Lipid metabolism Digestion and absorption of Lipids Digestion

Lipid metabolism Digestion and absorption of Lipids
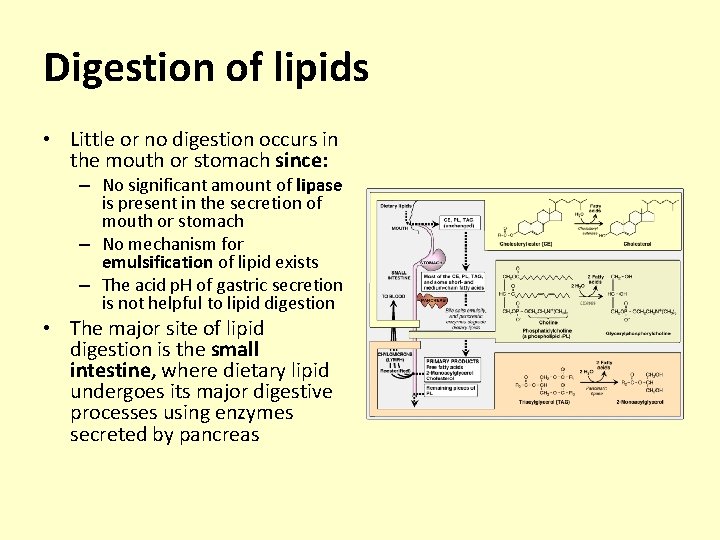
Digestion of lipids • Little or no digestion occurs in the mouth or stomach since: – No significant amount of lipase is present in the secretion of mouth or stomach – No mechanism for emulsification of lipid exists – The acid p. H of gastric secretion is not helpful to lipid digestion • The major site of lipid digestion is the small intestine, where dietary lipid undergoes its major digestive processes using enzymes secreted by pancreas
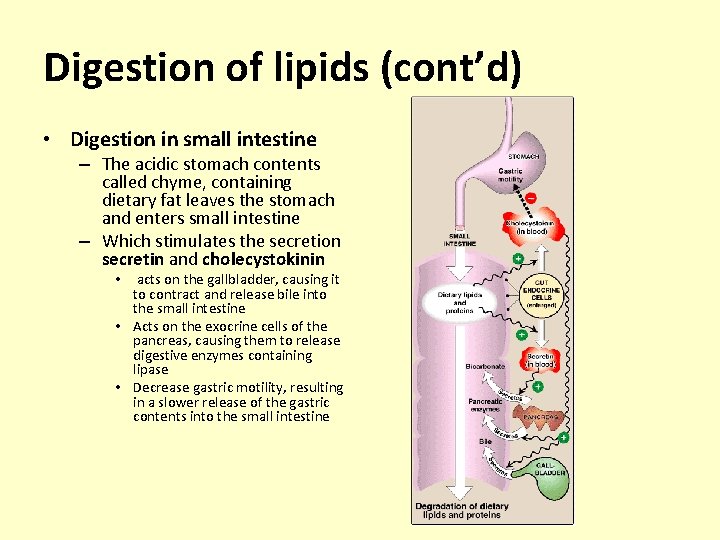
Digestion of lipids (cont’d) • Digestion in small intestine – The acidic stomach contents called chyme, containing dietary fat leaves the stomach and enters small intestine – Which stimulates the secretion secretin and cholecystokinin acts on the gallbladder, causing it to contract and release bile into the small intestine • Acts on the exocrine cells of the pancreas, causing them to release digestive enzymes containing lipase • Decrease gastric motility, resulting in a slower release of the gastric contents into the small intestine •
Digestion of lipids (cont’d) – Secretin cause the pancreas to release a solution enriched in bicarbonate that helps neutralize the p. H of the acidic chyme and changes the p. H to the alkaline side, which is necessary for the activity of pancreatic and intestinal enzymes – Pancreatic juice and bile enter the upper small intestine, the duodenum, by way of the pancreatic and bile ducts
Digestion of lipids (cont’d) – The major function of bile is to provide the emulsifying agents: • The bile salts • Phosphotidylcholine – Both these are powerful emulsifying agents. They emulsify the triacylglycerols into small droplets – Three lipid digestive enzymes secreted by pancreas are: • Pancreatic lipase • Cholesterol esterase • Phospholipase-A 2
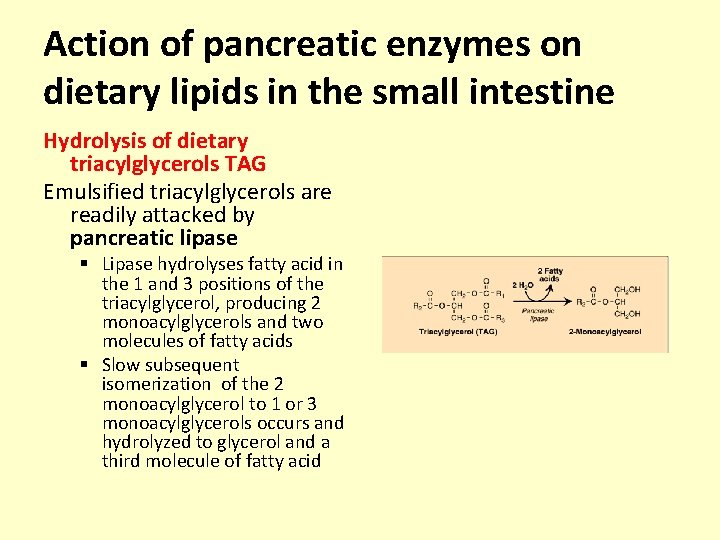
Action of pancreatic enzymes on dietary lipids in the small intestine Hydrolysis of dietary triacylglycerols TAG Emulsified triacylglycerols are readily attacked by pancreatic lipase § Lipase hydrolyses fatty acid in the 1 and 3 positions of the triacylglycerol, producing 2 monoacylglycerols and two molecules of fatty acids § Slow subsequent isomerization of the 2 monoacylglycerol to 1 or 3 monoacylglycerols occurs and hydrolyzed to glycerol and a third molecule of fatty acid
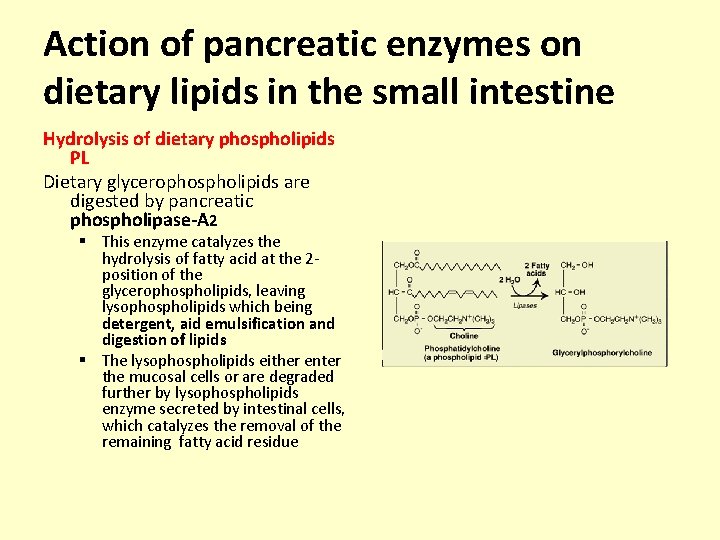
Action of pancreatic enzymes on dietary lipids in the small intestine Hydrolysis of dietary phospholipids PL Dietary glycerophospholipids are digested by pancreatic phospholipase-A 2 § This enzyme catalyzes the hydrolysis of fatty acid at the 2 position of the glycerophospholipids, leaving lysophospholipids which being detergent, aid emulsification and digestion of lipids § The lysophospholipids either enter the mucosal cells or are degraded further by lysophospholipids enzyme secreted by intestinal cells, which catalyzes the removal of the remaining fatty acid residue
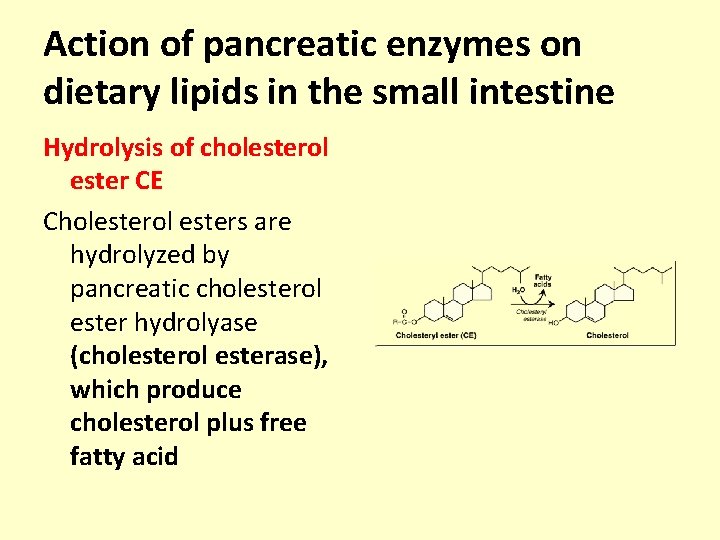
Action of pancreatic enzymes on dietary lipids in the small intestine Hydrolysis of cholesterol ester CE Cholesterol esters are hydrolyzed by pancreatic cholesterol ester hydrolyase (cholesterol esterase), which produce cholesterol plus free fatty acid
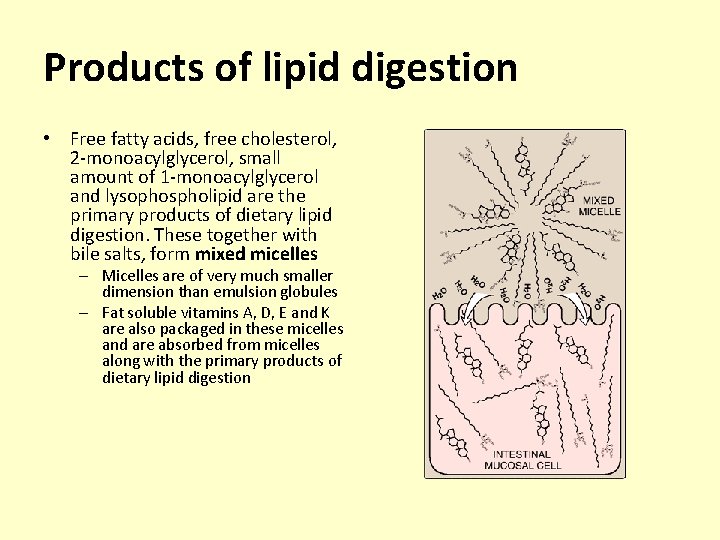
Products of lipid digestion • Free fatty acids, free cholesterol, 2 -monoacylglycerol, small amount of 1 -monoacylglycerol and lysophospholipid are the primary products of dietary lipid digestion. These together with bile salts, form mixed micelles – Micelles are of very much smaller dimension than emulsion globules – Fat soluble vitamins A, D, E and K are also packaged in these micelles and are absorbed from micelles along with the primary products of dietary lipid digestion
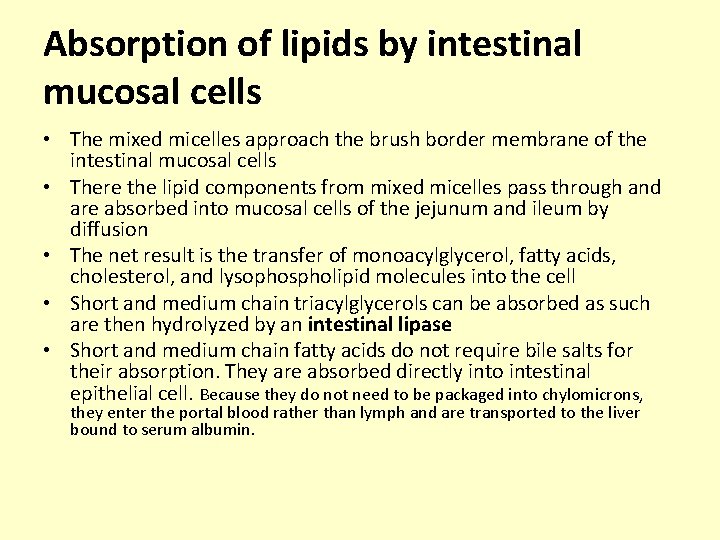
Absorption of lipids by intestinal mucosal cells • The mixed micelles approach the brush border membrane of the intestinal mucosal cells • There the lipid components from mixed micelles pass through and are absorbed into mucosal cells of the jejunum and ileum by diffusion • The net result is the transfer of monoacylglycerol, fatty acids, cholesterol, and lysophospholipid molecules into the cell • Short and medium chain triacylglycerols can be absorbed as such are then hydrolyzed by an intestinal lipase • Short and medium chain fatty acids do not require bile salts for their absorption. They are absorbed directly into intestinal epithelial cell. Because they do not need to be packaged into chylomicrons, they enter the portal blood rather than lymph and are transported to the liver bound to serum albumin.
