Lipid Peroxidation Introduction Oxidative deterioration of lipids containing















- Slides: 28

Lipid Peroxidation




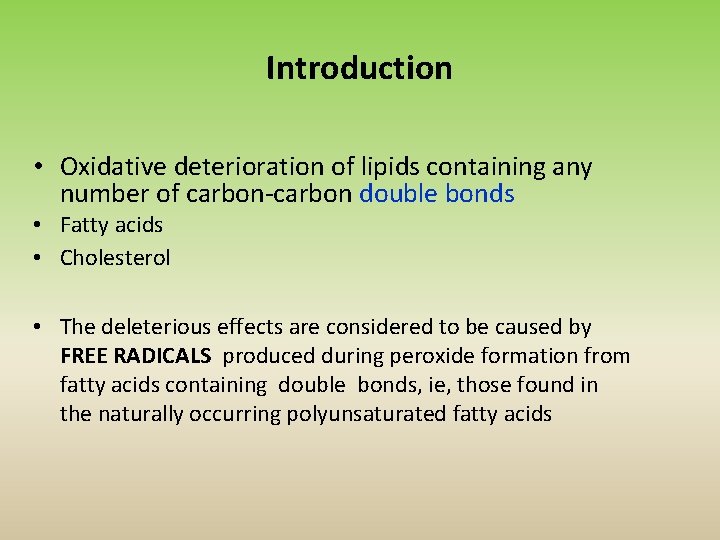
Introduction • Oxidative deterioration of lipids containing any number of carbon-carbon double bonds • Fatty acids • Cholesterol • The deleterious effects are considered to be caused by FREE RADICALS produced during peroxide formation from fatty acids containing double bonds, ie, those found in the naturally occurring polyunsaturated fatty acids

Free radicals A free radical is any species capable of independent existence that contains one or more UNPAIRED ELECTRONS ● designation: R● ● to be paramagnetic ● renowned for their high chemical reactivity Forming: X − e- X●+ (oxidation)

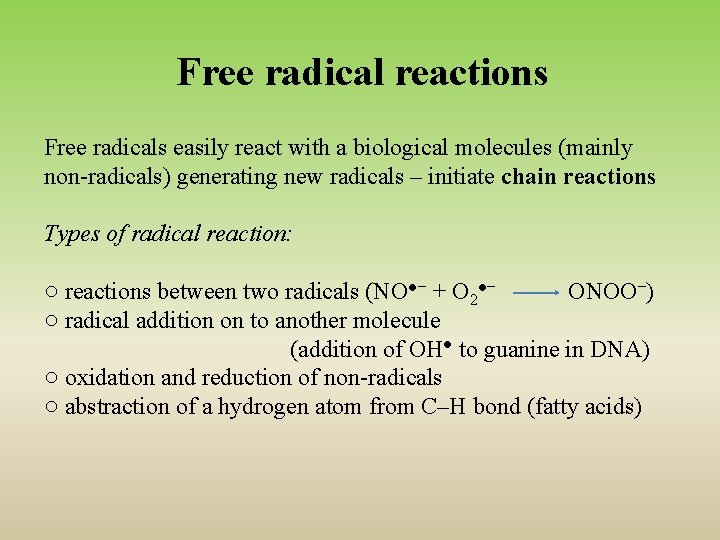
Free radical reactions Free radicals easily react with a biological molecules (mainly non-radicals) generating new radicals – initiate chain reactions Types of radical reaction: ○ reactions between two radicals (NO●− + O 2●− ONOO−) ○ radical addition on to another molecule (addition of OH● to guanine in DNA) ○ oxidation and reduction of non-radicals ○ abstraction of a hydrogen atom from C–H bond (fatty acids)

