Lipids and Carbohydrates Chemistry U 4 AOS 2

Lipids and Carbohydrates Chemistry U 4 AOS 2
Introduction to lipids Lipids are a diverse group of compounds that are insoluble in water but soluble in organic solvents such as ethanol. The most common types of lipid are triglycerides (sometimes known as true fats or neutral fats), but other important lipids include waxes, steroids and cholesterol. Like carbohydrates, lipids contain carbon, hydrogen and oxygen, but they have a higher proportion of hydrogen and a lower proportion of oxygen.
The structure of triglycerides
Saturated and unsaturated
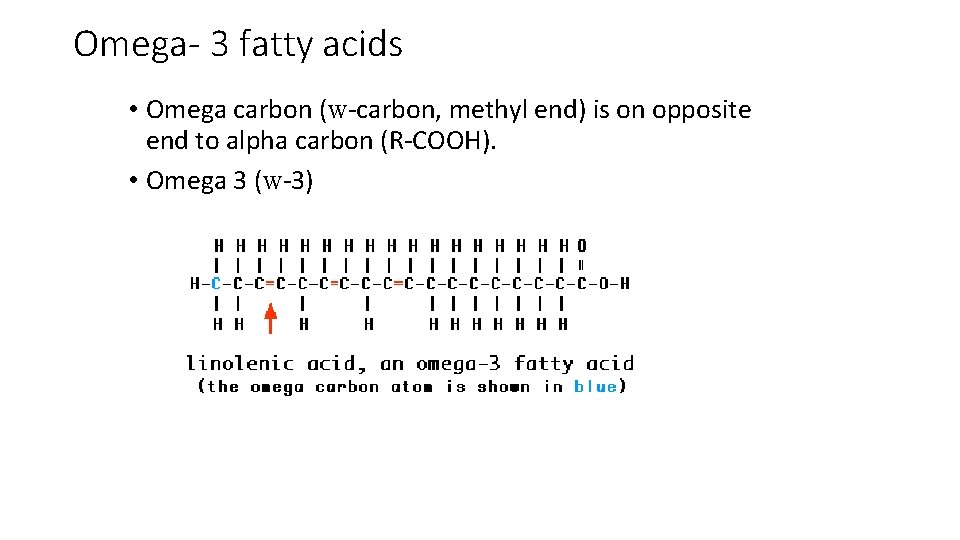
Omega- 3 fatty acids • Omega carbon (w-carbon, methyl end) is on opposite end to alpha carbon (R-COOH). • Omega 3 (w-3)

Omega-6 fatty acids • Identify the alpha carbon and omega carbon. • Why is this structure known as omega-6? • Which one of the two will have a greater kink in its structure? • So which one will have a higher MP?
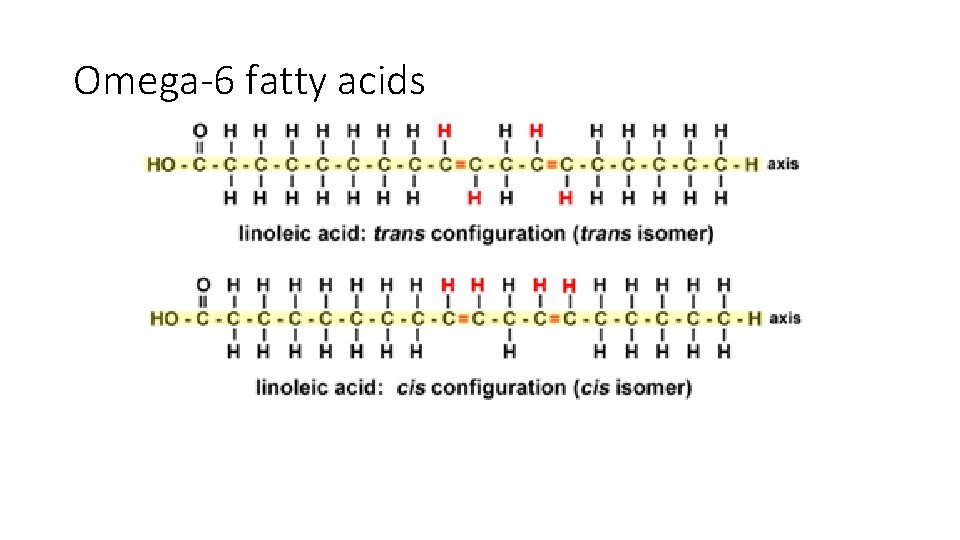
Omega-6 fatty acids

Melting points of lipids • Fats- solids at room temperature and from animal sources • Oils – liquids and mostly from vegetables • Melting points of fatty acids determines the uses of them, especially when using them in the kitchen. • What do we know about factors effecting MP? • • • Molecular mass Intermolecualr forces (mainly dispersion) Carbon=Carbon double bonds Cis-trans arrangement So can we convert saturated animal fats into solids? • We can react them with Hydrogen in the presence of a catalyst to create more saturated carbons making them solids to different temperatures (polyunsaturated margarines made from vegetable oils)

Role of lipids The major biological role of lipids is as an energy source. Lipids provide more than twice the amount of energy as carbohydrates – about 38 k. J/g. Lipids are stored in adipose tissue, which has several important roles, including: heat insulation – in mammals, adipose tissue underneath the skin helps reduce heat loss. protection – adipose tissue around delicate organs such as the kidneys acts as a cushion against impacts.
