LIPIDS CLASSIFICATION STRUCTURE AND BIOLOGICAL ROLE MICELLES WATER

LIPIDS CLASSIFICATION, STRUCTURE AND BIOLOGICAL ROLE.
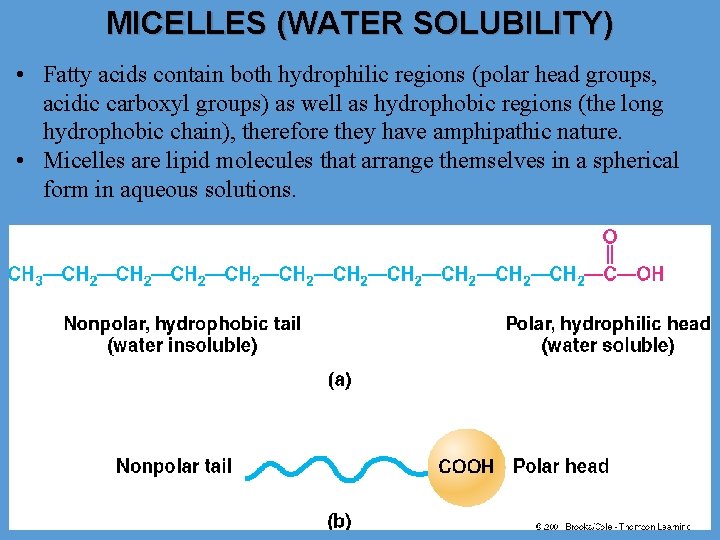
MICELLES (WATER SOLUBILITY) • Fatty acids contain both hydrophilic regions (polar head groups, acidic carboxyl groups) as well as hydrophobic regions (the long hydrophobic chain), therefore they have amphipathic nature. • Micelles are lipid molecules that arrange themselves in a spherical form in aqueous solutions.
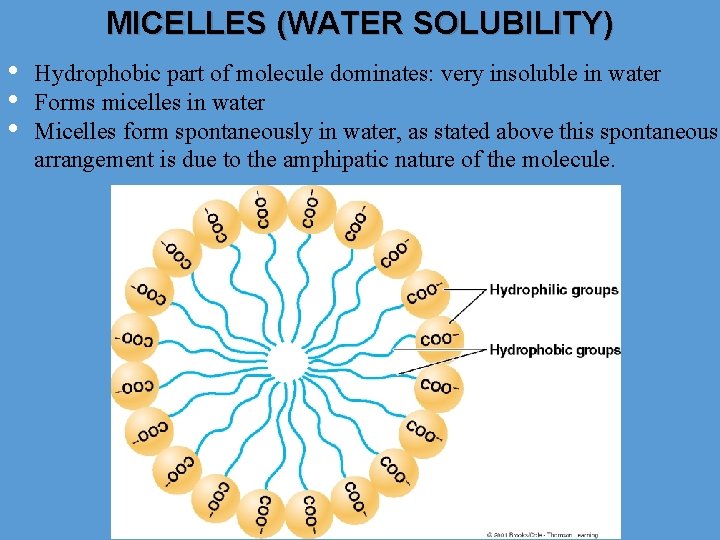
MICELLES (WATER SOLUBILITY) • • • Hydrophobic part of molecule dominates: very insoluble in water Forms micelles in water Micelles form spontaneously in water, as stated above this spontaneous arrangement is due to the amphipatic nature of the molecule.