Absorption of lipids by intestinal mucosal cells (cont’d)
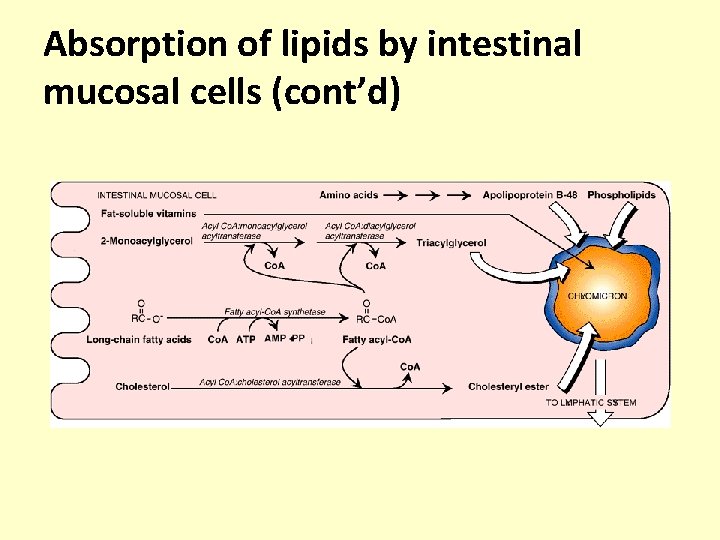
Absorption of lipids by intestinal mucosal cells (cont’d) • After absorption within the intestinal wall, the following events occur – 1 -monoacylglycerols are further hydrolyzed to produce free glycerol and fatty acids by an intestinal lipase (glycerol ester hydrolase) – 2 -monoacylglycerols are reconverted to triacyglycerols the fatty acids required for this synthesis can arise from three sources; • Absorbed from the lumen • Produced by hydrolysis of absorbed monoacylglycerols • Synthesized in the mucosal cells

Absorption of lipids by intestinal mucosal cells (cont’d) • The utilization of fatty acids for resynthesis of, triacyglycerols first requires their conversion to active form acyl-Co. A by the action of acyl-Co. A synthetase (thiokinase) • The absorbed lysophospholipids and cholesterol are also reacylated with acyl-Co. A to regenerate PL and CE • The free glycerol released in the intestinal lumen is not reutilized but passes directly to the portal vein. However, the glycerol-3 -phosphate, formed within the intestinal cells by the glucose, can be reutilized for triacylglycerol synthesis.

Transport • Triacylglycerol, phospholipid, cholesterol esters synthesized in the intestinal mucosa and absorbed fat soluble vitamins are transported from the mucosal cells into the lymph in the form of lipoprotein known as chylomicrons because they are insoluble in water
Transport (Cont’d) • Chylomicrons are composed of: – – Triacylglycerols (85 to 90%) Cholesterol and cholesterol ester (5%) Phospholipids (7%) Protein (apolipoprotein B (2%) • Although the amounts of protein and phospholipid in chylomicrons are small, they are essential for the transport of fat from the mucosal cells and if their synthesis • The chylomicrons pass from lymph into the blood through the thoratic duct. After a fatty meal, the plasma is milky in appearance due to the presence of these particles
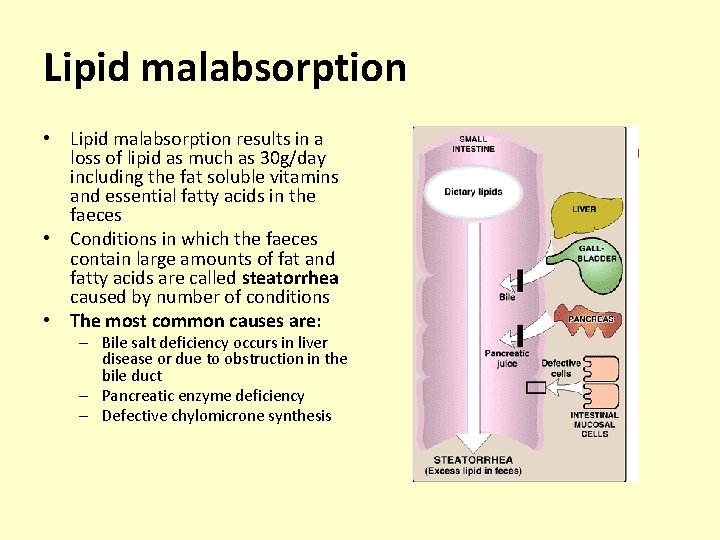
Lipid malabsorption • Lipid malabsorption results in a loss of lipid as much as 30 g/day including the fat soluble vitamins and essential fatty acids in the faeces • Conditions in which the faeces contain large amounts of fat and fatty acids are called steatorrhea caused by number of conditions • The most common causes are: – Bile salt deficiency occurs in liver disease or due to obstruction in the bile duct – Pancreatic enzyme deficiency – Defective chylomicrone synthesis

Lipid metabolism
Lipid metabolism • Depending on cell demands – Fatty acids are synthesized and stored in fat cells – Fatty acids are catabolized to liberate energy • The synthesis of FA begins with acetyl-Co. A • Catabolism of FA begins with carboxyl group • The pathways of lipid synthesis and catabolism differ in important ways – Synthesis take place in thee cytosol, while catabolism takes place in the mitochondrion – NADPH is the donor of high energy electrons in lipid synthesis, whereas FAD and NAD are electron acceptors in lipid catabolism – Different activating ligands are used (coenzyme A is used in catabolism, while the acyl carrier protein is used in anabolism)