Essential and Non-essential fatty acids • Non-essential fatty acids are the ones that unused certain fatty acids can be converted to when in demand. So essentially, your body is able to convert fatty acids into other fatty acids for non-essential ones.

• Essential fatty acids are unable to be produced by the body (just like essential AA’s) • These are all polyunsaturated fats (with more than one C=C double bond) • Omega-3 linolenic acid • Omega-6 linoleic acid • Omega-6 arachidonic acid • Only need these in small amounts to produce certain hormones. Namely the blood clotting hormones, prostaglandins and thromboxane.
The structure of phospholipids This is not required specifically for U 4 but is of interest and links to Biology!
Emulsion test for lipids

Components of lipids
Glossary
What’s the keyword?
Mystery substance
Multiple-choice quiz
Introducing carbohydrates Carbohydrates are a group of substances used as both energy sources and structural materials in organisms. All carbohydrates contain carbon, hydrogen and oxygen, with the general formula: Cx(H 2 O)y. There are three main groups of carbohydrates: monosaccharides – these are simple sugars, with the general formula (CH 20)n, where n can be 3– 7 disaccharides – these are ‘double sugars’, formed from two monosaccharides polysaccharides – these are large molecules formed from many monosaccharides.
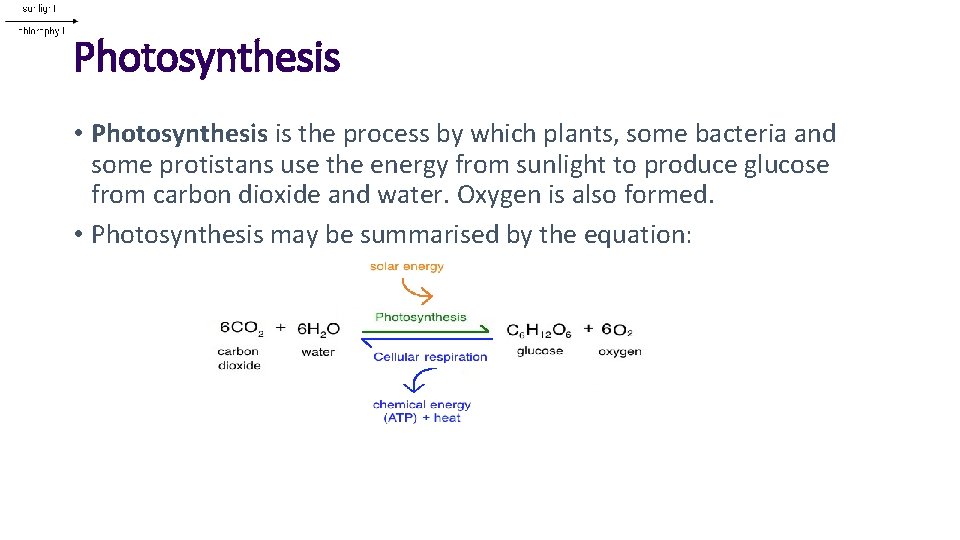
Photosynthesis • Photosynthesis is the process by which plants, some bacteria and some protistans use the energy from sunlight to produce glucose from carbon dioxide and water. Oxygen is also formed. • Photosynthesis may be summarised by the equation:
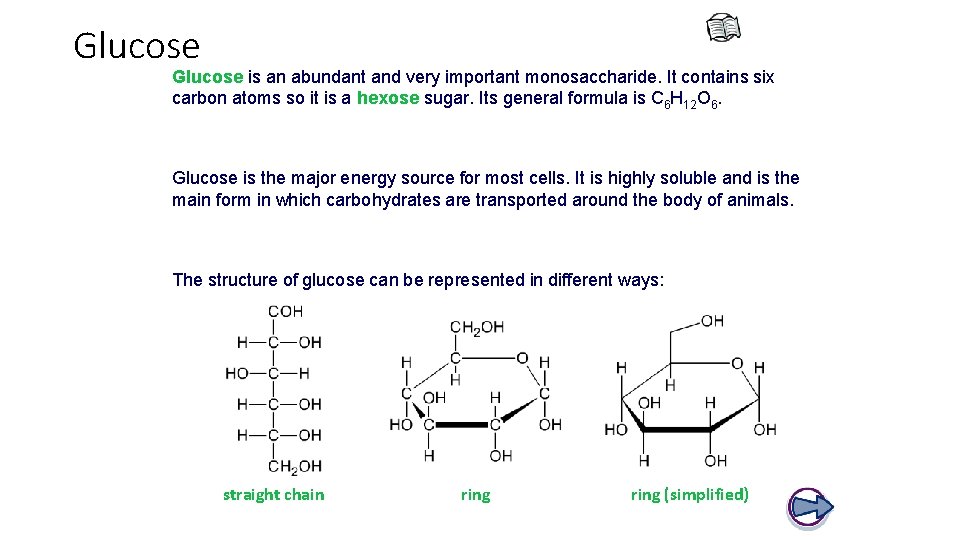
Glucose is an abundant and very important monosaccharide. It contains six carbon atoms so it is a hexose sugar. Its general formula is C 6 H 12 O 6. Glucose is the major energy source for most cells. It is highly soluble and is the main form in which carbohydrates are transported around the body of animals. The structure of glucose can be represented in different ways: straight chain ring (simplified)
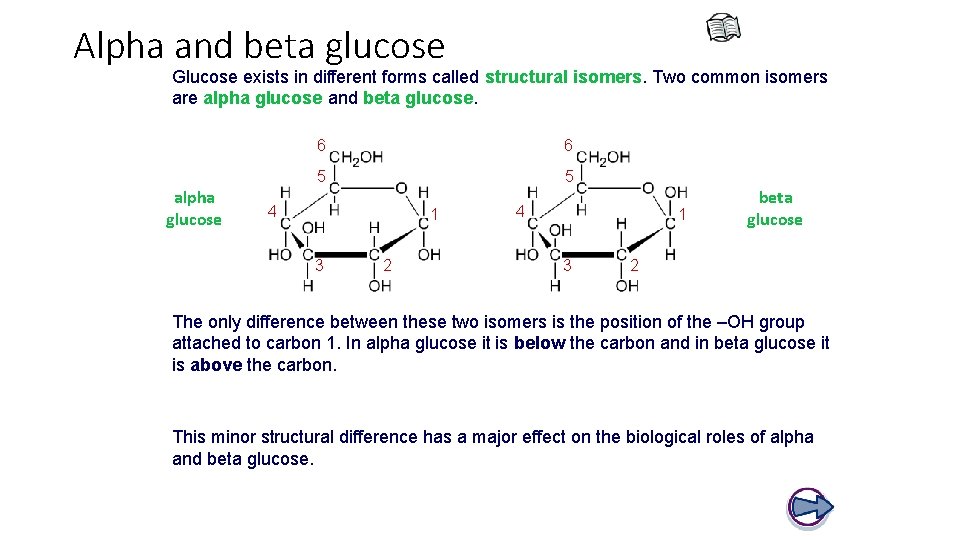
Alpha and beta glucose Glucose exists in different forms called structural isomers. Two common isomers are alpha glucose and beta glucose. alpha glucose 6 6 5 5 4 1 3 2 4 1 3 beta glucose 2 The only difference between these two isomers is the position of the –OH group attached to carbon 1. In alpha glucose it is below the carbon and in beta glucose it is above the carbon. This minor structural difference has a major effect on the biological roles of alpha and beta glucose.