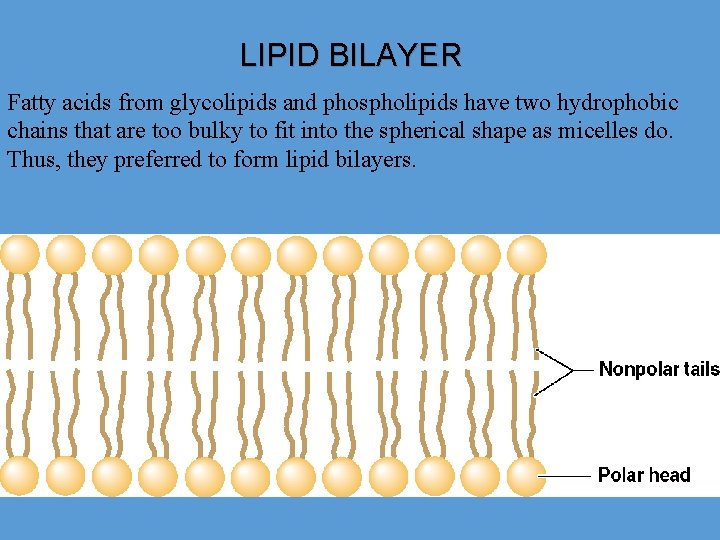
LIPID BILAYER Fatty acids from glycolipids and phospholipids have two hydrophobic chains that are too bulky to fit into the spherical shape as micelles do. Thus, they preferred to form lipid bilayers.

PROPERTIES OF FATTY ACIDS • THE PHYSICAL PROPERTIES OF FATTY ACIDS ARE LARGELY DETERMINED BY THE LENGTH AND DEGREE OF UNSATURATION OF THE HYDROCARBON CHAIN. • FATS CONTAINING SATURATED FATTY ACIDS ARE SOLID AT ORDINARY ROOM TEMPERATURE. THE ANIMAL FATS BELONG TO THIS CATEGORY. • MOST PLANT FATS POSSESS UNSATURATED FATTY ACIDS AND ARE LIQUID AT ROOM TEMPERATURE. • THE MELTING POINT OF FATS DEPENDS ON THE CHAIN LENGTH OF THE CONSTITUENT FATTY ACID AND THE DEGREE OF UNSATURATION. 5
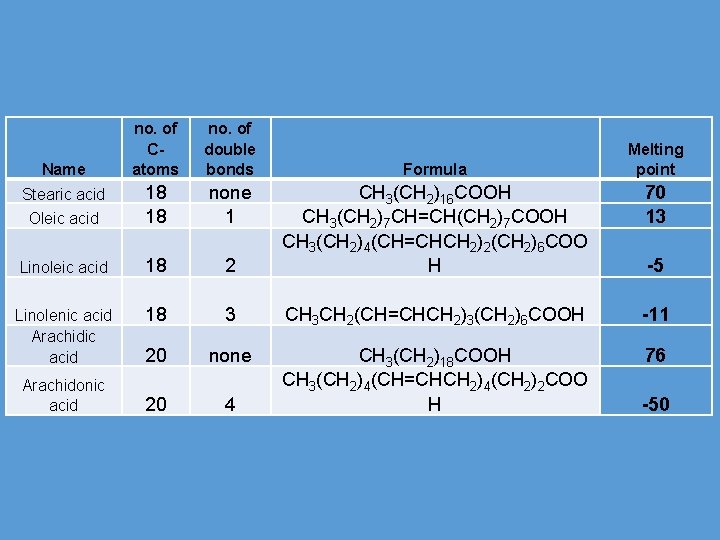