Lipids are Involved in Generation and Storage of Energy • The metabolic oxidation of lipids release large amounts of energy through production of acetyl Co. A, NADH and FADH • lipids represent an efficient way of storing chemical energy
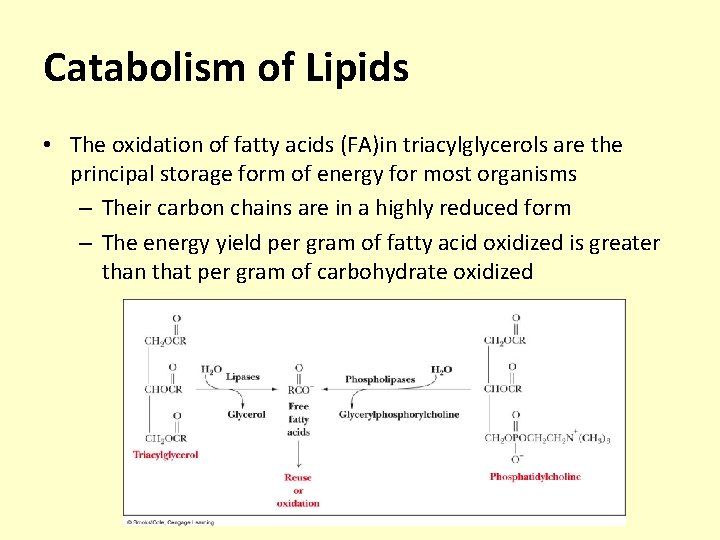
Catabolism of Lipids • The oxidation of fatty acids (FA)in triacylglycerols are the principal storage form of energy for most organisms – Their carbon chains are in a highly reduced form – The energy yield per gram of fatty acid oxidized is greater than that per gram of carbohydrate oxidized
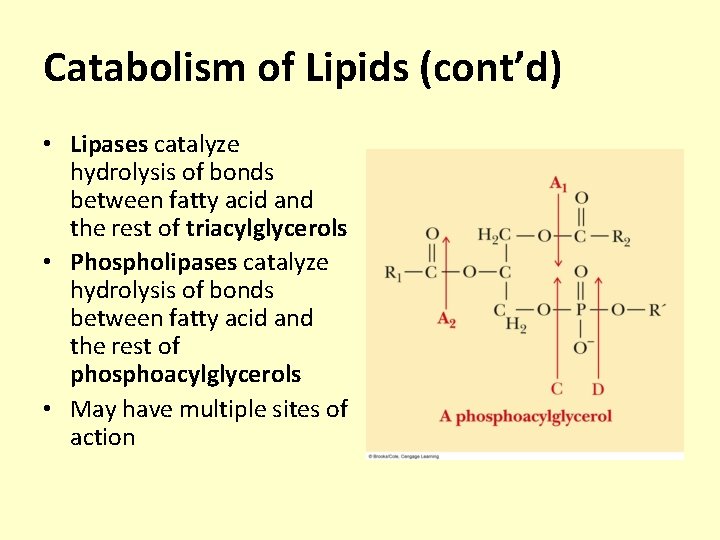
Catabolism of Lipids (cont’d) • Lipases catalyze hydrolysis of bonds between fatty acid and the rest of triacylglycerols • Phospholipases catalyze hydrolysis of bonds between fatty acid and the rest of phosphoacylglycerols • May have multiple sites of action
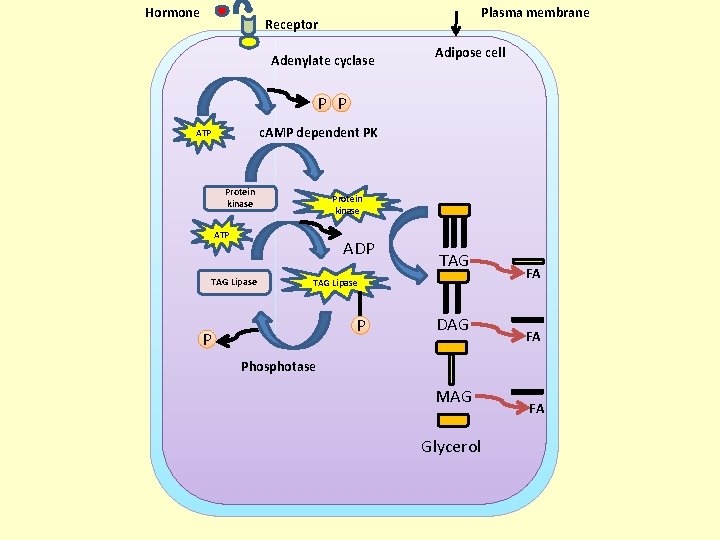
Hormone Plasma membrane Receptor Adenylate cyclase Adipose cell P P c. AMP dependent PK ATP Protein kinase ATP ADP TAG Lipase P P DAG FA FA Phosphotase MAG Glycerol FA
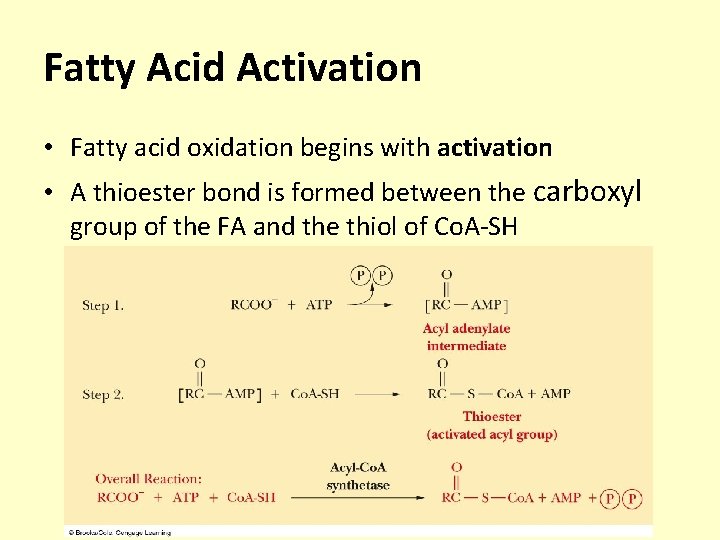
Fatty Acid Activation • Fatty acid oxidation begins with activation • A thioester bond is formed between the carboxyl group of the FA and the thiol of Co. A-SH
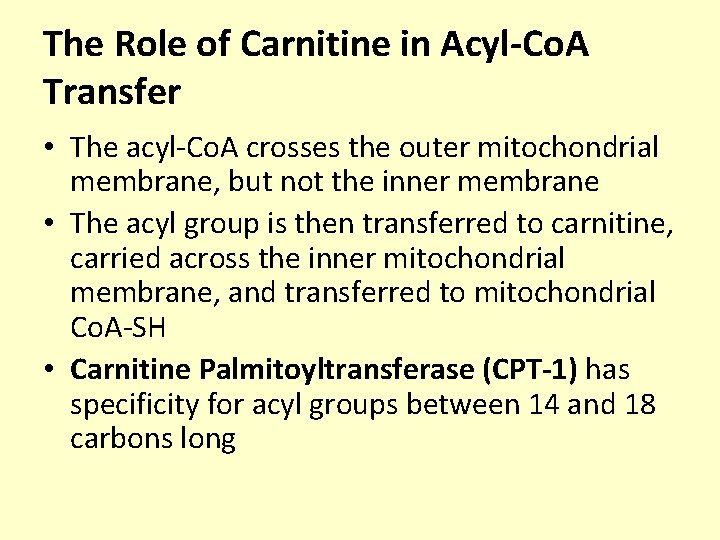
The Role of Carnitine in Acyl-Co. A Transfer • The acyl-Co. A crosses the outer mitochondrial membrane, but not the inner membrane • The acyl group is then transferred to carnitine, carried across the inner mitochondrial membrane, and transferred to mitochondrial Co. A-SH • Carnitine Palmitoyltransferase (CPT-1) has specificity for acyl groups between 14 and 18 carbons long
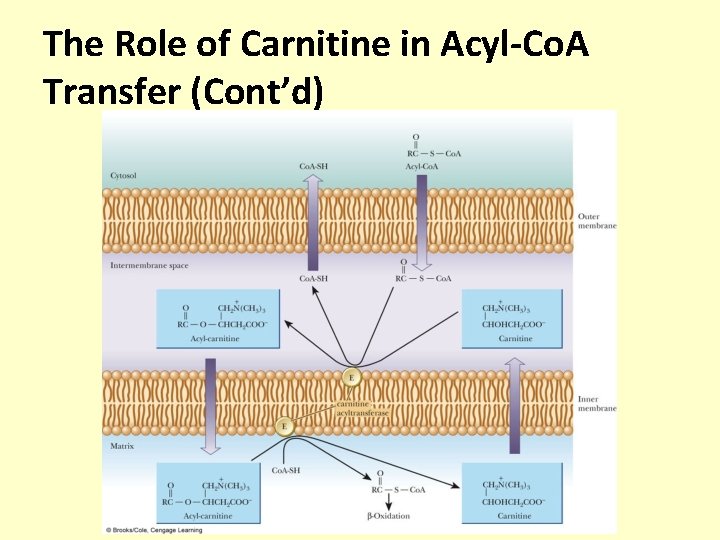
The Role of Carnitine in Acyl-Co. A Transfer (Cont’d)

-Oxidation • -Oxidation: a series of reactions that cleaves carbon atoms two at a time from the carboxyl end of a fatty acid • The complete cycle of one -oxidation requires four enzymes – Reaction 1: Oxidation of the , carbon-carbon single bond to a carbon-carbon double bond – Reaction 2: Hydration of the carbon-carbon double bond – Reaction 3: Oxidation of the -hydroxyl group to a carbonyl group – Reaction 4: Cleavage of the carbon chain by a reverse Claisen reaction
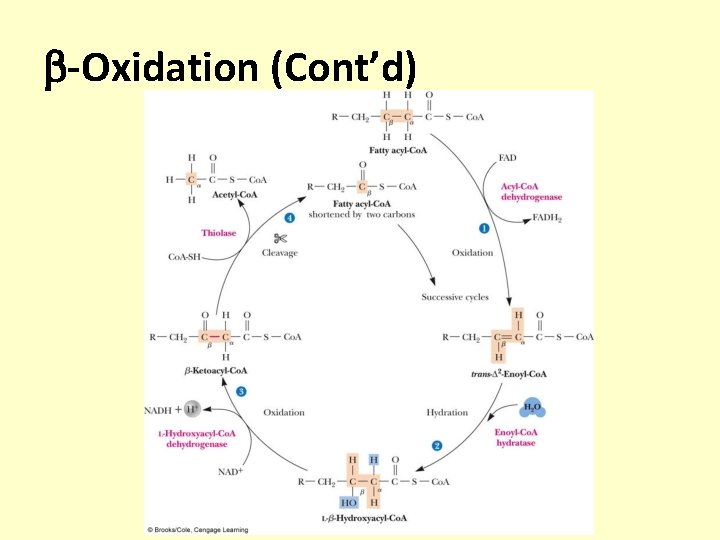
-Oxidation (Cont’d)
Summary • Fatty acids are activated and transported to the mitochondrial matrix for further catabolism • The breakdown of fatty acids takes place in the mitochondrial matrix and proceeds by successive removal of two-carbon units as acetyl-Co. A • Each Cleavage of a two-carbon moiety requires a four-step reaction sequences called -oxidation
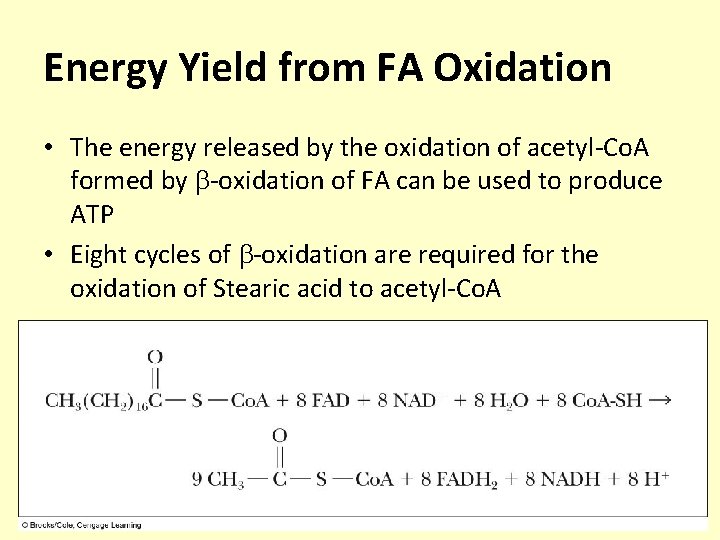
Energy Yield from FA Oxidation • The energy released by the oxidation of acetyl-Co. A formed by -oxidation of FA can be used to produce ATP • Eight cycles of -oxidation are required for the oxidation of Stearic acid to acetyl-Co. A
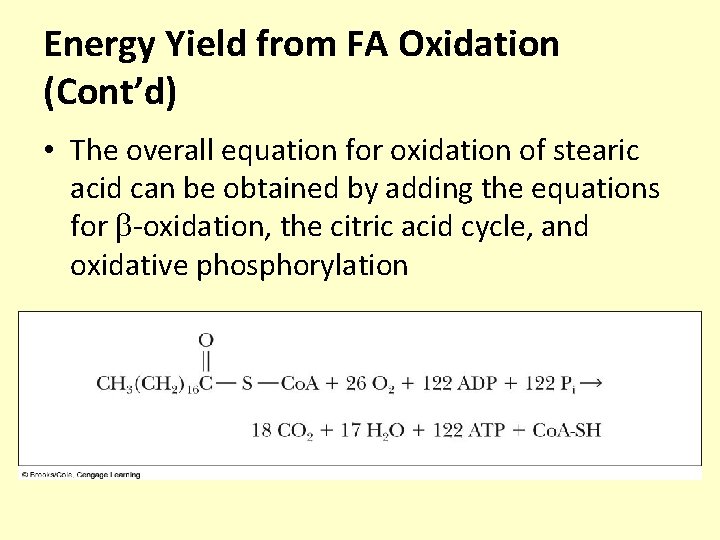
Energy Yield from FA Oxidation (Cont’d) • The overall equation for oxidation of stearic acid can be obtained by adding the equations for -oxidation, the citric acid cycle, and oxidative phosphorylation
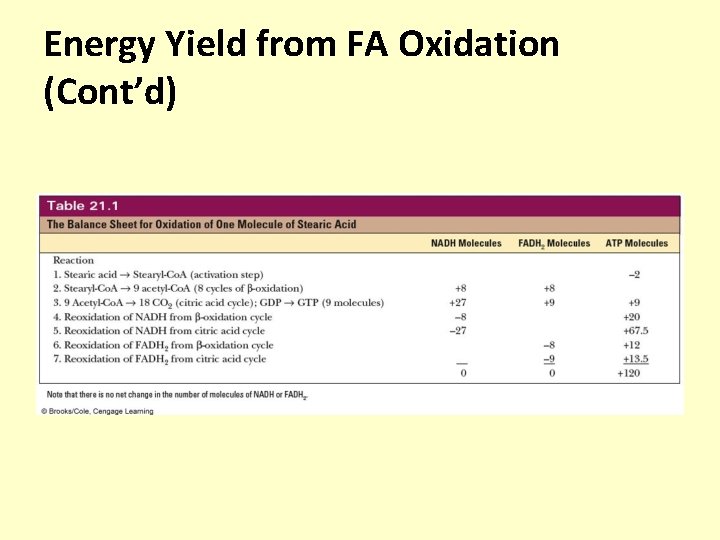