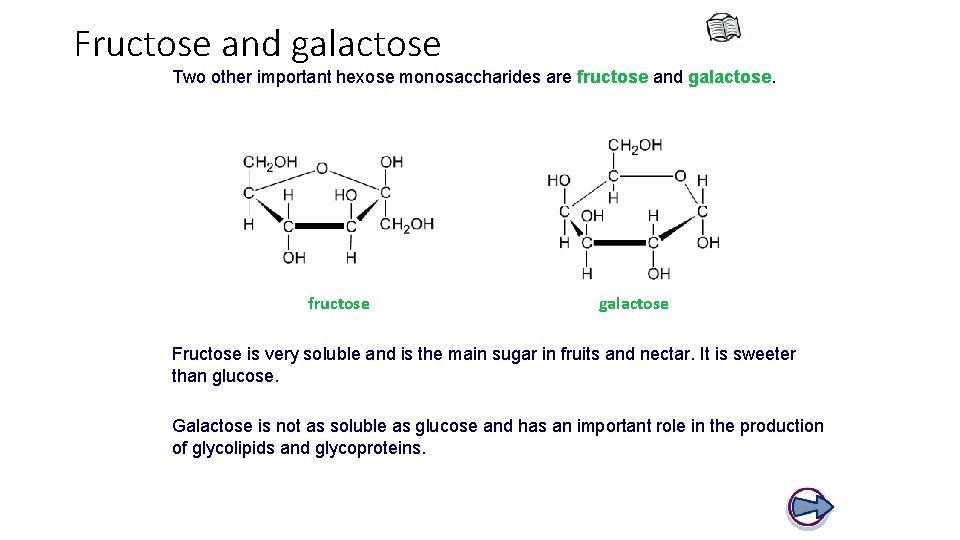
Fructose and galactose Two other important hexose monosaccharides are fructose and galactose. fructose galactose Fructose is very soluble and is the main sugar in fruits and nectar. It is sweeter than glucose. Galactose is not as soluble as glucose and has an important role in the production of glycolipids and glycoproteins.
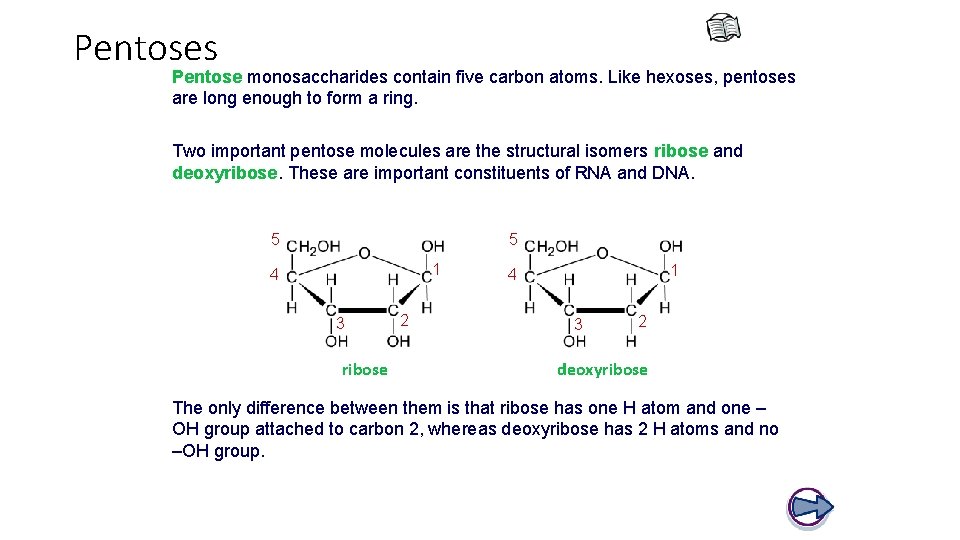
Pentoses Pentose monosaccharides contain five carbon atoms. Like hexoses, pentoses are long enough to form a ring. Two important pentose molecules are the structural isomers ribose and deoxyribose. These are important constituents of RNA and DNA. 5 5 1 4 3 ribose 2 1 4 3 2 deoxyribose The only difference between them is that ribose has one H atom and one – OH group attached to carbon 2, whereas deoxyribose has 2 H atoms and no –OH group.
