Lipid Extraction from Cell Membrane Qualitative Analysis Using
Lipid Extraction from Cell Membrane & Qualitative Analysis Using TLC Method Jung-Min Choi Dept. of Biochemistry Laboratory Animal Research Center and Mouse Genetics Lab of YONSEI University

Introduction – Lipids? 1) 2) 3) 4) 5) The term lipid is used to denote fats and fatlike substances and is synonymous with the terms lipoids or lipins. the simplest lipids are the fats, which are triesters made up one glycerol and three fatty acid The term fats is also used as a general synonym for lipids, so the more precise terms triacylglycerols or triglycerides are preferable for the simplest lipids. It includes a great varieties. The main component is triglycerides(>95%), others include phospholipids, wax, fatty acids, cholesterin etc. Lipids are usually defined as food components that are insoluble in water (relatively non-polar chemical) but soluble in organic solvents such as chloroform. exist in the free form(such as fats or oil), or associated (combined with carbohydrates, or protein in food). Which complicates their extraction and structural identification. Triacylglycerols are used primarily for energy storage in animals. More complex lipids, the phospholipids, glycolipids, and cholesterol, are the major constituents of biological cell membranes. Essential structural component of living cells (along with proteins and carbohydrates).
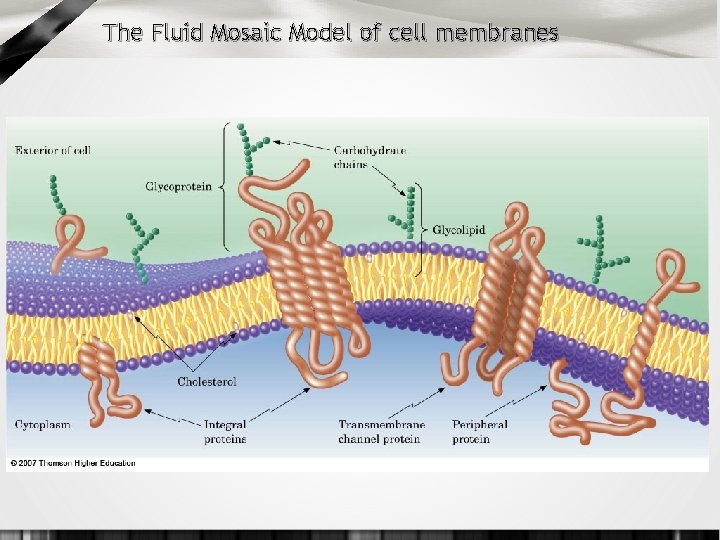
The Fluid Mosaic Model of cell membranes

Fats and Oils • Esters can be called fats or oils, depending upon the degree of unsaturation in the compound. • There are several ways of distinguishing between fats and oils: physical properties and structural formulas are two of them. • The physical distinction is that fats are solid at room temperature and oils are generally liquid at room temperature. The structural difference is that fats are saturated (they have single bonds between all the carbon atoms) and oils are unsaturated (they contain some double bonds between carbon atoms). A third common distinction is that fats come from animals and oils come from vegetables. Keep in mind that, although useful, these distinctions are generalities and there are exceptions. Fats solids saturated animal Oils liquids unsaturated plant