Energy Yield from FA Oxidation (Cont’d)
Summary • The complete oxidation of FA by the citric acid cycle and the electron transport chain releases large amounts of energy • When we include the reoxidation of NADH and FADH 2 from -oxidation and the citric acid cycle, we obtain a net yield of 120 ATP for a single molecule of stearic acid
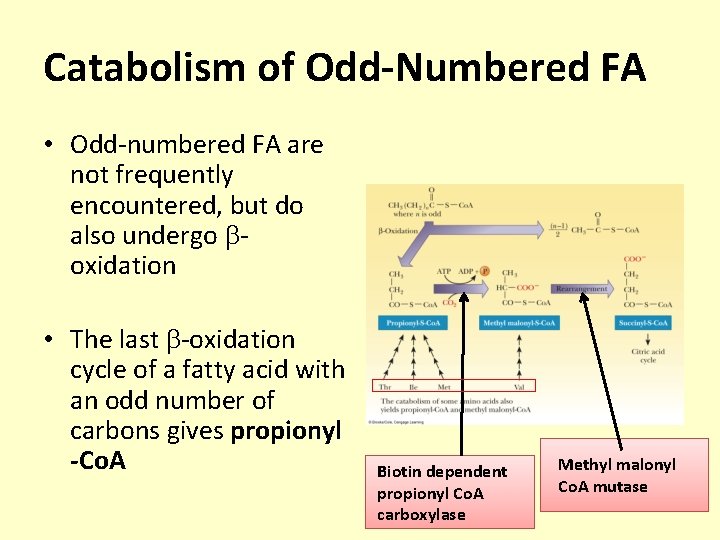
Catabolism of Odd-Numbered FA • Odd-numbered FA are not frequently encountered, but do also undergo oxidation • The last -oxidation cycle of a fatty acid with an odd number of carbons gives propionyl -Co. A Biotin dependent propionyl Co. A carboxylase Methyl malonyl Co. A mutase
Oxidation of an Unsaturated FA • A cis-trans isomerization is needed to convert unsaturated FA to acetyl-Co. A • This enzyme is known as an isomerase • Oxidation of unsaturated FA does not generate as much ATP relative to saturated FA with the same # of carbons
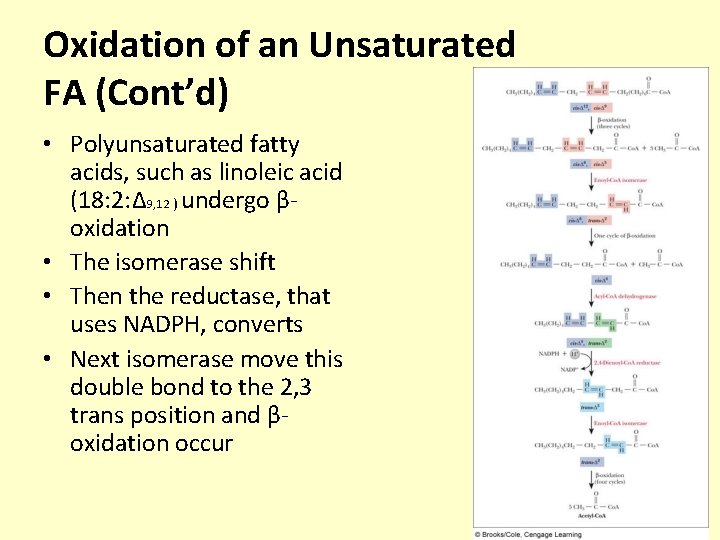
Oxidation of an Unsaturated FA (Cont’d) • Polyunsaturated fatty acids, such as linoleic acid (18: 2: ∆9, 12 ) undergo βoxidation • The isomerase shift • Then the reductase, that uses NADPH, converts • Next isomerase move this double bond to the 2, 3 trans position and βoxidation occur
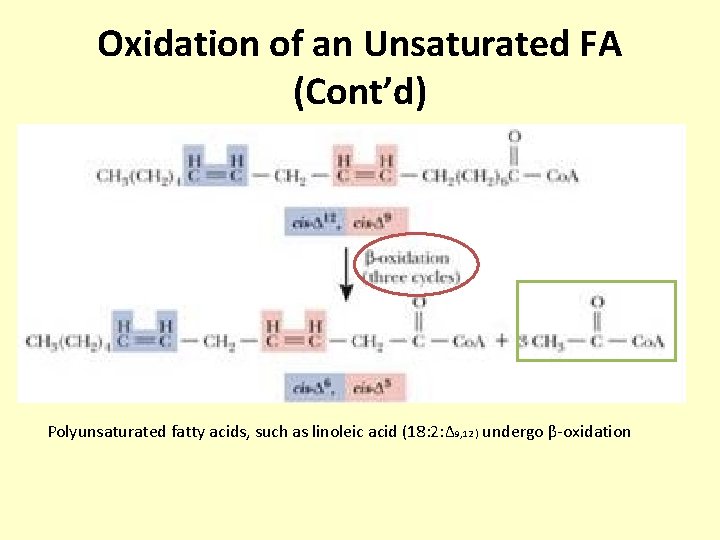
Oxidation of an Unsaturated FA (Cont’d) Polyunsaturated fatty acids, such as linoleic acid (18: 2: ∆9, 12 ) undergo β-oxidation
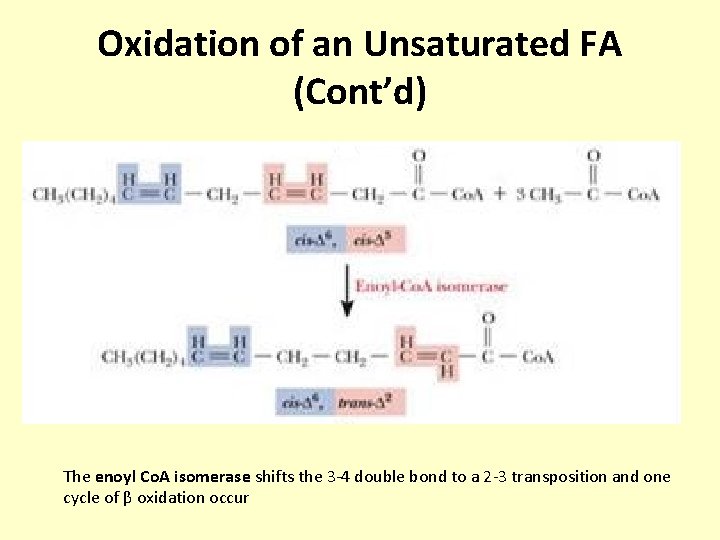
Oxidation of an Unsaturated FA (Cont’d) The enoyl Co. A isomerase shifts the 3 -4 double bond to a 2 -3 transposition and one cycle of β oxidation occur
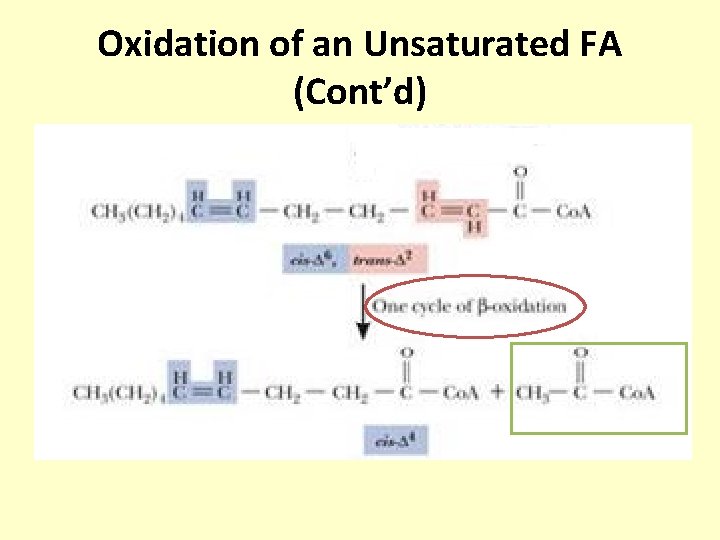
Oxidation of an Unsaturated FA (Cont’d)
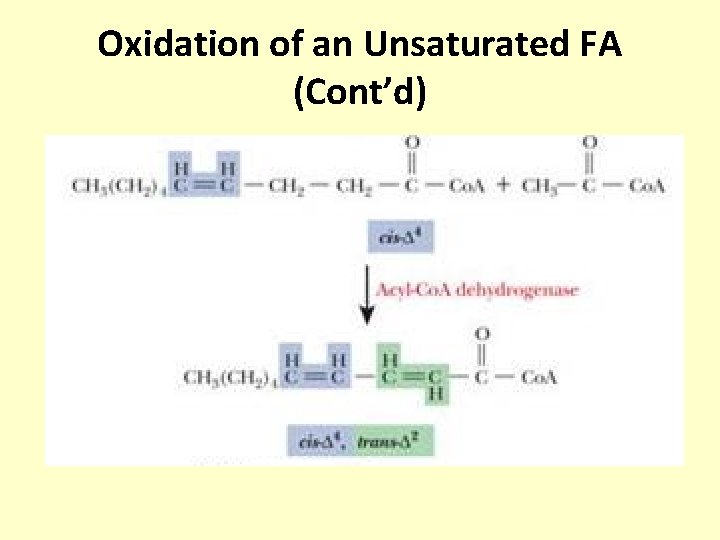
Oxidation of an Unsaturated FA (Cont’d)
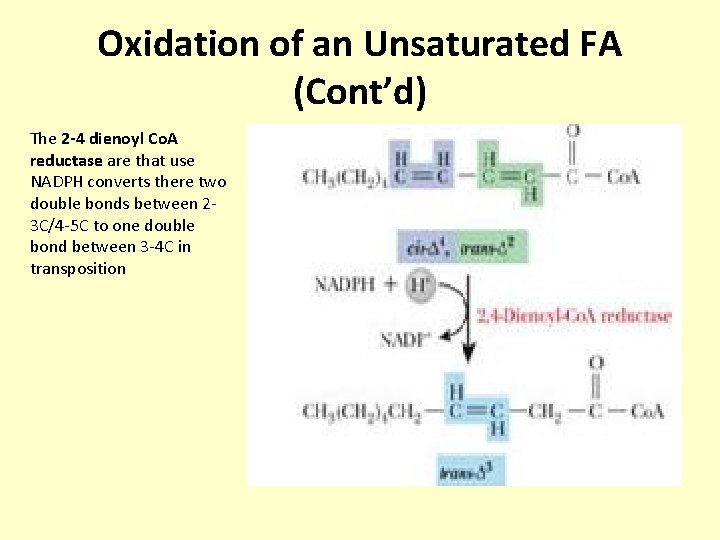
Oxidation of an Unsaturated FA (Cont’d) The 2 -4 dienoyl Co. A reductase are that use NADPH converts there two double bonds between 23 C/4 -5 C to one double bond between 3 -4 C in transposition
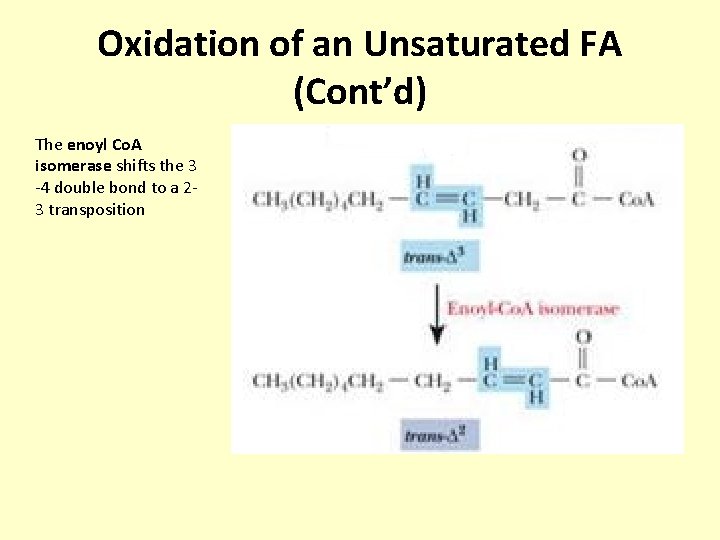
Oxidation of an Unsaturated FA (Cont’d) The enoyl Co. A isomerase shifts the 3 -4 double bond to a 23 transposition
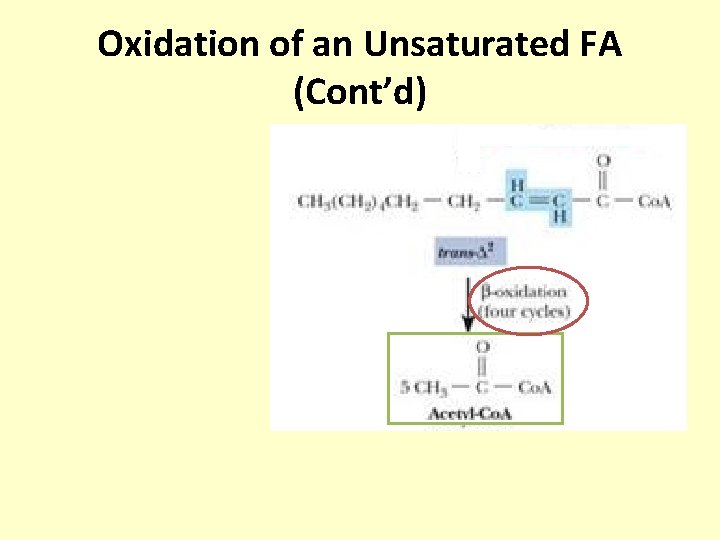
Oxidation of an Unsaturated FA (Cont’d)
Summary • FA with odd number of carbons produce propionyl-Co. A in the last step of the oxidation • Propionyl-Co. A can be converted to succinyl-Co. A, which plays a role in the citric acid cycle • The oxidation of unsaturated FA requires enzymes that catalyze isomerization around the double bonds so that oxidation can proceed

Ketone Bodies • Formation of ketone bodies occurs when the amount of acetyl-Co. A produced is excessive compared to the amount of oxaloacetate available to react with it – Intake high in lipids and low in carbohydrates – Diabetes not suitably controlled – Starvation • Ketone bodies are: are acetone, -hydroxybutyrate, and acetoacetate – Formed principally in liver mitochondria – Can be used as a fuel in most tissues and organs
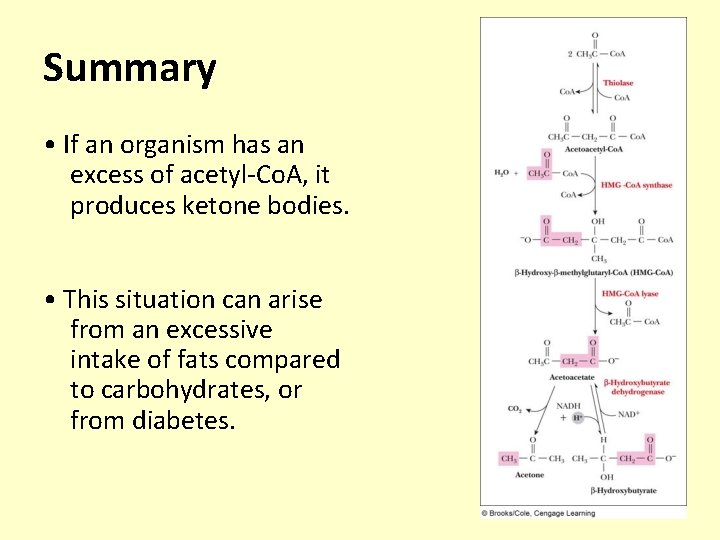
Summary • If an organism has an excess of acetyl-Co. A, it produces ketone bodies. • This situation can arise from an excessive intake of fats compared to carbohydrates, or from diabetes.