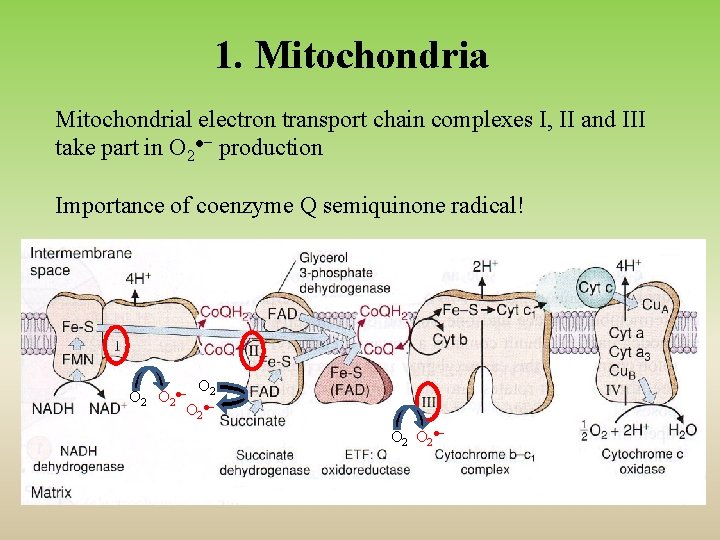
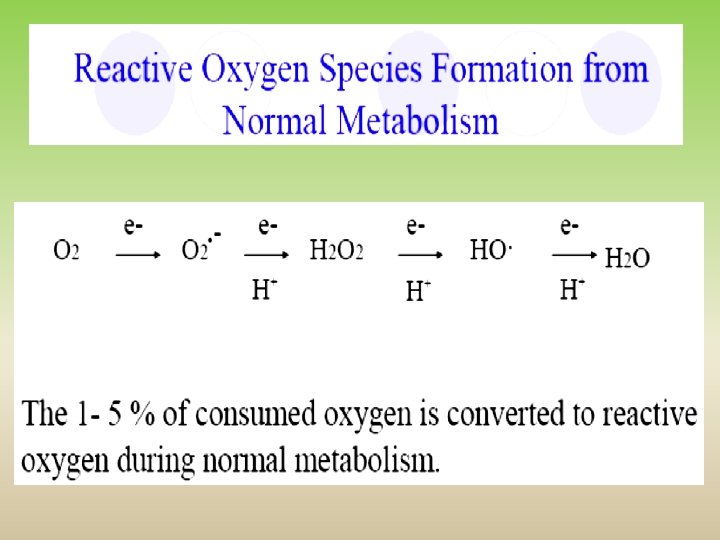
1. Mitochondrial electron transport chain complexes I, II and III take part in O 2●− production Importance of coenzyme Q semiquinone radical! O O 2●− 2 O 2●−

Mitochondria ● one of the most important sources of ROS production ● complexes of ETC can catalyse one electron reduction of O 2 to superoxide ● cytochrome c oxidase produces radical intermediates, although they are firmly bound to the enzyme ● NADH dehydrogenase (complex I) and cytochrome bc 1 (complex III) are major sites of superoxide production ● electron carrier coenzyme Q (ubiquinone) is during electron transport oxidized and afterwards reduced by single electron forming radical intermediate (semiquinone) and it can react with O 2 to give superoxide O 2●−

Free radical sources • Exogenous – Foods – Air pollutants – Radiation • Endogenous – Metabolism (mitochondria and peroxisomes) – Detoxification – cytochrome P 450 – Immune cells

Production of toxic compounds • Many secondary by-products of lipid oxidation are potential carcinogens • Hydroperoxides are known to damage DNA • Carbonyl compounds may affect cellular signal transduction • Aldehydes: 4 -hydroxynonenal, and MDA • Epoxides and hydrogen peroxide by-products are known carcinogens

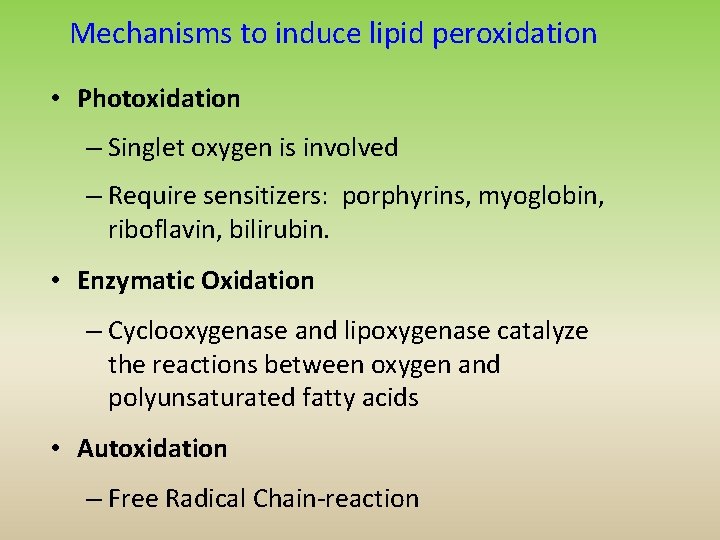
Mechanisms to induce lipid peroxidation • Photoxidation – Singlet oxygen is involved – Require sensitizers: porphyrins, myoglobin, riboflavin, bilirubin. • Enzymatic Oxidation – Cyclooxygenase and lipoxygenase catalyze the reactions between oxygen and polyunsaturated fatty acids • Autoxidation – Free Radical Chain-reaction

Reactive Oxygen Species and Free Radicals • Reactive Oxygen Species – Triplet oxygen – Superoxide – Singlet Oxygen – Hydroperoxyl radical – Hydrogen peroxide – Ozone • Peroxyl radical (ROO. ) • Alkoxyl radical (RO. ) • Iron-oxygen complexes (ferryl and perferryl radicals) • Thiyl radicals (RS. ) • Nitric oxide (. NO)