Lipid Classes • simple: FA’s esterified with glycerol • compound: same as simple, but with other compounds also attached • phospholipids: fats containing phosphoric acid and nitrogen (lecithin) • glycolipids: FA’s compounded with CHO, but no N • derived lipids: substances from the above derived by hydrolysis • sterols: large molecular wt. alcohols found in nature and combined w/FA’s (e. g. , cholesterol)
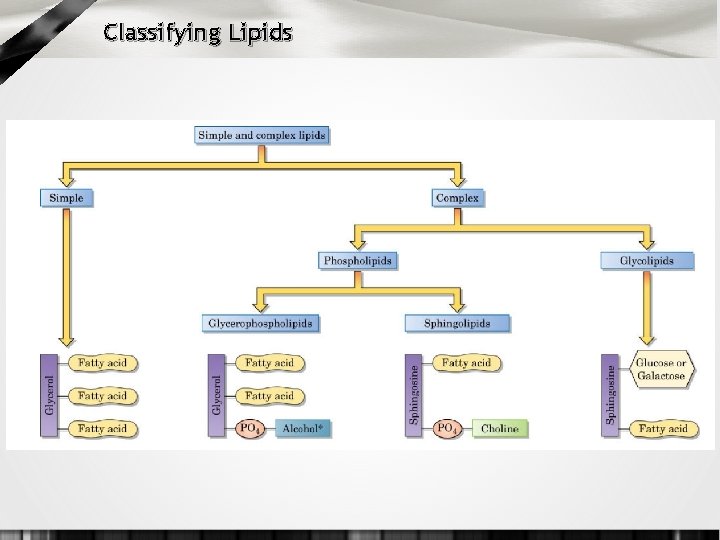
Classifying Lipids

Saturated vs. Unsaturated Fatty Acids • saturated: the SFA’s of a lipid have no double bonds between carbons in chain • polyunsaturated: more than one double bond in the chain • most common polyunsaturated fats contain the polyunsaturated fatty acids (PUFAs) oleic, linoleic and linolenic acid • unsaturated fats have lower melting points • stearic (SFA) melts at 70 o. C, oleic (PUFA) at 26 o. C • saturated fats tightly packed, clog arteries as atherosclerosis • because of double bonds, polyunsaturated fats do not pack well -- like building a wall with bricks vs. irregular -shaped objects • plant fats are much higher in PUFA’s than animal fats
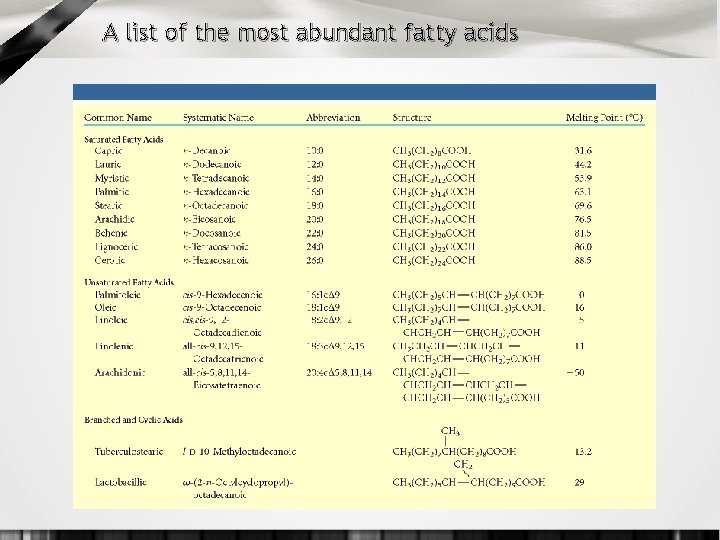
A list of the most abundant fatty acids

Lipid Digestion/Absorption • • Fats serve a structural function in cells, as sources of energy, and insulation the poor water solubility of lipids presents a problem for digestion: substrates are not easily accessible to digestive enzymes even if hydrolyzed, the products tend to aggregate to larger complexes that make poor contact with the cell surface and aren’t easily absorbed to overcome these problems, changes in the physical state of lipids are connected to chemical changes during digestion and absorption Enzymes Involved in Digestion of Lipids • • • lingual lipase: provides a stable interface with aqueous environment of stomach pancreatic lipase: major enzyme affecting triglyceride hydrolysis colipase: protein anchoring lipase to the lipid esterase: secreted by pancreas, acts on cholestrol esters, activated by bile phospholipases: cleave phospholipids, activated by trypsin