no. of Catoms no. of double bonds Oleic acid 18 18 none 1 Linoleic acid 18 Linolenic acid Arachidic acid Name Stearic acid Arachidonic acid Formula Melting point 70 13 2 CH 3(CH 2)16 COOH CH 3(CH 2)7 CH=CH(CH 2)7 COOH CH 3(CH 2)4(CH=CHCH 2)2(CH 2)6 COO H 18 3 CH 3 CH 2(CH=CHCH 2)3(CH 2)6 COOH -11 20 none 76 20 4 CH 3(CH 2)18 COOH CH 3(CH 2)4(CH=CHCH 2)4(CH 2)2 COO H -5 -50
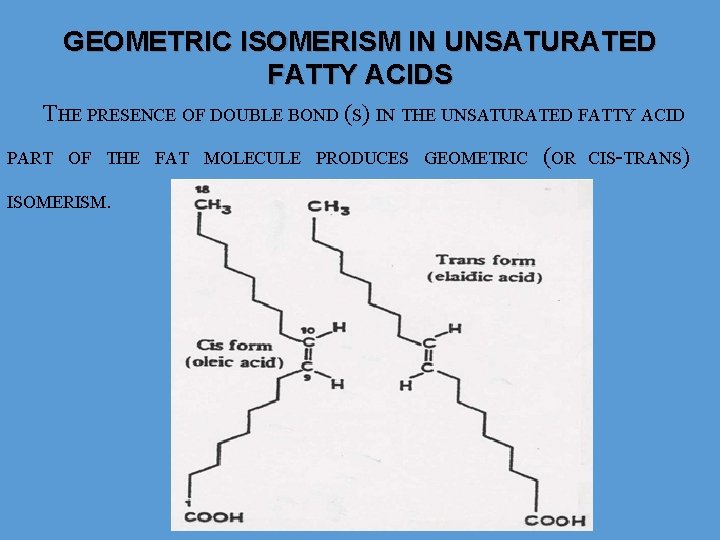
GEOMETRIC ISOMERISM IN UNSATURATED FATTY ACIDS THE PRESENCE OF DOUBLE BOND (S) IN THE UNSATURATED FATTY ACID PART OF THE FAT MOLECULE PRODUCES GEOMETRIC ISOMERISM. (OR CIS-TRANS)
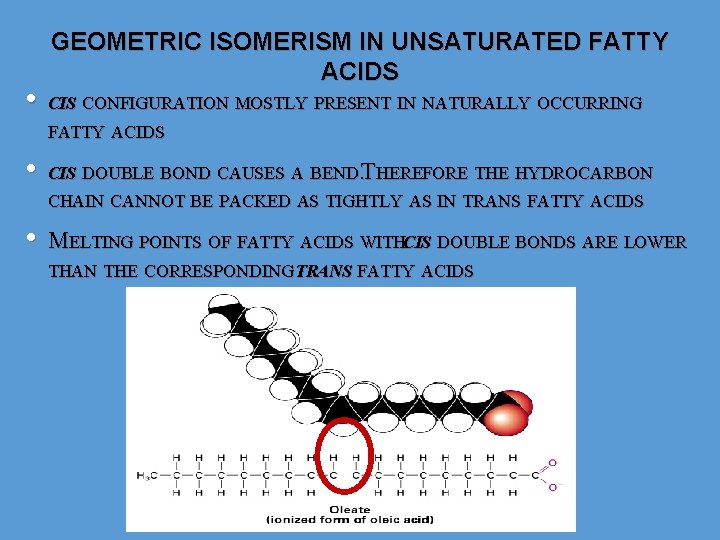
• GEOMETRIC ISOMERISM IN UNSATURATED FATTY ACIDS CIS CONFIGURATION MOSTLY PRESENT IN NATURALLY OCCURRING FATTY ACIDS • CIS DOUBLE BOND CAUSES A BEND. THEREFORE THE HYDROCARBON CHAIN CANNOT BE PACKED AS TIGHTLY AS IN TRANS FATTY ACIDS • MELTING POINTS OF FATTY ACIDS WITHCIS DOUBLE BONDS ARE LOWER THAN THE CORRESPONDING TRANS FATTY ACIDS
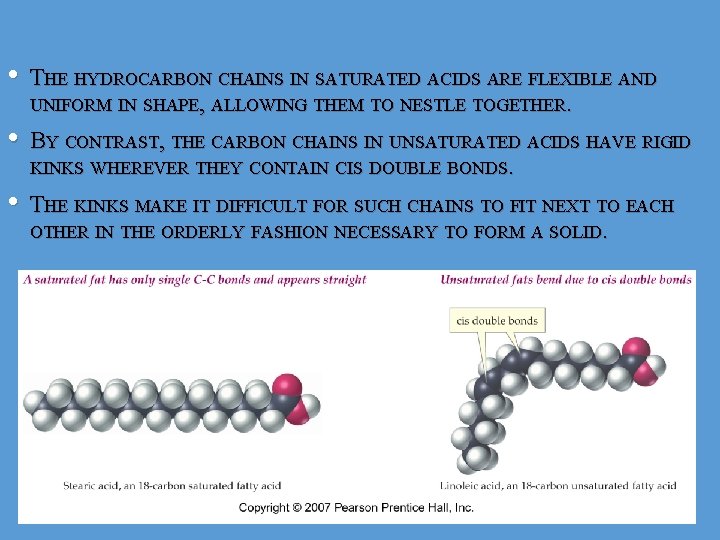