Fatty acid Biosynthesis • Biosynthesis is not exact reversal of β-oxidation • Biosynthetic reactions occur in the cytosol
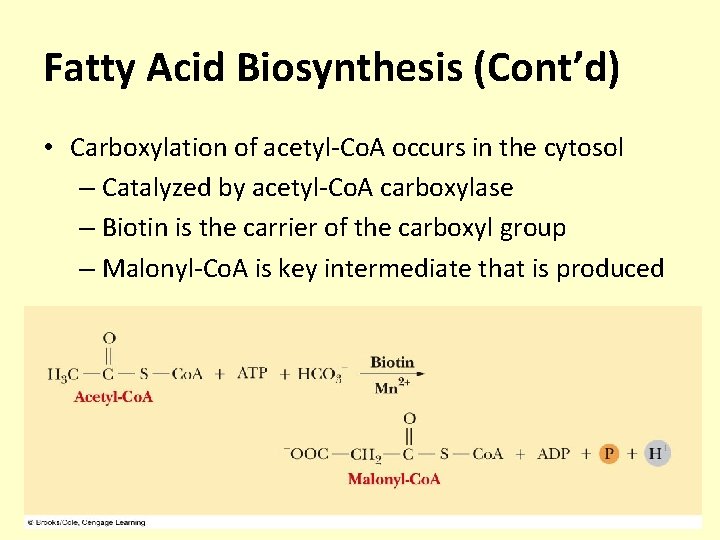
Fatty Acid Biosynthesis (Cont’d) • Carboxylation of acetyl-Co. A occurs in the cytosol – Catalyzed by acetyl-Co. A carboxylase – Biotin is the carrier of the carboxyl group – Malonyl-Co. A is key intermediate that is produced
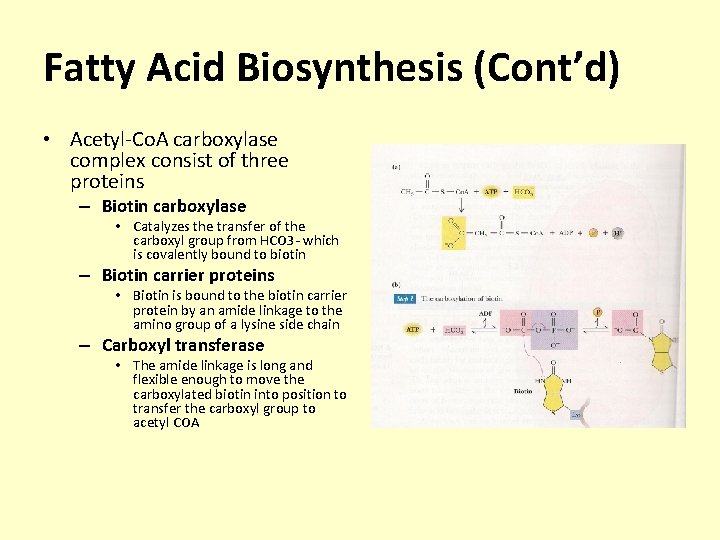
Fatty Acid Biosynthesis (Cont’d) • Acetyl-Co. A carboxylase complex consist of three proteins – Biotin carboxylase • Catalyzes the transfer of the carboxyl group from HCO 3 - which is covalently bound to biotin – Biotin carrier proteins • Biotin is bound to the biotin carrier protein by an amide linkage to the amino group of a lysine side chain – Carboxyl transferase • The amide linkage is long and flexible enough to move the carboxylated biotin into position to transfer the carboxyl group to acetyl COA
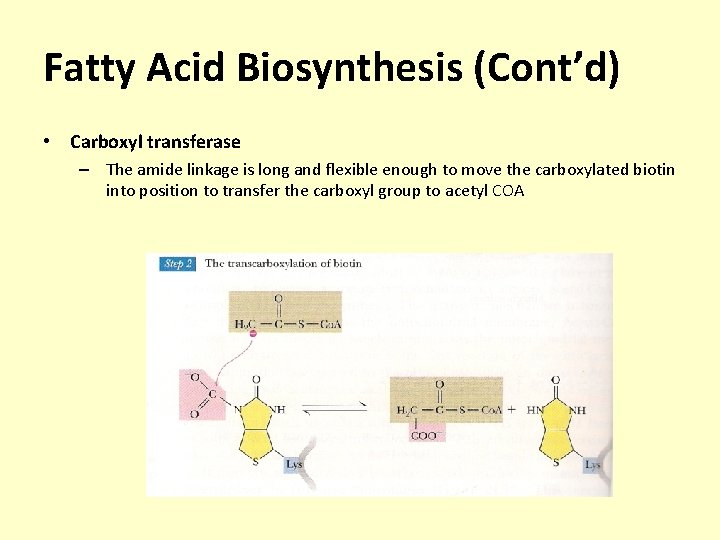
Fatty Acid Biosynthesis (Cont’d) • Carboxyl transferase – The amide linkage is long and flexible enough to move the carboxylated biotin into position to transfer the carboxyl group to acetyl COA
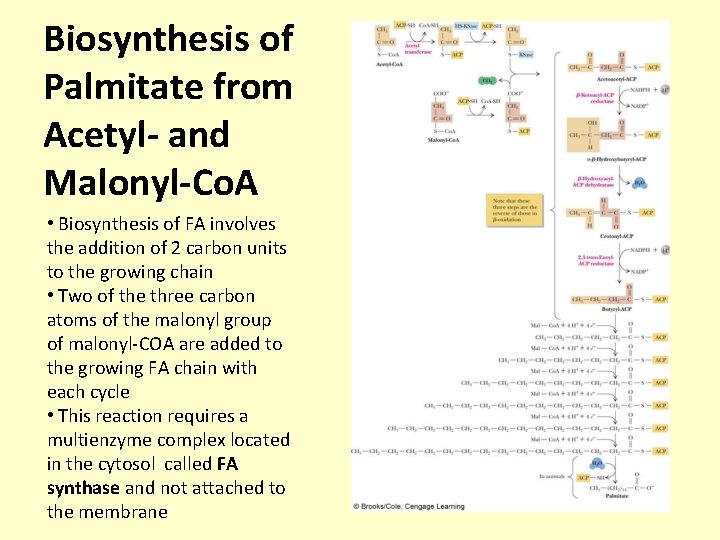
Biosynthesis of Palmitate from Acetyl- and Malonyl-Co. A • Biosynthesis of FA involves the addition of 2 carbon units to the growing chain • Two of the three carbon atoms of the malonyl group of malonyl-COA are added to the growing FA chain with each cycle • This reaction requires a multienzyme complex located in the cytosol called FA synthase and not attached to the membrane
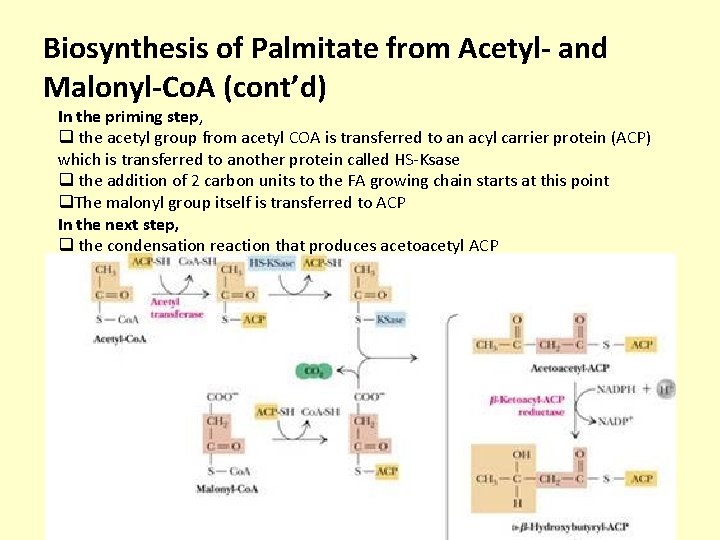
Biosynthesis of Palmitate from Acetyl- and Malonyl-Co. A (cont’d) In the priming step, q the acetyl group from acetyl COA is transferred to an acyl carrier protein (ACP) which is transferred to another protein called HS-Ksase q the addition of 2 carbon units to the FA growing chain starts at this point q. The malonyl group itself is transferred to ACP In the next step, q the condensation reaction that produces acetoacetyl ACP
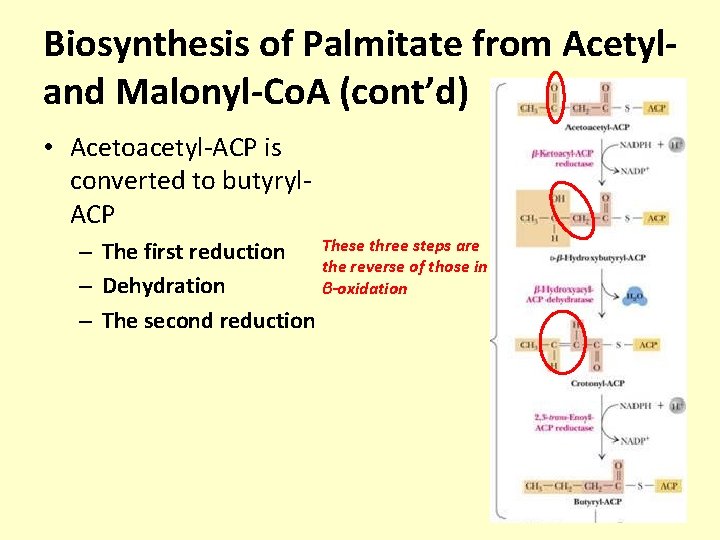
Biosynthesis of Palmitate from Acetyland Malonyl-Co. A (cont’d) • Acetoacetyl-ACP is converted to butyryl. ACP – The first reduction These three steps are the reverse of those in – Dehydration β-oxidation – The second reduction
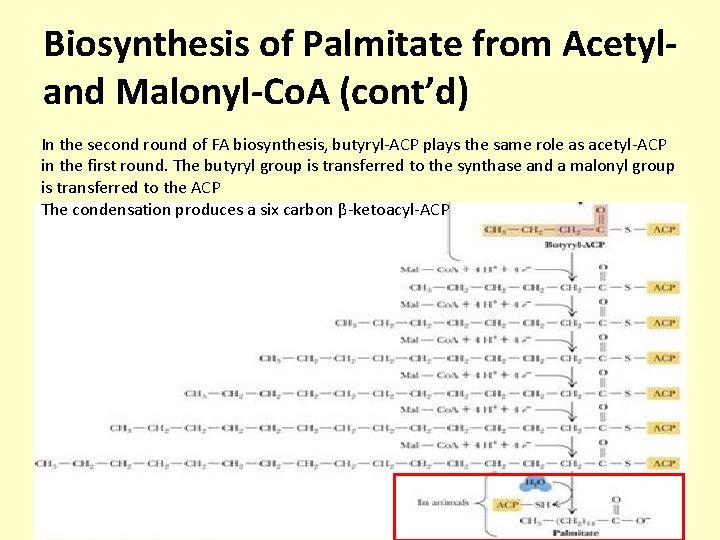
Biosynthesis of Palmitate from Acetyland Malonyl-Co. A (cont’d) In the second round of FA biosynthesis, butyryl-ACP plays the same role as acetyl-ACP in the first round. The butyryl group is transferred to the synthase and a malonyl group is transferred to the ACP The condensation produces a six carbon β-ketoacyl-ACP
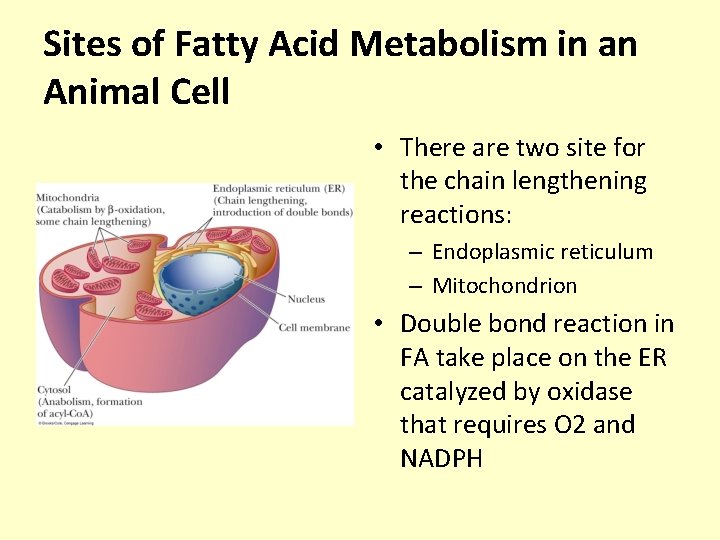
Sites of Fatty Acid Metabolism in an Animal Cell • There are two site for the chain lengthening reactions: – Endoplasmic reticulum – Mitochondrion • Double bond reaction in FA take place on the ER catalyzed by oxidase that requires O 2 and NADPH
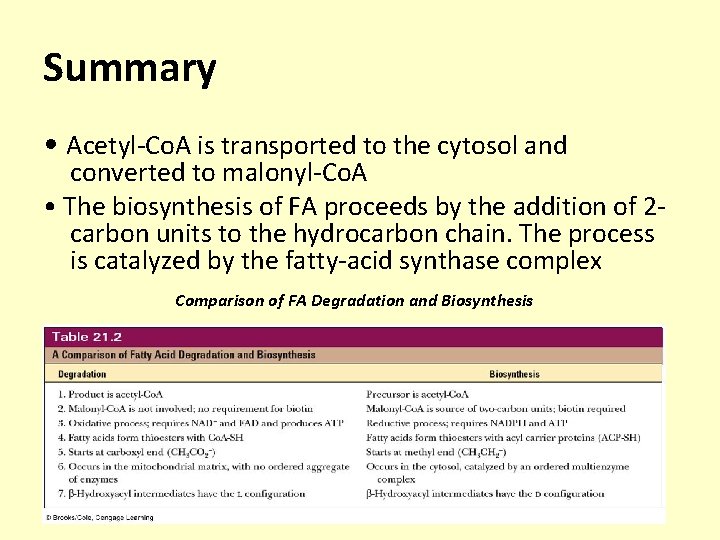