FA vary in Chain Length and Degree of Unsaturation • • More saturated fatty acids (in other words, no C=C double bonds present, the fatty acids are "saturated" with hydrogen) pack together better and are therefore more viscous. The membrane will be less fluid and therefore the Temperature of Melting (Tm) will be higher. On the other hand, if the membrane contains more un-saturated fatty acids (cis C=C double bonds are present) there will be poor packing of fatty acids. The membrane will be more fluid and the Tm will be lower if a fatty acid is saturated which means it only has single bonds then the shape of the molecule is straight. this means the molecules can pack tightly together even at high temperatures which means the fat will be solid at room temp. e. g. animal fat if a fatty acid is unsaturated which means it contains some double bonds then the shape of the molecule is bent or branched. this means the molecules cannot pack tightly together which means the fat will be liquid at room temp. e. g. olive oil. Short chain length and unsaturation enhance the fluidity of Fatty acid
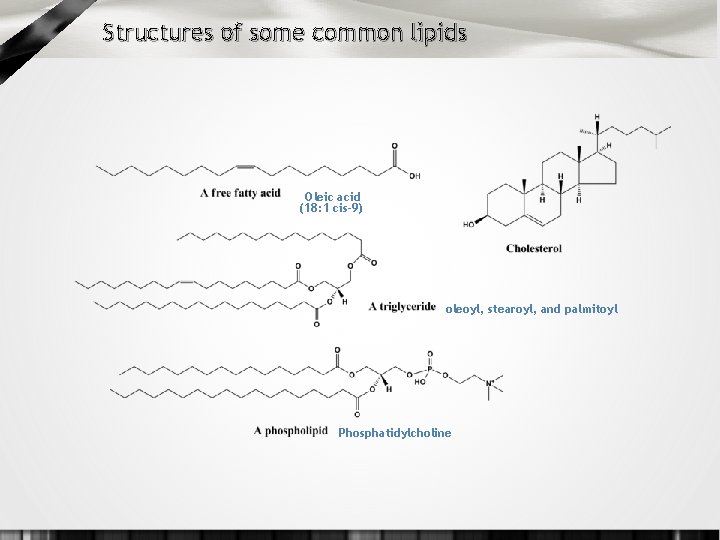
Structures of some common lipids Oleic acid (18: 1 cis-9) oleoyl, stearoyl, and palmitoyl Phosphatidylcholine

LIPID EXTRACTION • The aim of all extraction procedures is to separate cellular or fluid lipids from the other constituents, proteins, polysaccharides, small molecules (amino acids, sugars. . . ) but also to preserve these lipids for further analyses. • The Order of migration of lipids during chromatography depends primarily on polarity. The less polar the lipid, the greater its affinity for the nonpolar solvent (compare the polar silica gel) and hence the greater the migration.

