Lipids Fats Basic Structure Made up of 3

Lipids (Fats)
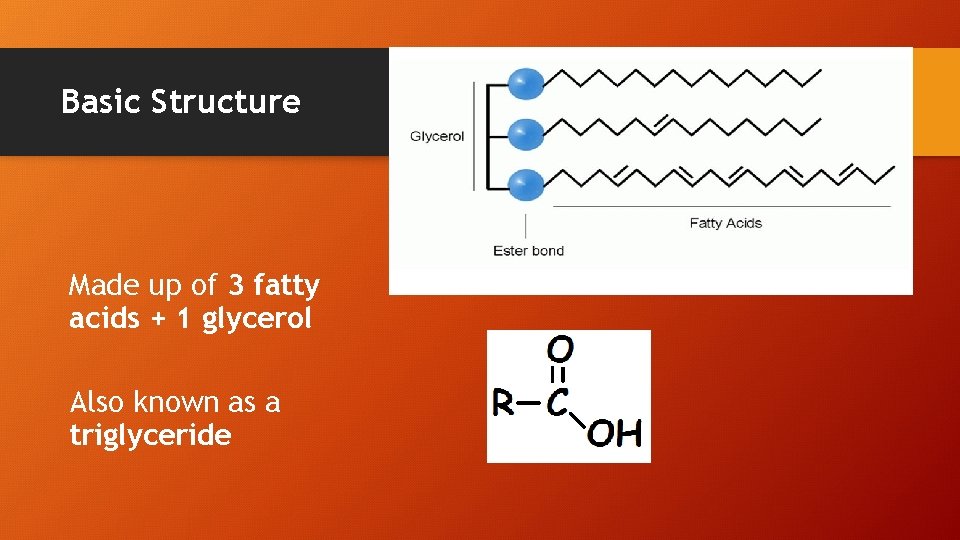
Basic Structure Made up of 3 fatty acids + 1 glycerol Also known as a triglyceride
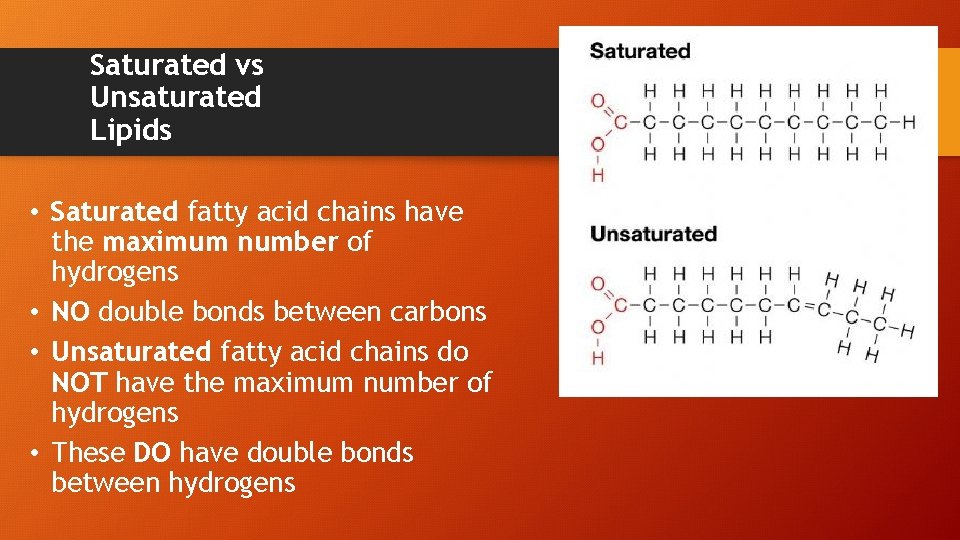
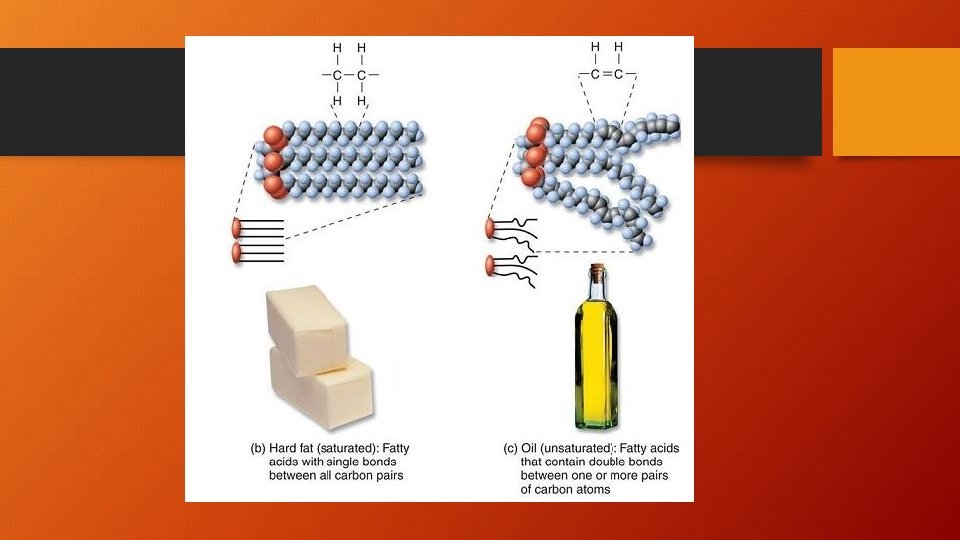
Saturated vs Unsaturated Lipids • Saturated fatty acid chains have the maximum number of hydrogens • NO double bonds between carbons • Unsaturated fatty acid chains do NOT have the maximum number of hydrogens • These DO have double bonds between hydrogens
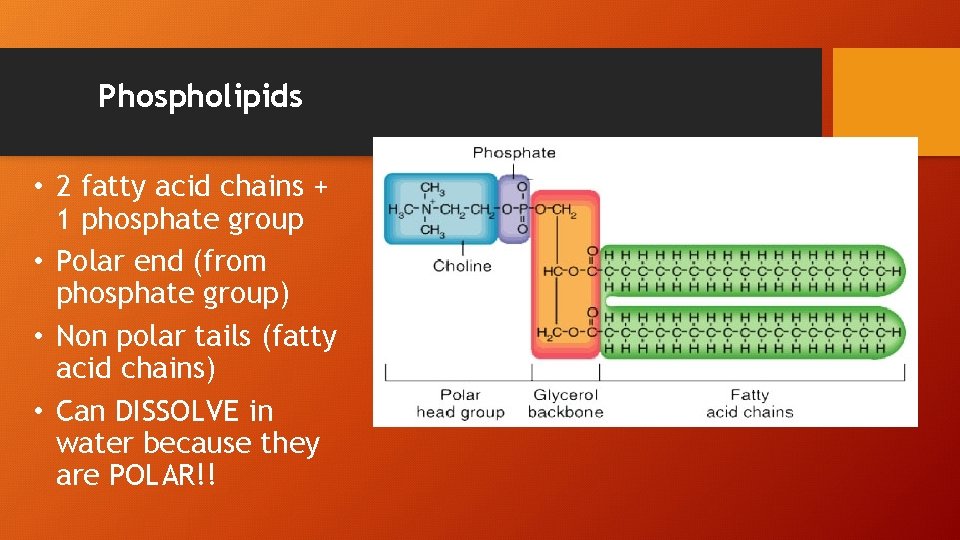
Phospholipids • 2 fatty acid chains + 1 phosphate group • Polar end (from phosphate group) • Non polar tails (fatty acid chains) • Can DISSOLVE in water because they are POLAR!!
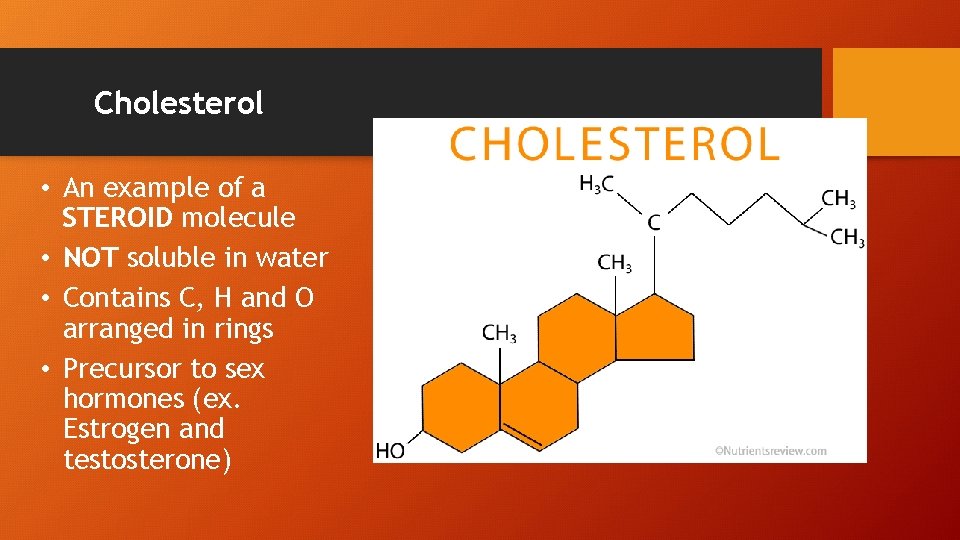
Cholesterol • An example of a STEROID molecule • NOT soluble in water • Contains C, H and O arranged in rings • Precursor to sex hormones (ex. Estrogen and testosterone)

Forming a Soap using a Fatty Acid • Soap is formed by a fatty acid + an inorganic base

Why will soap mix with water where a fat or other oily substance won’t? • Water is a polar molecule • Oil is a non polar molecule non polar things dissolve in other non polar things but NOT in polar things (like water) • If you add soap to water you can dissolve the oil in it… soap has one end that is polar and the rest is non polar.

What are FOUR important uses of lipids in your body? • Insulation and protection • Energy storage (long term) • Structure of cell membrane (phospholipids) • Basis of hormones (ex. Sex hormones)

How are phospholipids different from neutral fats? • Instead of a 3 rd fatty acid there is a phosphate group • Not electrically neutral • Soluble in water (have polar “head”)
- Slides: 10