Summary • Acetyl-Co. A is transported to the cytosol and converted to malonyl-Co. A • The biosynthesis of FA proceeds by the addition of 2 carbon units to the hydrocarbon chain. The process is catalyzed by the fatty-acid synthase complex Comparison of FA Degradation and Biosynthesis
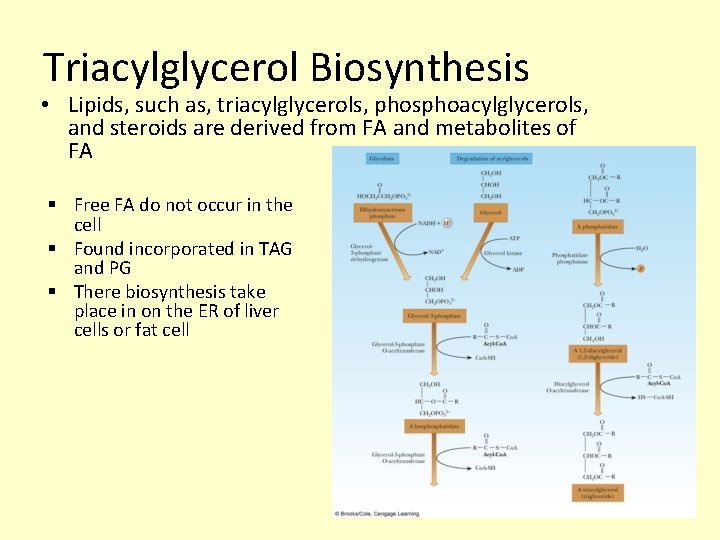
Triacylglycerol Biosynthesis • Lipids, such as, triacylglycerols, phosphoacylglycerols, and steroids are derived from FA and metabolites of FA § Free FA do not occur in the cell § Found incorporated in TAG and PG § There biosynthesis take place in on the ER of liver cells or fat cell
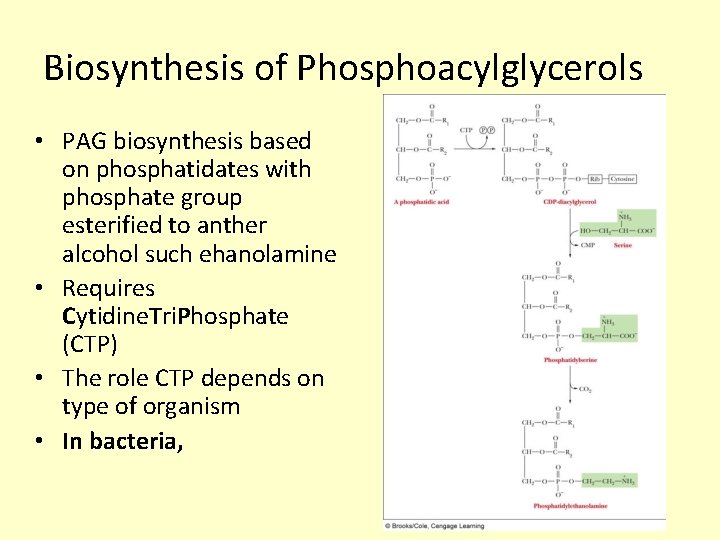
Biosynthesis of Phosphoacylglycerols • PAG biosynthesis based on phosphatidates with phosphate group esterified to anther alcohol such ehanolamine • Requires Cytidine. Tri. Phosphate (CTP) • The role CTP depends on type of organism • In bacteria,
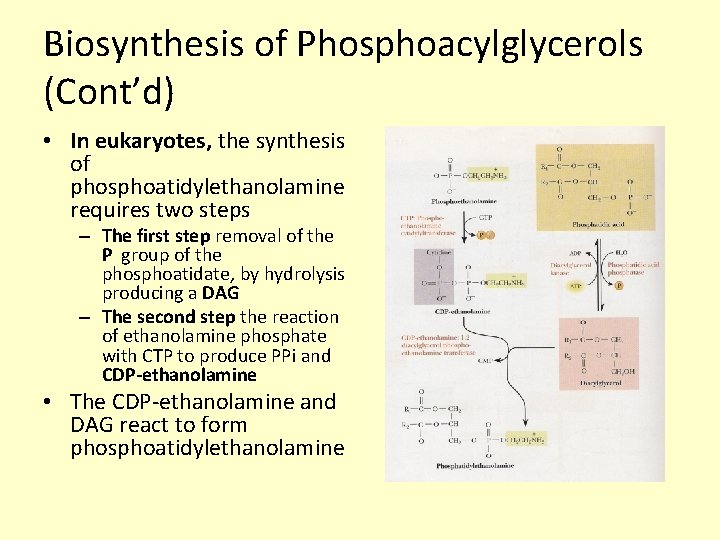
Biosynthesis of Phosphoacylglycerols (Cont’d) • In eukaryotes, the synthesis of phosphoatidylethanolamine requires two steps – The first step removal of the P group of the phosphoatidate, by hydrolysis producing a DAG – The second step the reaction of ethanolamine phosphate with CTP to produce PPi and CDP-ethanolamine • The CDP-ethanolamine and DAG react to form phosphoatidylethanolamine
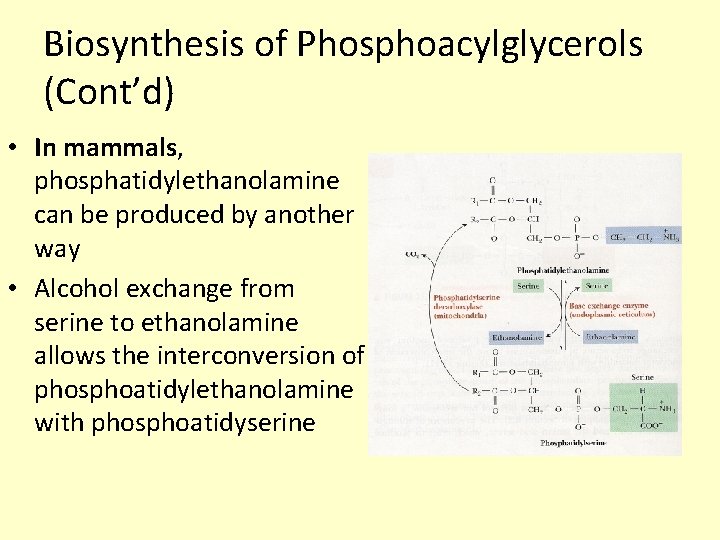
Biosynthesis of Phosphoacylglycerols (Cont’d) • In mammals, phosphatidylethanolamine can be produced by another way • Alcohol exchange from serine to ethanolamine allows the interconversion of phosphoatidylethanolamine with phosphoatidyserine
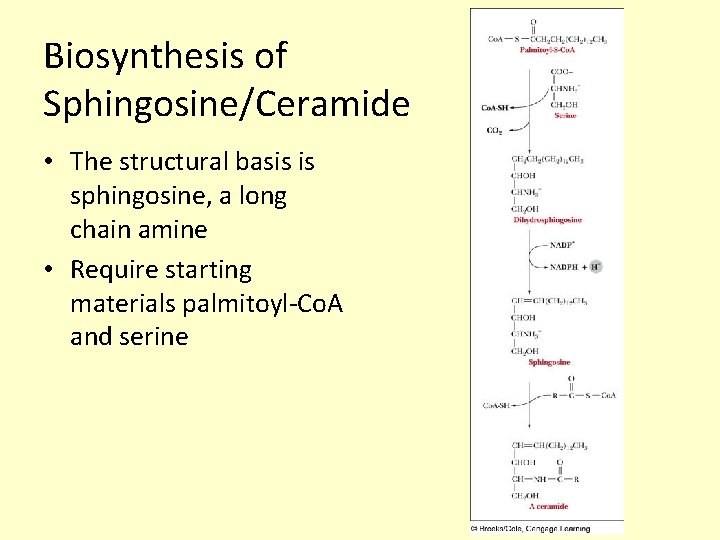
Biosynthesis of Sphingosine/Ceramide • The structural basis is sphingosine, a long chain amine • Require starting materials palmitoyl-Co. A and serine
Cholesterol Biosynthesis • All carbon atoms of cholesterol and steroids synthesized from it are derived from the twocarbon acetyl group of acetyl-Co. A • Involves many reaction steps • Involvement of isoprene units are key to the biosynthesis of steroids and other biomolecules known as terpenes
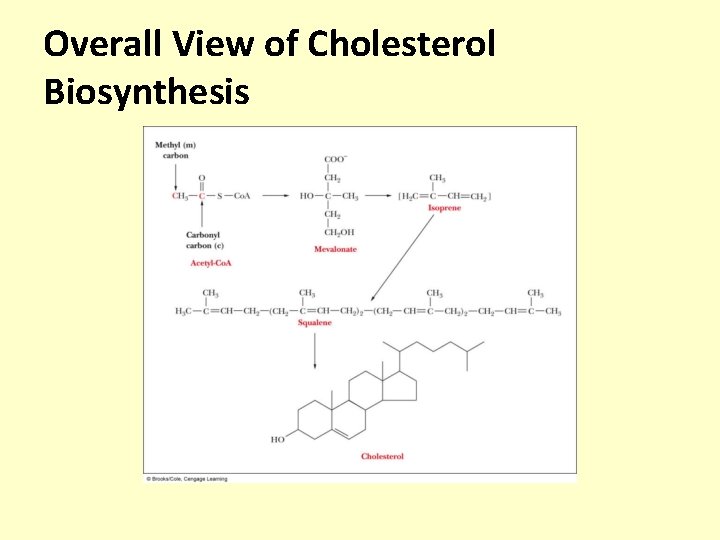
Overall View of Cholesterol Biosynthesis
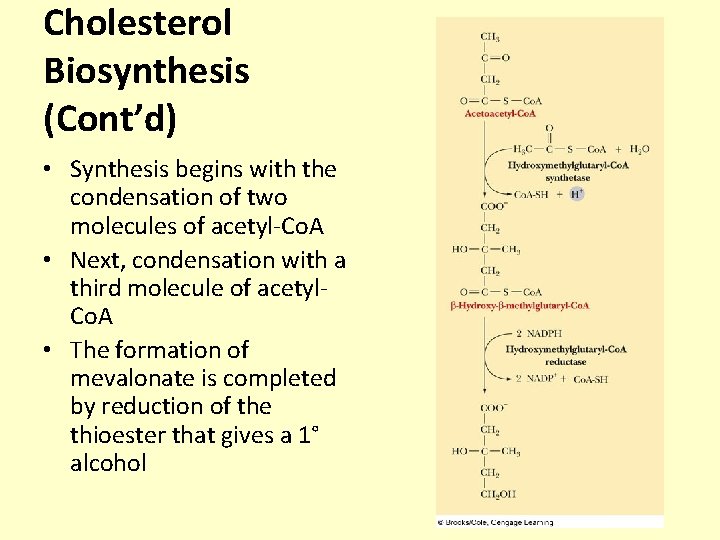
Cholesterol Biosynthesis (Cont’d) • Synthesis begins with the condensation of two molecules of acetyl-Co. A • Next, condensation with a third molecule of acetyl. Co. A • The formation of mevalonate is completed by reduction of the thioester that gives a 1° alcohol

Mevalonate to squalene • The pyrophosphorylation of the 1° alcohol of mevalonate (two moles of ATP) is followed by phosphorylation of the 3° alcohol (one mole of ATP), then the concerted decarboxylation and -elimination of phosphate ion gives isopentenyl pyrophosphate • Then there is an enzyme-catalyzed isomerization of the carbon-carbon double bond that gives dimethylallyl pyrophosphate • Isopentyl pyrophosphate is then converted to dimethylallyl pyrophosphate, which is followed by H+ loss to give farnesyl pyrophosphate • The joining together of two units of farnesyl pyrophosphate (C 15) units by a two-electron oxidation gives squalene (C 30)