Implications of Lipid Oxidation to Human Health

Oxidative Stress

Consequences of Lipid Peroxidation • Structural changes in membranes – Alter fluidity and ion channels – Alter membrane-bound signaling proteins – Increase membrane permeability • Form lipid oxidation products adducts/crosslinks with non lipids – e. g. , proteins and DNA • Cause direct toxicity – e. g. , 4 -hydroxynonenal, MDA • DNA damage and mutagenesis

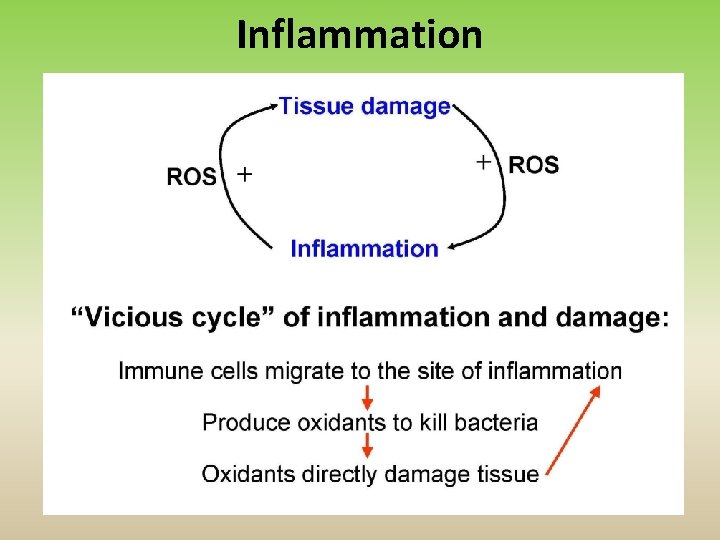
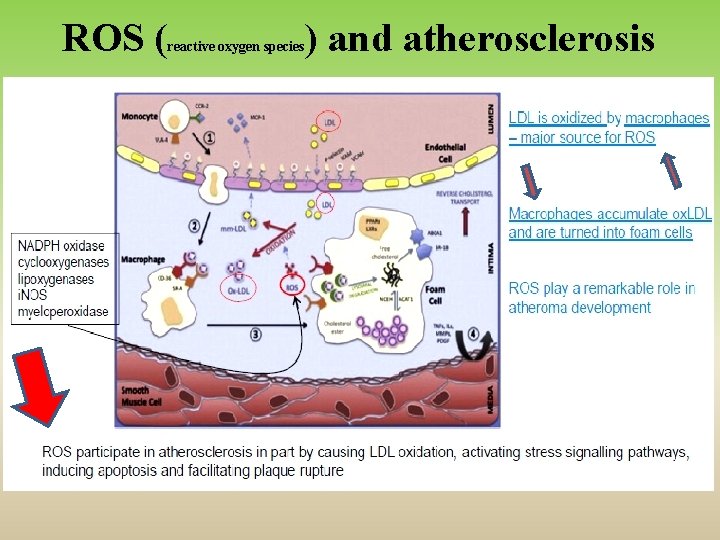
Pathological Conditions that Involve Oxidative Stress • Inflammation • Atherosclerosis • Ischemia/reperfusion injury • Cancer • Aging

Inflammation

ROS ( reactive oxygen species ) and atherosclerosis

Aging • The process(es) that occur during life which culminate in changes that decrease an individual’s ability to handle biological challenges

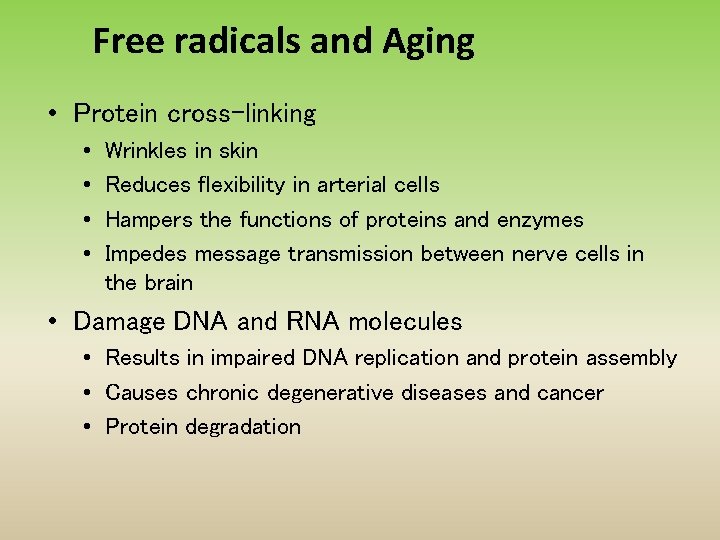
Free radicals and Aging • Protein cross-linking • • Wrinkles in skin Reduces flexibility in arterial cells Hampers the functions of proteins and enzymes Impedes message transmission between nerve cells in the brain • Damage DNA and RNA molecules • Results in impaired DNA replication and protein assembly • Causes chronic degenerative diseases and cancer • Protein degradation

