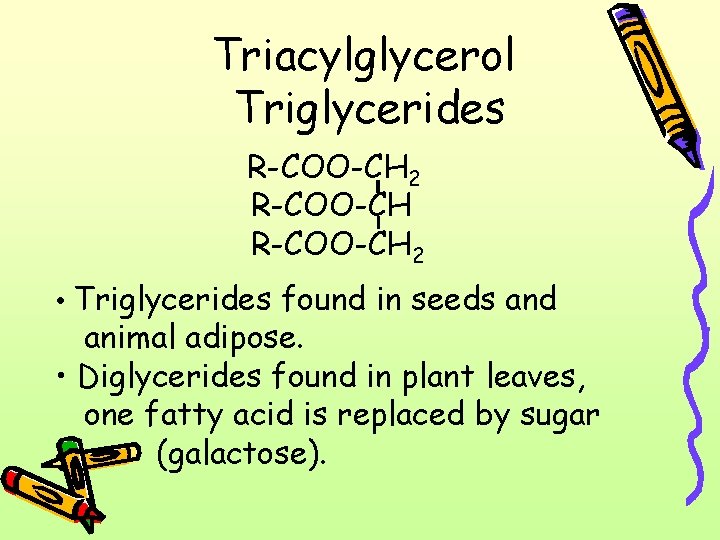
Triacylglycerol Triglycerides R-COO-CH 2 • Triglycerides found in seeds and animal adipose. • Diglycerides found in plant leaves, one fatty acid is replaced by sugar (galactose).
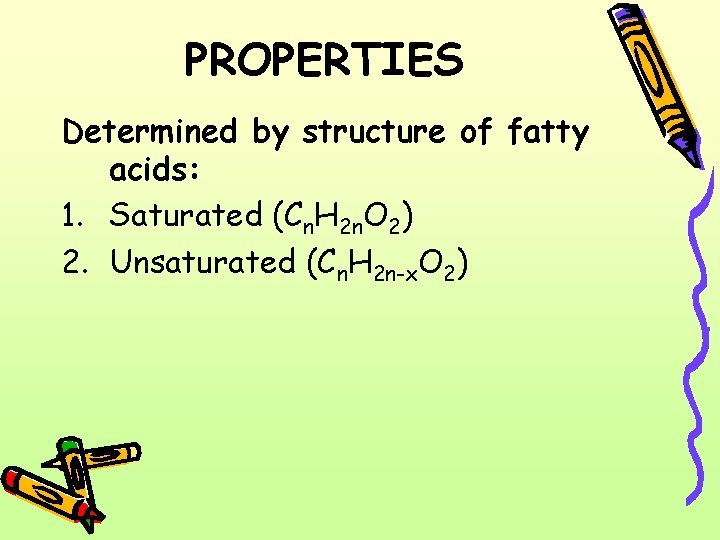
PROPERTIES Determined by structure of fatty acids: 1. Saturated (Cn. H 2 n. O 2) 2. Unsaturated (Cn. H 2 n-x. O 2)
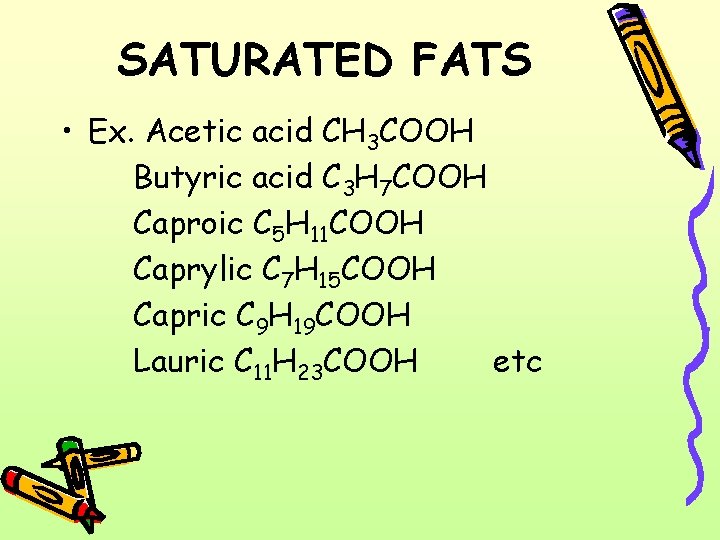
SATURATED FATS • Ex. Acetic acid CH 3 COOH Butyric acid C 3 H 7 COOH Caproic C 5 H 11 COOH Caprylic C 7 H 15 COOH Capric C 9 H 19 COOH Lauric C 11 H 23 COOH etc
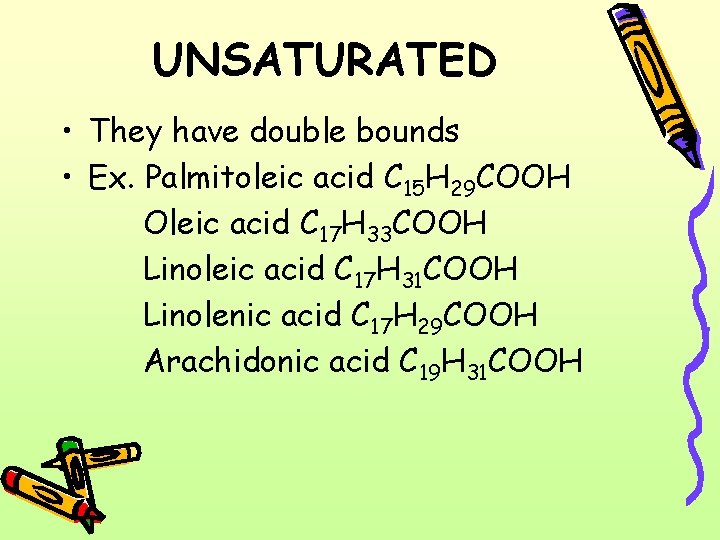
UNSATURATED • They have double bounds • Ex. Palmitoleic acid C 15 H 29 COOH Oleic acid C 17 H 33 COOH Linoleic acid C 17 H 31 COOH Linolenic acid C 17 H 29 COOH Arachidonic acid C 19 H 31 COOH
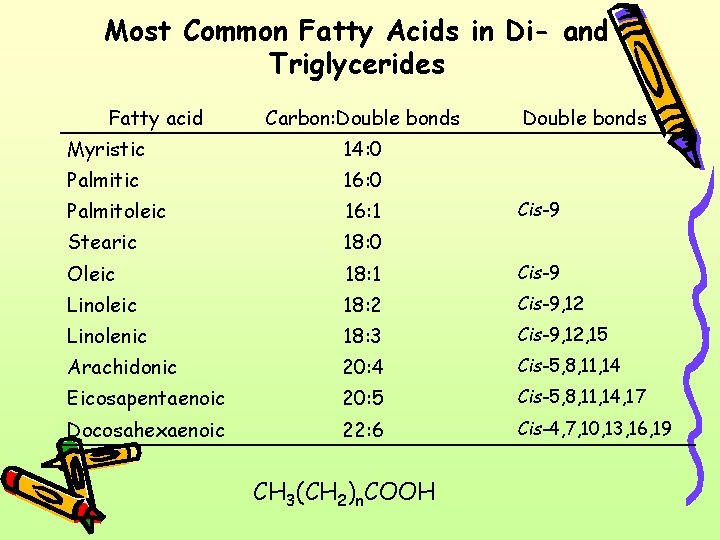