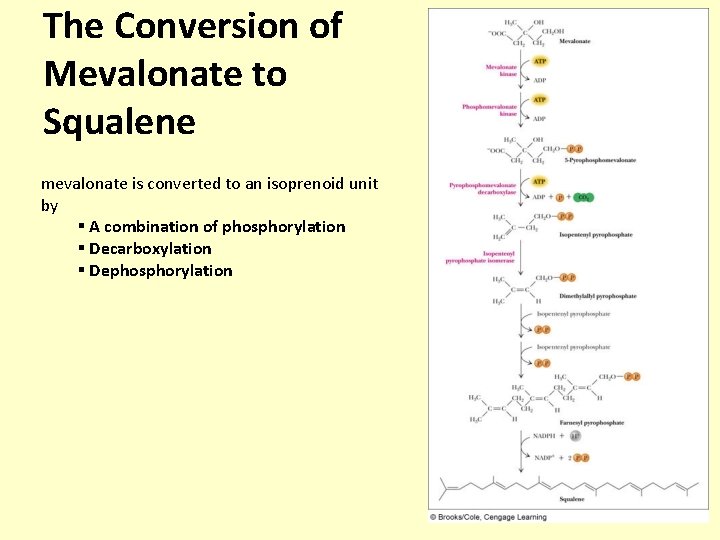
The Conversion of Mevalonate to Squalene mevalonate is converted to an isoprenoid unit by § A combination of phosphorylation § Decarboxylation § Dephosphorylation
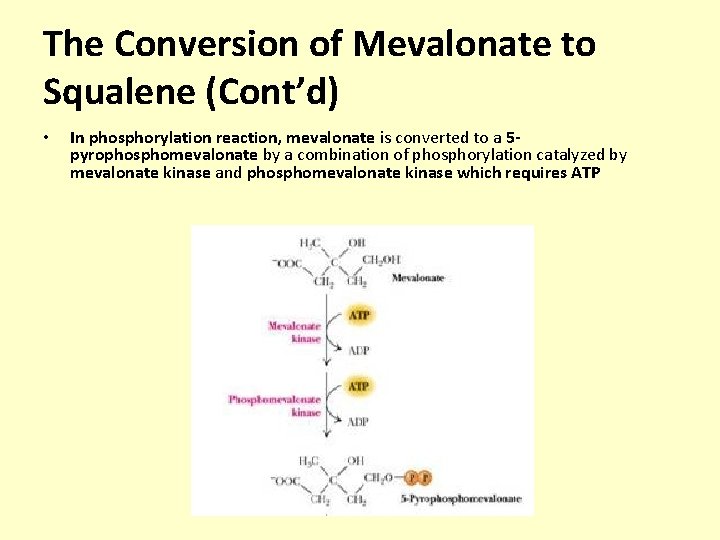
The Conversion of Mevalonate to Squalene (Cont’d) • In phosphorylation reaction, mevalonate is converted to a 5 pyrophosphomevalonate by a combination of phosphorylation catalyzed by mevalonate kinase and phosphomevalonate kinase which requires ATP
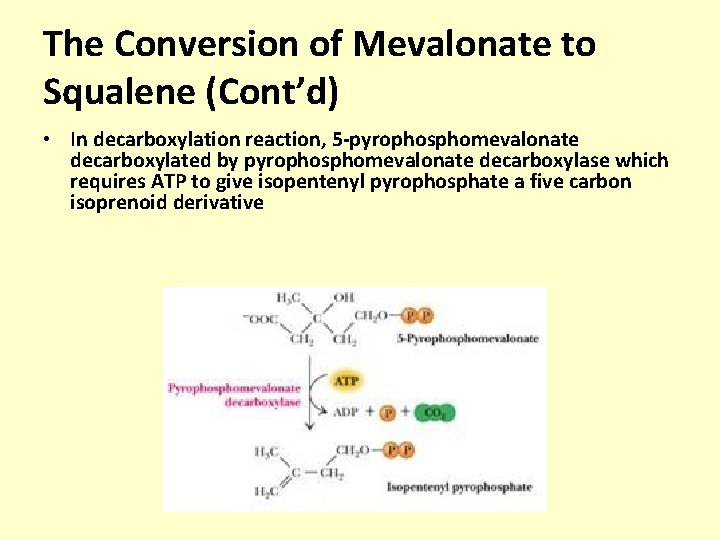
The Conversion of Mevalonate to Squalene (Cont’d) • In decarboxylation reaction, 5 -pyrophosphomevalonate decarboxylated by pyrophosphomevalonate decarboxylase which requires ATP to give isopentenyl pyrophosphate a five carbon isoprenoid derivative
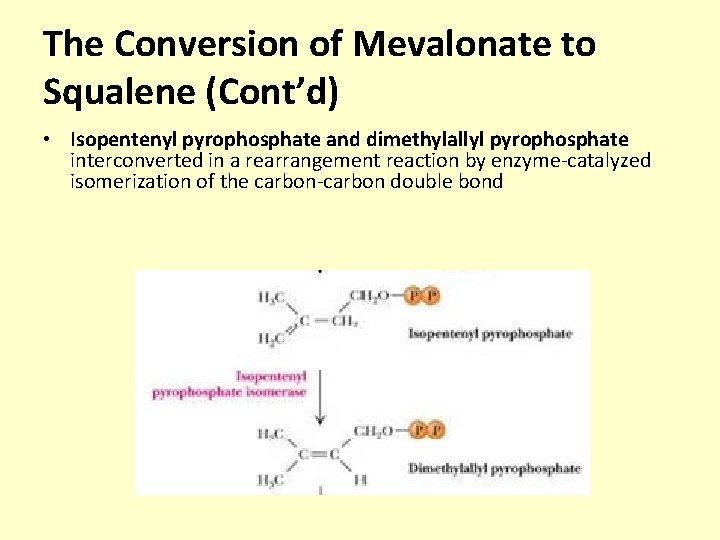
The Conversion of Mevalonate to Squalene (Cont’d) • Isopentenyl pyrophosphate and dimethylallyl pyrophosphate interconverted in a rearrangement reaction by enzyme-catalyzed isomerization of the carbon-carbon double bond
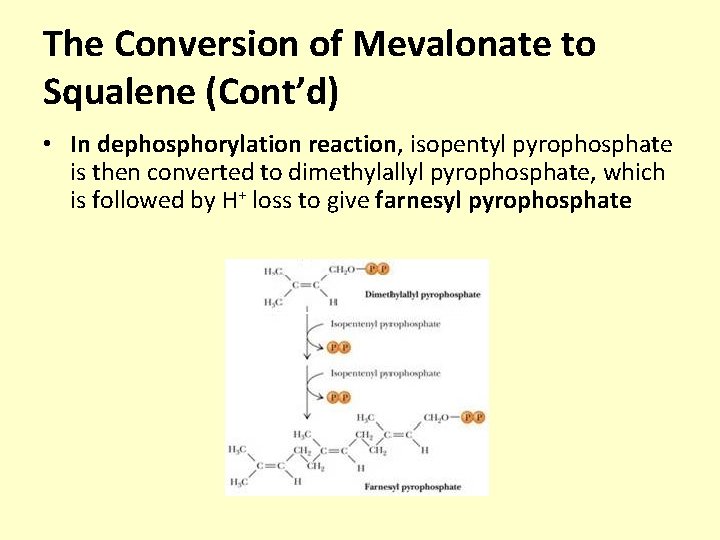
The Conversion of Mevalonate to Squalene (Cont’d) • In dephosphorylation reaction, isopentyl pyrophosphate is then converted to dimethylallyl pyrophosphate, which is followed by H+ loss to give farnesyl pyrophosphate
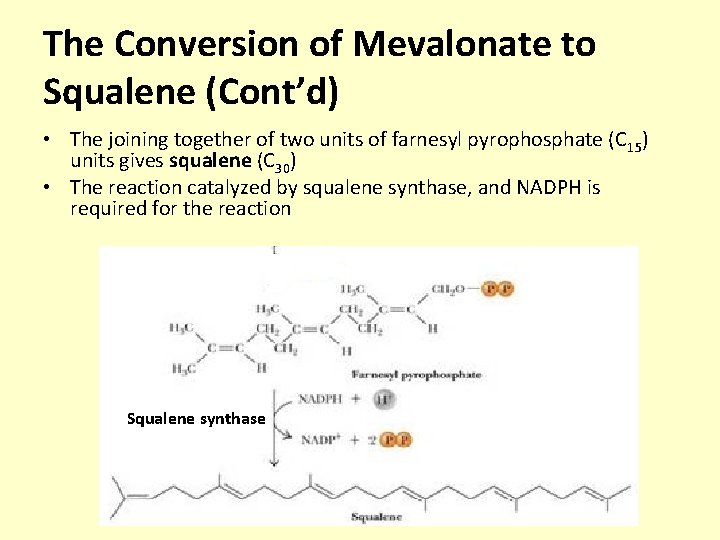
The Conversion of Mevalonate to Squalene (Cont’d) • The joining together of two units of farnesyl pyrophosphate (C 15) units gives squalene (C 30) • The reaction catalyzed by squalene synthase, and NADPH is required for the reaction Squalene synthase
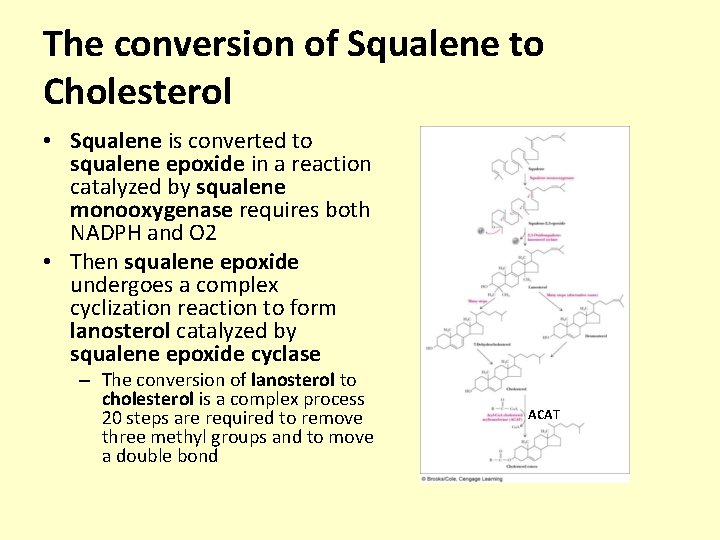
The conversion of Squalene to Cholesterol • Squalene is converted to squalene epoxide in a reaction catalyzed by squalene monooxygenase requires both NADPH and O 2 • Then squalene epoxide undergoes a complex cyclization reaction to form lanosterol catalyzed by squalene epoxide cyclase – The conversion of lanosterol to cholesterol is a complex process 20 steps are required to remove three methyl groups and to move a double bond ACAT
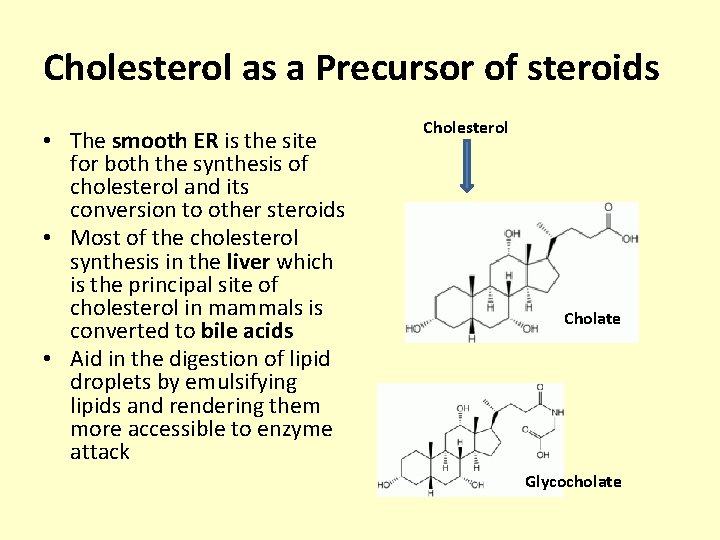
Cholesterol as a Precursor of steroids • The smooth ER is the site for both the synthesis of cholesterol and its conversion to other steroids • Most of the cholesterol synthesis in the liver which is the principal site of cholesterol in mammals is converted to bile acids • Aid in the digestion of lipid droplets by emulsifying lipids and rendering them more accessible to enzyme attack Cholesterol Cholate Glycocholate
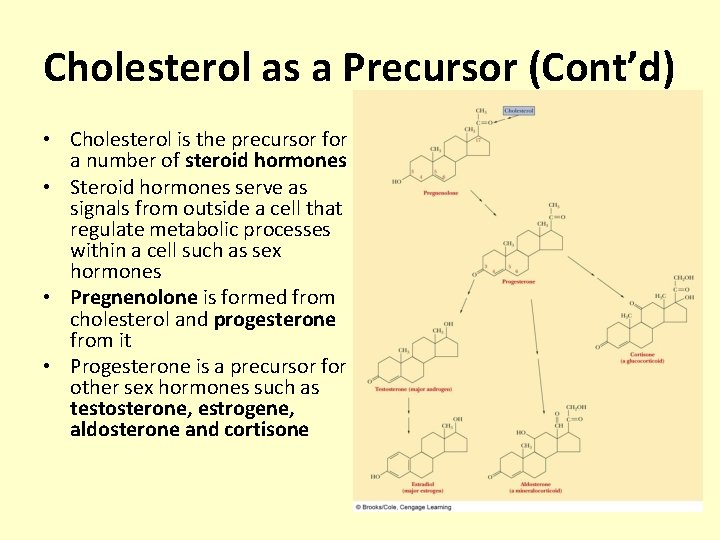
Cholesterol as a Precursor (Cont’d) • Cholesterol is the precursor for a number of steroid hormones • Steroid hormones serve as signals from outside a cell that regulate metabolic processes within a cell such as sex hormones • Pregnenolone is formed from cholesterol and progesterone from it • Progesterone is a precursor for other sex hormones such as testosterone, estrogene, aldosterone and cortisone
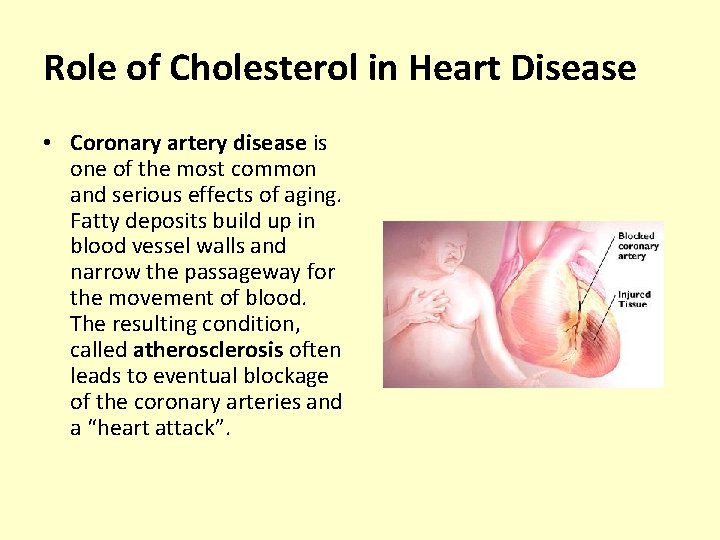