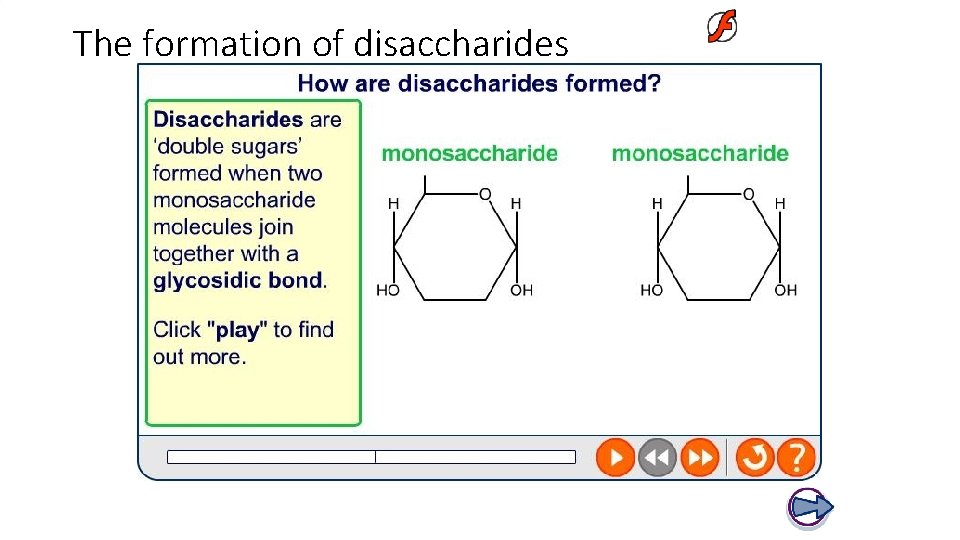
The formation of disaccharides
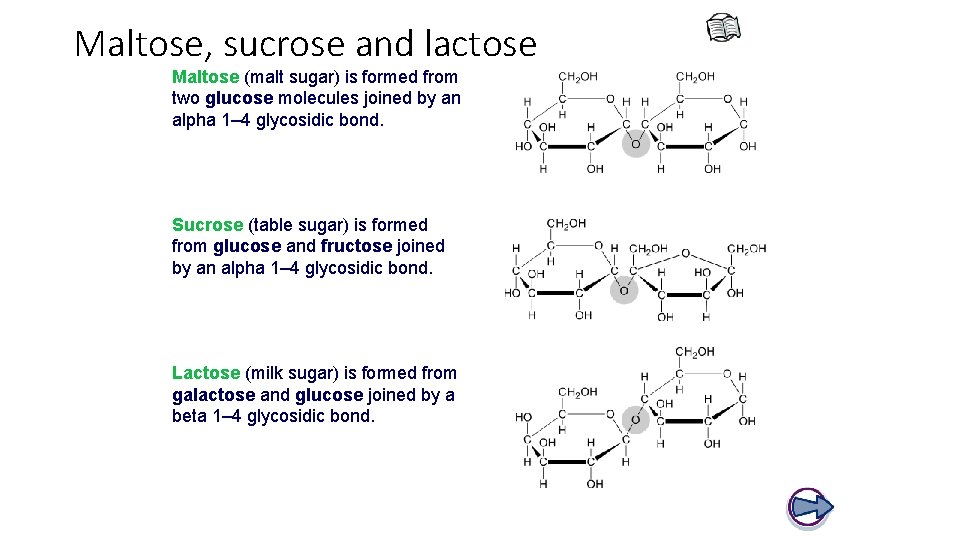
Maltose, sucrose and lactose Maltose (malt sugar) is formed from two glucose molecules joined by an alpha 1– 4 glycosidic bond. Sucrose (table sugar) is formed from glucose and fructose joined by an alpha 1– 4 glycosidic bond. Lactose (milk sugar) is formed from galactose and glucose joined by a beta 1– 4 glycosidic bond.
Benedict’s test for reducing sugars
Mono- and disaccharides
What are polysaccharides? Polysaccharides are polymers containing many monosaccharides linked by glycosidic bonds. Like disaccharides, polysaccharides are formed by condensation reactions. Polysaccharides are mainly used as an energy store and as structural components of cells. The major polysaccharides are starch and cellulose in plants, and glycogen in animals.
