LineAngle Notation for Depicting Chemical Structures A shorthand

- Slides: 44

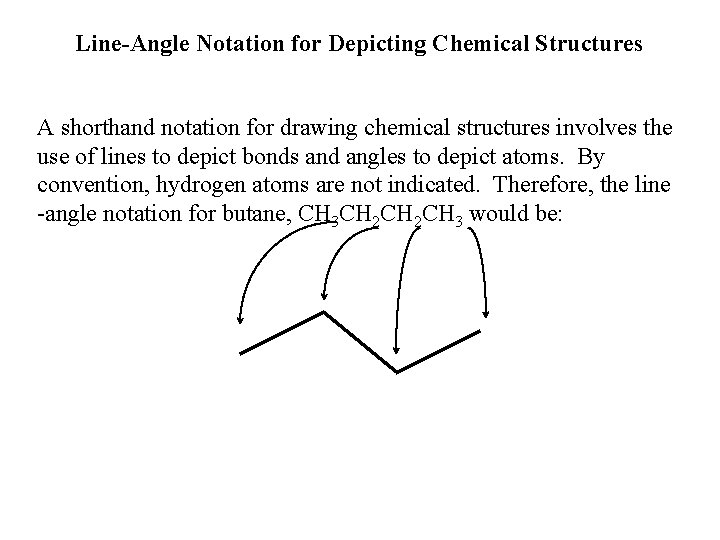
Line-Angle Notation for Depicting Chemical Structures A shorthand notation for drawing chemical structures involves the use of lines to depict bonds and angles to depict atoms. By convention, hydrogen atoms are not indicated. Therefore, the line -angle notation for butane, CH 3 CH 2 CH 3 would be:

Organic Functional Groups

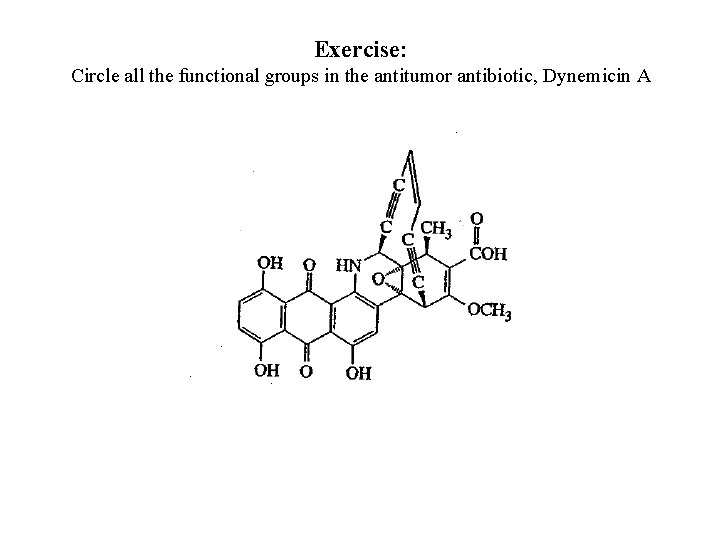
Exercise: Circle all the functional groups in the antitumor antibiotic, Dynemicin A

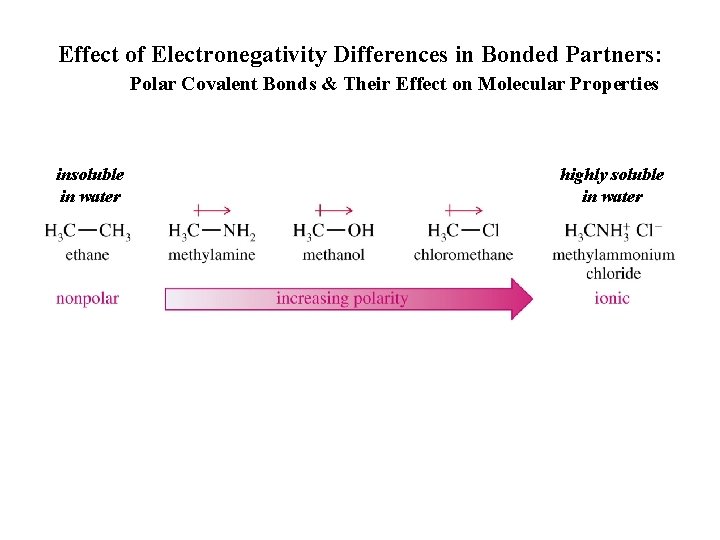
Effect of Electronegativity Differences in Bonded Partners: Polar Covalent Bonds & Their Effect on Molecular Properties insoluble in water highly soluble in water