Role of Cholesterol in Heart Disease • Coronary artery disease is one of the most common and serious effects of aging. Fatty deposits build up in blood vessel walls and narrow the passageway for the movement of blood. The resulting condition, called atherosclerosis often leads to eventual blockage of the coronary arteries and a “heart attack”.

Role of Cholesterol in Heart Disease • Atherosclerosis • Both diet and genetics cause atherosclerosis • Lipids are transported in the blood stream by lipoproteins
Atherosclerosis • Can and does, occur in almost any artery in the body. But in the heart it’s effects can be crucial. “The body depends on a strong pumping heart to circulate life-giving blood, and this includes to the heart muscle itself. If the coronary arteries become blocked, the cardiac muscle begins to fail, and so the blood circulation decreases, which includes the circulation to the heart muscle itself. ”

Causes • • • High blood cholesterol High blood pressure Smoking Obesity Lack of physical activity

Risk factors Uncontrollable • Sex • Hereditary • Race • Age Controllable • • • High blood cholesterol High blood pressure Smoking Obesity Lack of physical activity
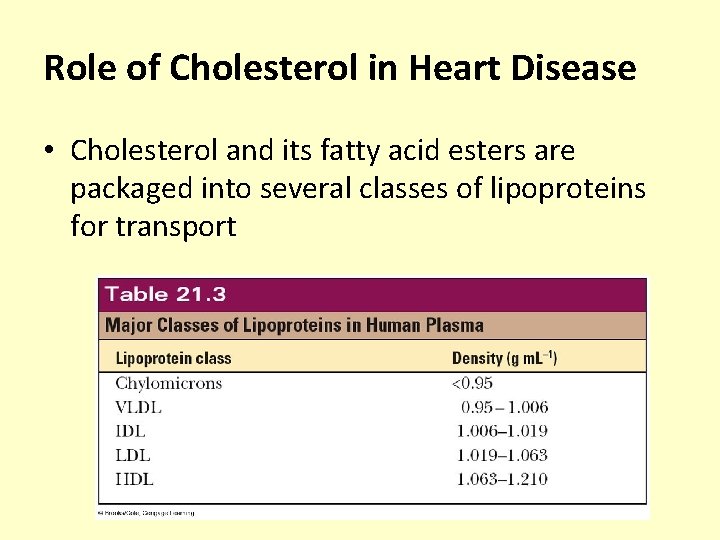
Role of Cholesterol in Heart Disease • Cholesterol and its fatty acid esters are packaged into several classes of lipoproteins for transport
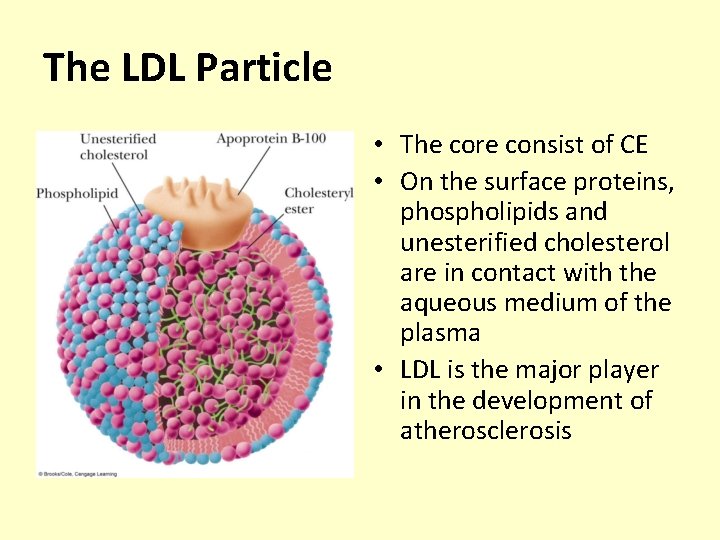
The LDL Particle • The core consist of CE • On the surface proteins, phospholipids and unesterified cholesterol are in contact with the aqueous medium of the plasma • LDL is the major player in the development of atherosclerosis
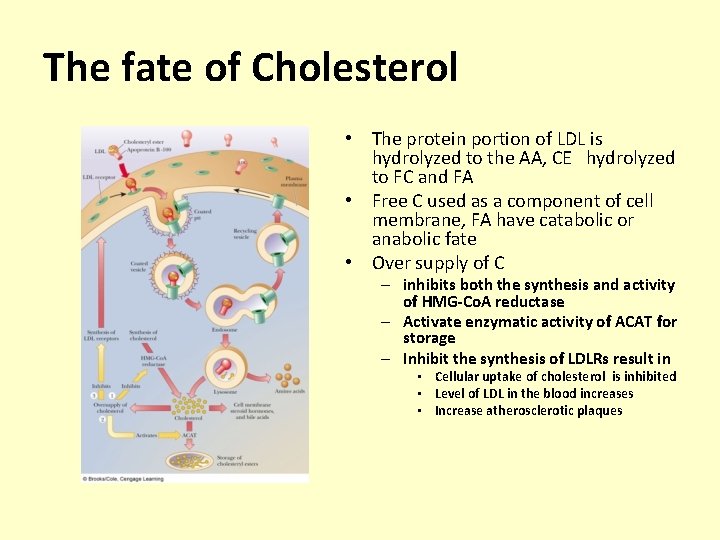
The fate of Cholesterol • The protein portion of LDL is hydrolyzed to the AA, CE hydrolyzed to FC and FA • Free C used as a component of cell membrane, FA have catabolic or anabolic fate • Over supply of C – inhibits both the synthesis and activity of HMG-Co. A reductase – Activate enzymatic activity of ACAT for storage – Inhibit the synthesis of LDLRs result in • Cellular uptake of cholesterol is inhibited • Level of LDL in the blood increases • Increase atherosclerotic plaques

High Cholesterol: a naturally occurring fat Cell membrane component produced in liver Carried in blood by lipoproteins. Low-density lipoprotein (LDL) bad cholesterol High-density lipoprotein (HDL) good cholesterol • Cholesterol in diet • • •

How is high cholesterol treated? (Prevention) • Healthy Diet, weight loss, and regular exercise • Stop smoking, reduce dietary saturated fat intake • Medicines to treat high blood fat (lipid) levels • Check cholesterol regularly • Get regular medical checkups. • Control your blood pressure. • Manage stress.
Summary • The biosynthesis of cholesterol proceeds by the condensation of five-carbon isoprenoid units • Isoprenoid units in turn are derived from the reaction of three acetyl-Co. A units • Once cholesterol is formed, it serves as a precursor for other steroids • Cholesterol must be packaged for transport in the bloodstream. Some of these forms of cholesterol play a role in heart disease
- Slides: 84