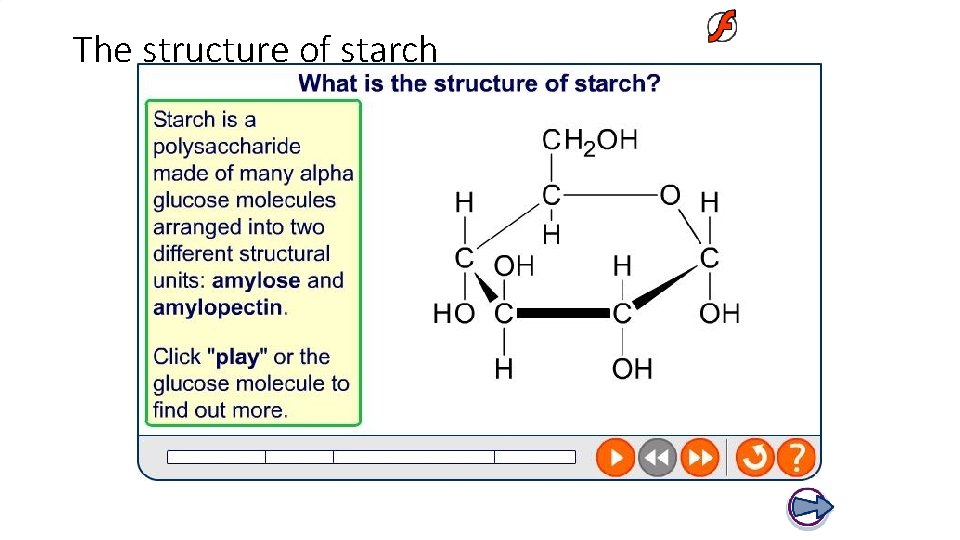
The structure of starch

Properties and uses of starch Starch is the major carbohydrate storage molecule in plants. It is usually stored as intracellular starch grains in organelles called plastids. Plastids include green chloroplasts (e. g. in leaves) and colourless amyloplasts (e. g. in potatoes). Starch is produced from glucose made during photosynthesis. It is broken down during respiration to provide energy and is also a source of carbon for producing other molecules.
Iodine test for starch

What is cellulose? Cellulose is another polysaccharide and is the main part of plant cell walls. It is the most abundant organic polymer. Unlike starch, cellulose is very strong, and prevents cells from bursting when they take in excess water. Cellulose consists of long chains of beta glucose molecules joined by beta 1– 4 glycosidic bonds. The glucose chains form rope-like microfibrils, which are layered to form a network.
The structure of cellulose
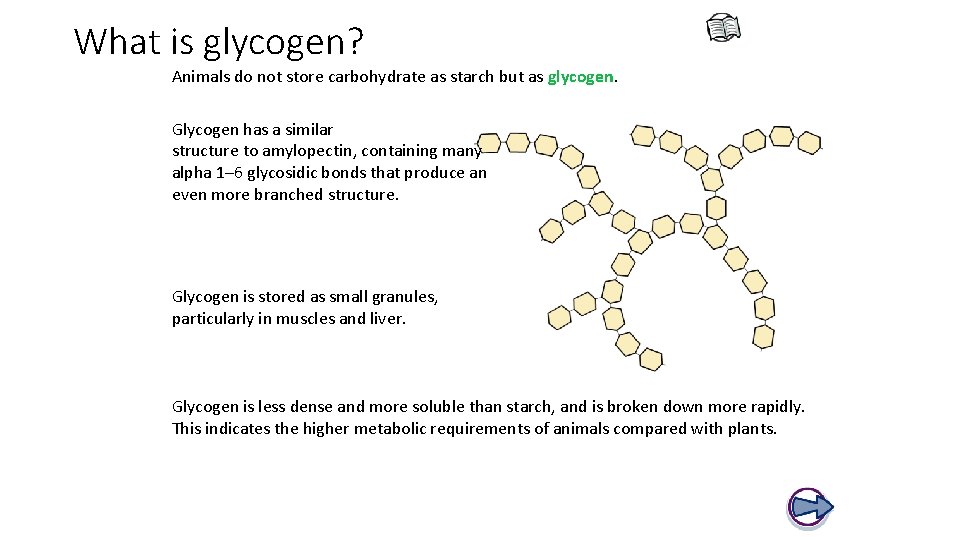
What is glycogen? Animals do not store carbohydrate as starch but as glycogen. Glycogen has a similar structure to amylopectin, containing many alpha 1– 6 glycosidic bonds that produce an even more branched structure. Glycogen is stored as small granules, particularly in muscles and liver. Glycogen is less dense and more soluble than starch, and is broken down more rapidly. This indicates the higher metabolic requirements of animals compared with plants.
Polysaccharides: true or false?
Glossary
What’s the keyword?
What’s the carbohydrate?
Multiple-choice quiz
- Slides: 40