• THE HYDROCARBON CHAINS IN SATURATED ACIDS ARE FLEXIBLE AND UNIFORM IN SHAPE, ALLOWING THEM TO NESTLE TOGETHER. • BY CONTRAST, THE CARBON CHAINS IN UNSATURATED ACIDS HAVE RIGID KINKS WHEREVER THEY CONTAIN CIS DOUBLE BONDS. • THE KINKS MAKE IT DIFFICULT FOR SUCH CHAINS TO FIT NEXT TO EACH OTHER IN THE ORDERLY FASHION NECESSARY TO FORM A SOLID. 9
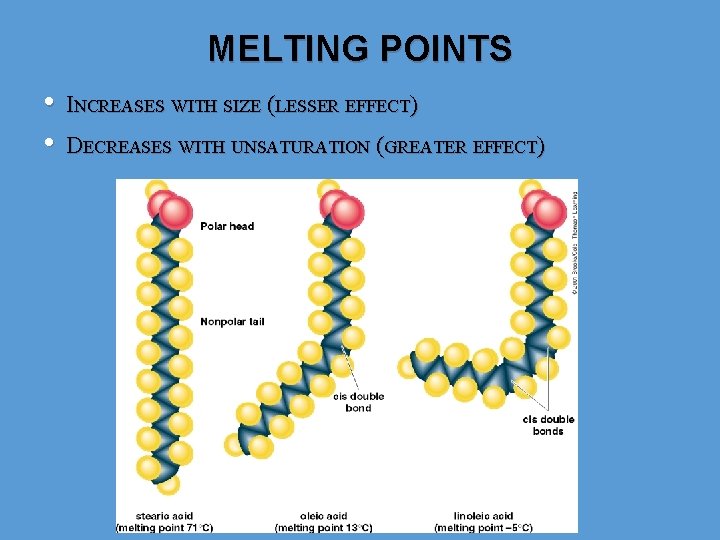
MELTING POINTS • • INCREASES WITH SIZE (LESSER EFFECT) DECREASES WITH UNSATURATION (GREATER EFFECT)
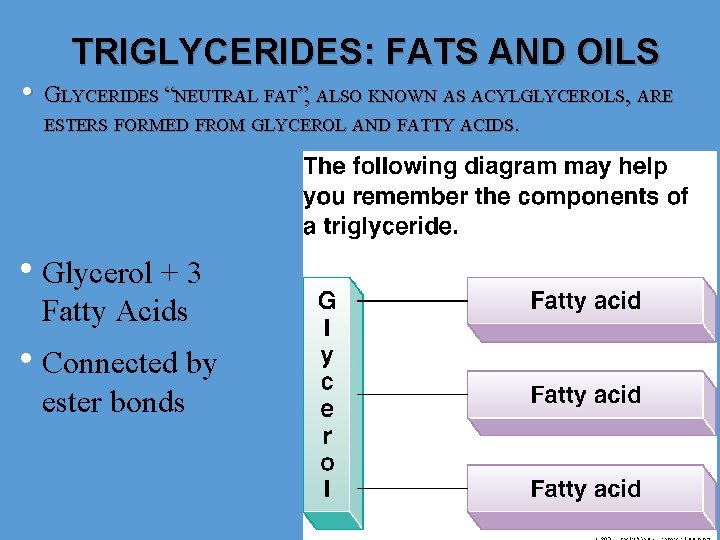
• TRIGLYCERIDES: FATS AND OILS GLYCERIDES “NEUTRAL FAT”, ALSO KNOWN AS ACYLGLYCEROLS, ARE ESTERS FORMED FROM GLYCEROL AND FATTY ACIDS. • Glycerol + 3 Fatty Acids • Connected by ester bonds
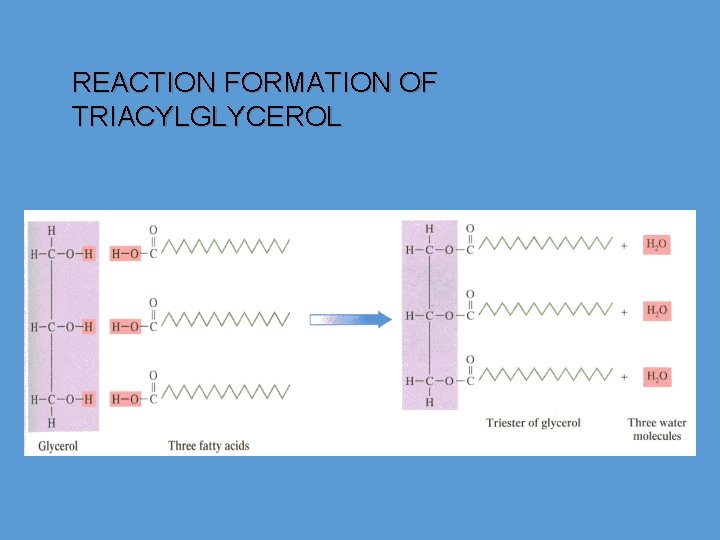
REACTION FORMATION OF TRIACYLGLYCEROL

FAT OR OIL • Glycerol has three hydroxyl functional groups, which can be esterified with one, two, or three fatty acids to form monoglycerides, diglycerides and triglycerides. • Vegetable oils and animal fats contain mostly triglycerides, but are broken down by natural enzymes (lipases) into mono and diglycerides and free fatty acids and glycerol. • The simplest lipids constructed from fatty acids are the triglycerides, also referred to as triacylglycerols, fats, or neutral fats. • Those containing the same kind of fatty acid in all three positions are called simple triglycerides. • Simple triglycerides of 16: 0, 18: 0, and 18: 1, for example, are tristearin, tripalmitin, and triolein, respectively. • Most naturally occurring triglycerides are mixed; they contain two or more different fatty acids. • Because the polar hydroxyls of glycerol and the polar carboxylates of the fatty acids are bound in ester linkages, triglycerides are nonpolar, hydrophobic molecules, essentially insoluble in water.