Chromatography sheet material Thin layer chromatography (TLC) adsorbent sheet is a thin layer of fine silica powder (pure sand) spread uniformly over an inert support such as a sheet of glass or plastic. Adsorption chromatography separates lipids of different polarity
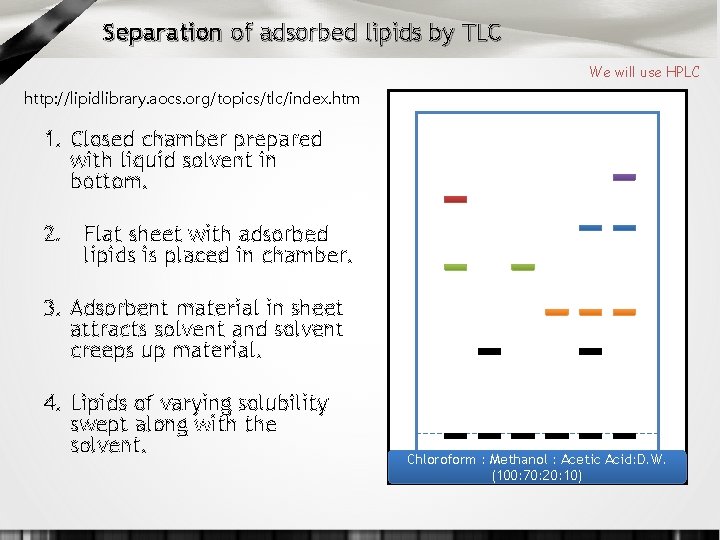
Separation of adsorbed lipids by TLC We will use HPLC http: //lipidlibrary. aocs. org/topics/tlc/index. htm 1. Closed chamber prepared with liquid solvent in bottom. 2. Flat sheet with adsorbed lipids is placed in chamber. 3. Adsorbent material in sheet attracts solvent and solvent creeps up material. 4. Lipids of varying solubility swept along with the solvent. Chloroform : Methanol : Acetic Acid: D. W. (100: 70: 20: 10)
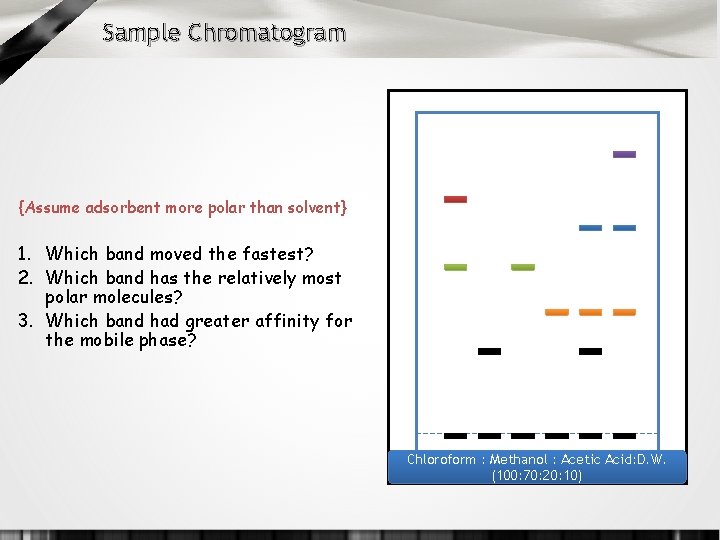
Sample Chromatogram {Assume adsorbent more polar than solvent} 1. Which band moved the fastest? 2. Which band has the relatively most polar molecules? 3. Which band had greater affinity for the mobile phase? Chloroform : Methanol : Acetic Acid: D. W. (100: 70: 20: 10)

Procedure • Lipid Extraction from mammalian cells 1) Ice-Cold PBS로 Culture Plates를 씻어준다 2) Trypsin-EDTA를 이용하여 plate에서 cell pellet을 얻는다 3) 1, 200~2000 rmp에서 5분간 원심 분리하여 cell pellet을 얻는 다. 4) Ice-cold PBS로 pellet을 풀어준 후 1, 200~2, 000 rpm에서 5분간 원심 분리 5) Pellet을 피해서 조심스럽게 상층액을 제거한다. 6) 얻어낸 pellet에 Chloroform : Me. OH (1: 2) 혼합액 (400 ul)을 넣고 Vortex 7) 동량의 Chloroform을 넣고 다시 Vortex 8) 동량의 D. W. 를 넣고 다시 Vortex 9) 3, 000 ~ 4, 000 rpm에서 10분간 원심분리 10) 하층 (Chloroform)층을 조심스럽게 덜어 낸다 이 다음 STEP부터 교재 참고 ㅋ

Further Study 1. Try to explain the relative order of Rf values you obtained for the standard lipid. What is the main factor that affects the difference of migration? 2. How does iodine react to produce the red-brown spots on a developed chromatogram? Why do some lipids give darker spots than others? 3. Write the order of migratory distance of chromatography for each set of FAMEs below. (short long) (1) 14: 0, 20: 0, 12: 0, 16: 0, 18: 0 (2) 16: 1∆9, 16: 0, 16: 2∆9, 12 (3) 16: 0, 18: 0, 16: 1∆9, 18: 1∆9
- Slides: 17