Antioxidative defense An antioxidant is any substance that delays, prevents or removes oxidative damage to a target molecule There is no universal best antioxidant! Their relative importance depends upon: Which, how, where ROS is generated and what target of damage is measured

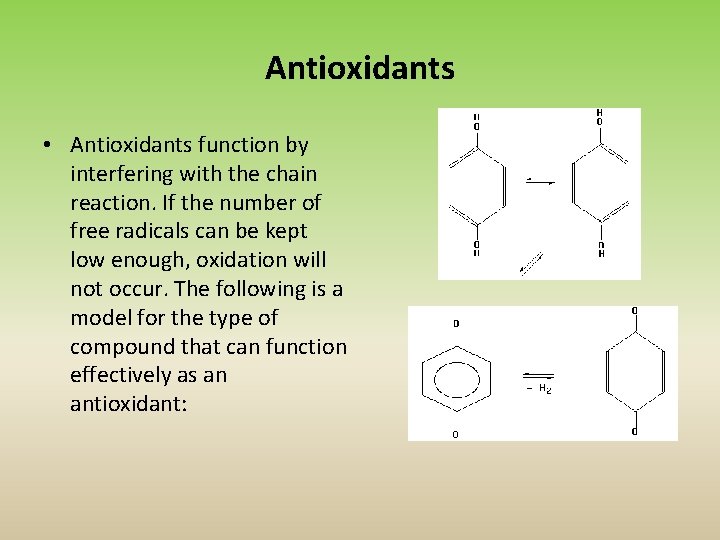
Antioxidants • Antioxidants function by interfering with the chain reaction. If the number of free radicals can be kept low enough, oxidation will not occur. The following is a model for the type of compound that can function effectively as an antioxidant:

Main mechanisms for inhibition of oxidative reactions 1. Interrupt the free-radical chain mechanism 2. Function as being preferentially oxidized - poor protection 3. Reducing agents 4. Chelating agents for free iron

Ideal Antioxidants • No harmful physiological effects • Not contribute an objectionable flavor, odor, or color to the product • Effective in low concentration • Fat soluble • Carry-through effect – no destruction during processing • Readily available • Economical • Non-absorbable by the body

Antioxidant Defenses in Biological Systems • Fat-soluble cellular membrane consists – Vitamin E – beta-carotene – Coenzyme Q (10) • Water soluble antioxidant scavengers – Vitamin C – Glutathione peroxidase, – Superoxide dismutase – Catalase

Thank you for attention!
 Ninds-airen criteria
Ninds-airen criteria Alkaligenes viscolactis in milk causes
Alkaligenes viscolactis in milk causes Wear vs deterioration in software engineering
Wear vs deterioration in software engineering What are the causes of food spoilage
What are the causes of food spoilage Bilirubin bruise
Bilirubin bruise Deamination of glutamine
Deamination of glutamine Fructose breakdown pathway
Fructose breakdown pathway Uncouple oxidative phosphorylation
Uncouple oxidative phosphorylation Uncouplers of oxidative phosphorylation
Uncouplers of oxidative phosphorylation Pentose phosphate pathway non oxidative phase
Pentose phosphate pathway non oxidative phase Anionation
Anionation Chart
Chart Uncouplers of oxidative phosphorylation
Uncouplers of oxidative phosphorylation Plp mechanism transamination
Plp mechanism transamination Corrosion oxidation or reduction
Corrosion oxidation or reduction Catabolism of amino acids
Catabolism of amino acids Histidine catabolism
Histidine catabolism Substrate level phosphorylation vs oxidative
Substrate level phosphorylation vs oxidative Uncouplers of oxidative phosphorylation
Uncouplers of oxidative phosphorylation Concept map of oxidative phosphorylation
Concept map of oxidative phosphorylation The stages of cellular respiration
The stages of cellular respiration Oxidative phosphorylation energy yield
Oxidative phosphorylation energy yield Non-oxidative phase of pentose phosphate pathway
Non-oxidative phase of pentose phosphate pathway Oxidative phosphorylation
Oxidative phosphorylation Azide electron transport chain
Azide electron transport chain Oxidative desizing
Oxidative desizing Deamination
Deamination Transamination and oxidative deamination
Transamination and oxidative deamination Inhibitor of oxidative phosphorylation
Inhibitor of oxidative phosphorylation