FAT OR OIL • • DEPENDS ON MELTING POINT- SOLID OR LIQUID AT ROOM TEMPERATURE DEPENDS MOSTLY ON DEGREE OF UNSATURATION ANIMAL FATS MORE SATURATED, PLANT OILS MORE UNSATURATED FATS ARE SOLID AND OILS ARE LIQUID AT ROOM TEMPERATURE
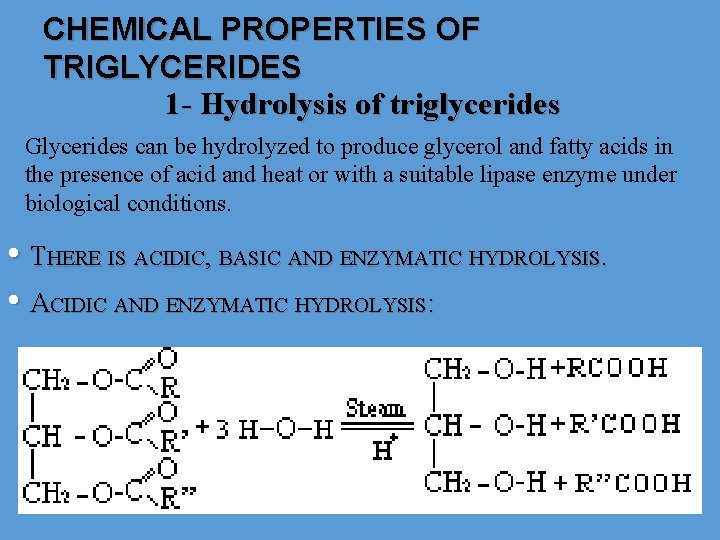
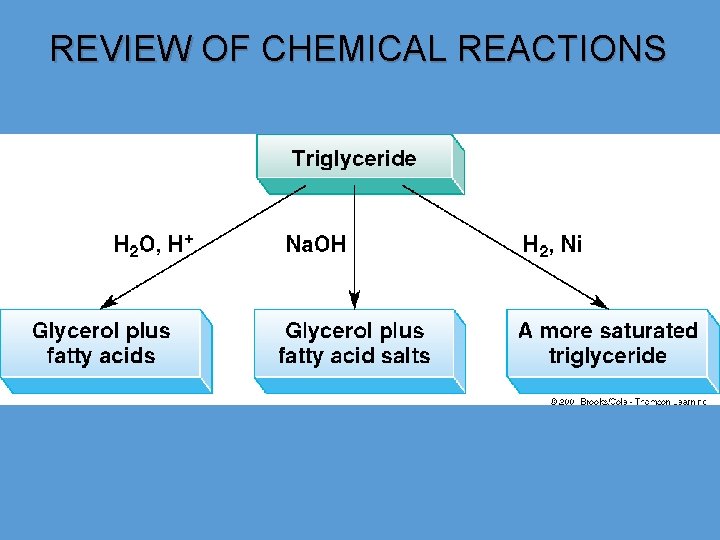
CHEMICAL PROPERTIES OF TRIGLYCERIDES 1 - Hydrolysis of triglycerides Glycerides can be hydrolyzed to produce glycerol and fatty acids in the presence of acid and heat or with a suitable lipase enzyme under biological conditions. • THERE IS ACIDIC, BASIC AND ENZYMATIC HYDROLYSIS. • ACIDIC AND ENZYMATIC HYDROLYSIS:
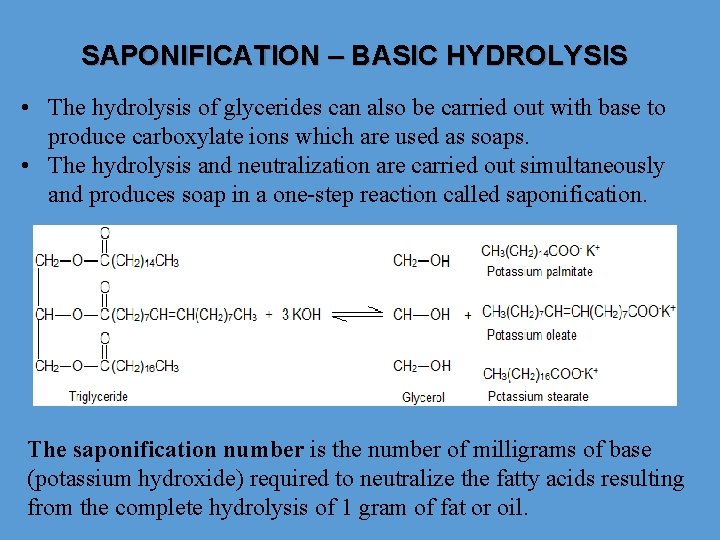
SAPONIFICATION – BASIC HYDROLYSIS • The hydrolysis of glycerides can also be carried out with base to produce carboxylate ions which are used as soaps. • The hydrolysis and neutralization are carried out simultaneously and produces soap in a one-step reaction called saponification. The saponification number is the number of milligrams of base (potassium hydroxide) required to neutralize the fatty acids resulting from the complete hydrolysis of 1 gram of fat or oil.