Most Common Fatty Acids in Di- and Triglycerides Fatty acid Carbon: Double bonds Myristic 14: 0 Palmitic 16: 0 Palmitoleic 16: 1 Stearic 18: 0 Oleic 18: 1 Cis-9 Linoleic 18: 2 Cis-9, 12 Linolenic 18: 3 Cis-9, 12, 15 Arachidonic 20: 4 Cis-5, 8, 11, 14 Eicosapentaenoic 20: 5 Cis-5, 8, 11, 14, 17 Docosahexaenoic 22: 6 Cis-4, 7, 10, 13, 16, 19 CH 3(CH 2)n. COOH Cis-9
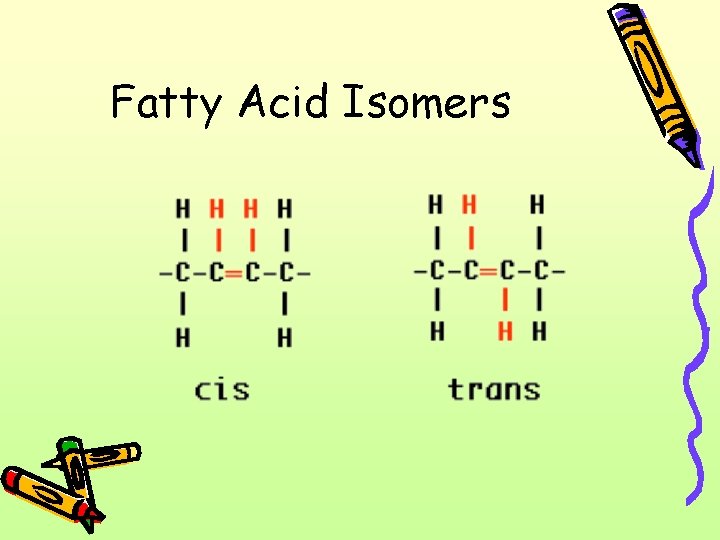
Fatty Acid Isomers
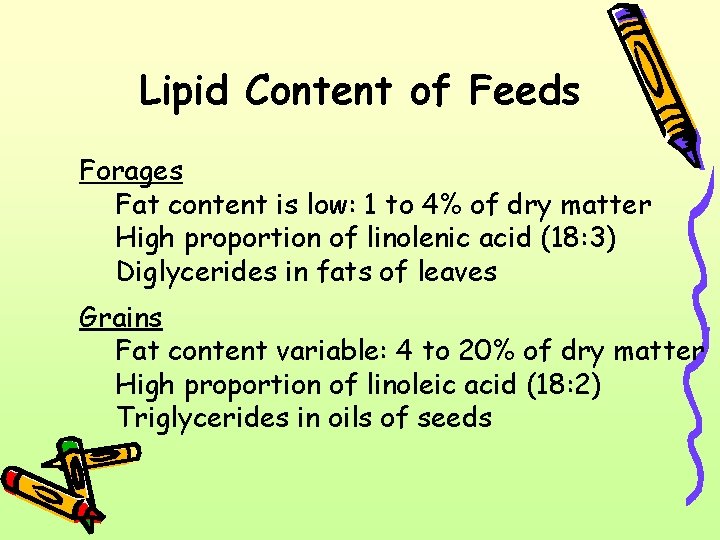
Lipid Content of Feeds Forages Fat content is low: 1 to 4% of dry matter High proportion of linolenic acid (18: 3) Diglycerides in fats of leaves Grains Fat content variable: 4 to 20% of dry matter High proportion of linoleic acid (18: 2) Triglycerides in oils of seeds
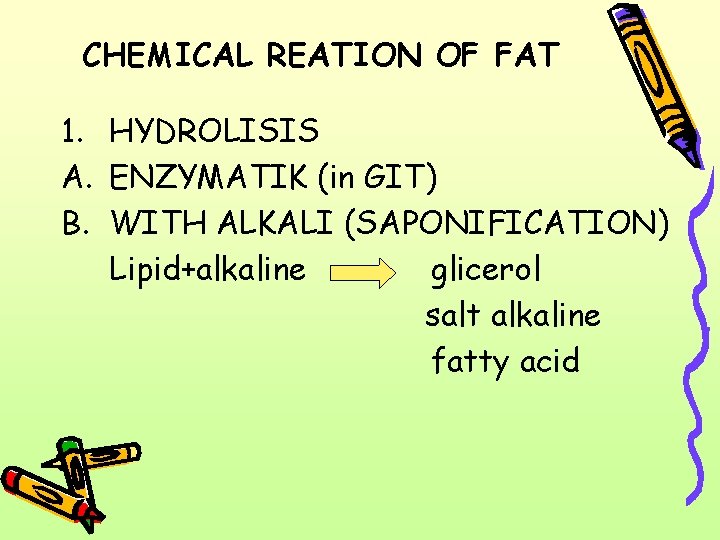
CHEMICAL REATION OF FAT 1. HYDROLISIS A. ENZYMATIK (in GIT) B. WITH ALKALI (SAPONIFICATION) Lipid+alkaline glicerol salt alkaline fatty acid
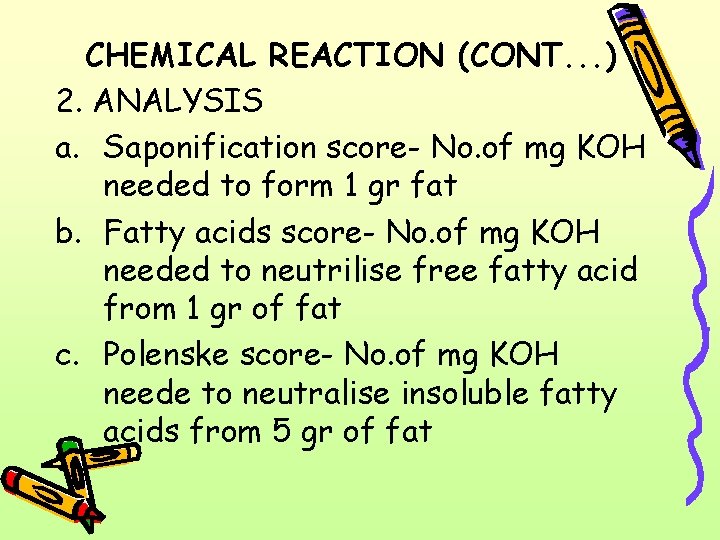
CHEMICAL REACTION (CONT. . . ) 2. ANALYSIS a. Saponification score- No. of mg KOH needed to form 1 gr fat b. Fatty acids score- No. of mg KOH needed to neutrilise free fatty acid from 1 gr of fat c. Polenske score- No. of mg KOH neede to neutralise insoluble fatty acids from 5 gr of fat
CHEMICAL REACTION OF FAT d. Iodine score (unsaturation level) - gr iodine absorbed by 100 gr lipid (each double bounds of fat can take 2 iodine atoms) e. Ranacidity Test (level of rancidity) - Peroxida score (using KI) - Tiobarbiturate test - Oven Schaal test

RANCIDITY 1. HYDROLITIC Presence of lipase causes this reaction to form fatty acid+glicerol NV of feed is not affected 2. OXIDATIVE Occured by the presence of oxigen Energi value of fat reduced

PREVENTION 1. ANTIOXIDANT Compund preventing the occurence of lipid oxidation (rancidity) # PRIMER (to stop chained reaction of free radical formation to release H) # SEKUNDER (to prevent the action of prooxidant) 2. HYROGENISATION ---- lessreaktve 3. Good storage
- Slides: 17