CHEMICAL PROPERTIES OF TRIGLYCERIDES 2 - REACTION WITH I 2 • Used to quantitate unsaturation in fats • Product is colorless, I 2 is colored Iodine number is the number of grams of iodine consumed by 100 g of fat. A higher iodine value indicates a higher degree of unsaturation.

CHEMICAL PROPERTIES OF TRIGLYCERIDES 3 - HYDROGENATION. • Fatty acid chains in glycerides having double bonds are susceptible to attack by atmospheric oxygen, resulting in rancid taste, which is not acceptable to consumers. • Oils and fats molecules having multiple double bonds are more susceptible to rancidity as compared with those having single or nil double bond. • To minimize number of double bonds; hydrogenation reaction is achieved to give single bond from double bonds. • Hydrogenation process not only enhances stability of oils and fats but also offers improved taste due to softness of hydrogenated solid product. • Edible products such as margarines, low fat spreads, cake shortenings and confectionery fats are made with oils containing certain concentration of solid fat content that could give good mouth feel during consumption. • Today, hydrogenation reaction of oils and fatty acids is carried out using supported nickel as most appropriate catalyst to decrease the unsaturation.
REVIEW OF CHEMICAL REACTIONS
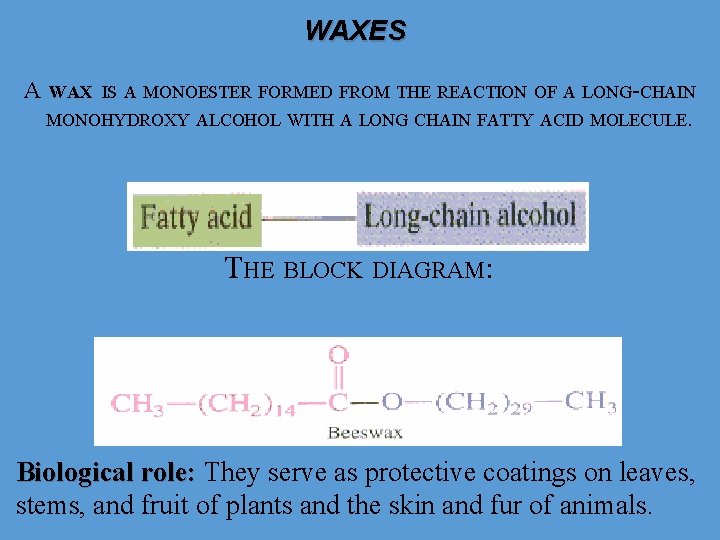
WAXES А WAX IS А MONOESTER FORMED FROM THE REACTION OF А LONG-CHAIN MONOHYDROXY ALCOHOL WITH А LONG CHAIN FATTY ACID MOLECULE. THE BLOCK DIAGRAM: Biological role: They serve as protective coatings on leaves, stems, and fruit of plants and the skin and fur of animals.

WAXES • Waxes are completely water-insoluble and generally solid at room temperatures. • Their strongly hydrophobic nature allows them to function as water repellents on the leaves of some plants, on feathers of birds, and on the cuticles of certain insects. • The most commonly known animal wax is beeswax. • A major component of the beeswax used in constructing honeycombs is the ester myricyl palmitate. • Waxes are used to make candles, which used for lighting and decoration. • Also, waxes are used as finishes and coatings for wood products. • Biological waxes find a variety of applications in the pharmaceutical, cosmetic, and other industries. • Lanolin, beeswax, carnauba wax are widely used in the manufacture of lotions, ointments, and polishes.

- Slides: 22