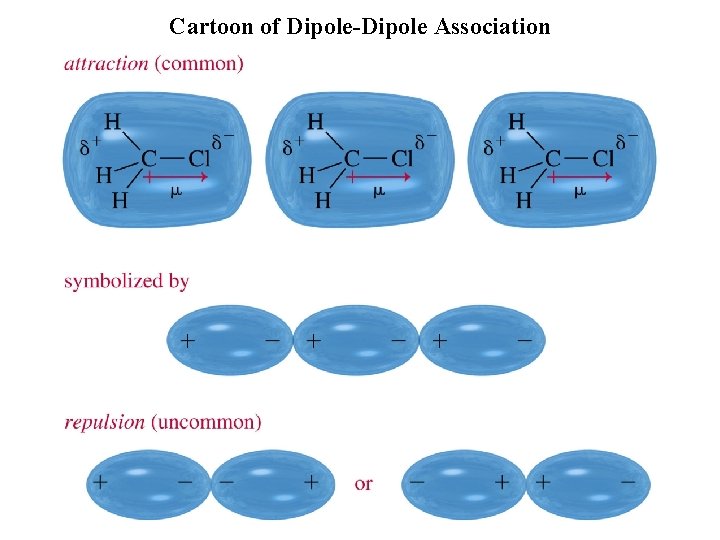
Cartoon of Dipole-Dipole Association

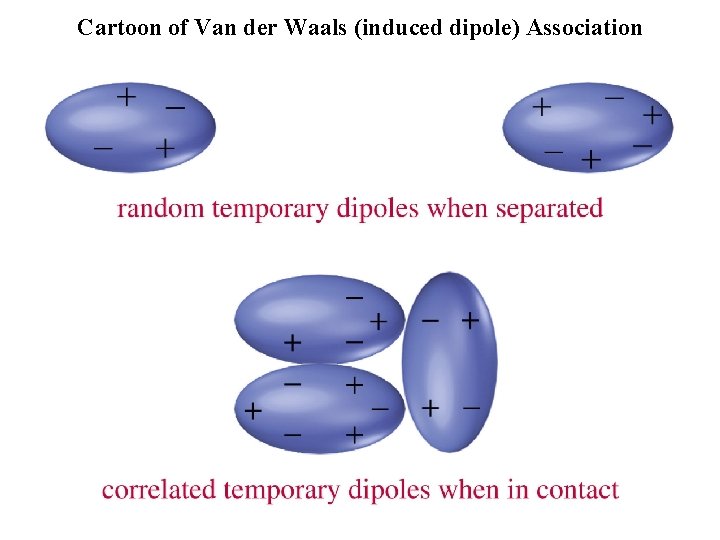
Cartoon of Van der Waals (induced dipole) Association

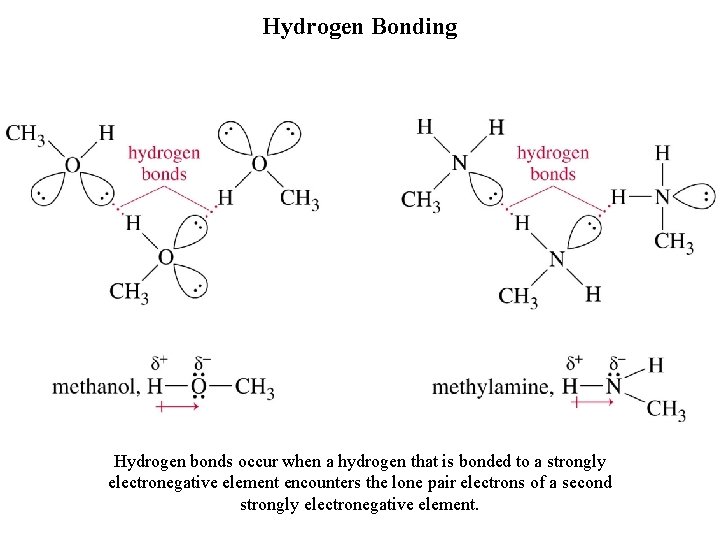
Hydrogen Bonding Hydrogen bonds occur when a hydrogen that is bonded to a strongly electronegative element encounters the lone pair electrons of a second strongly electronegative element.

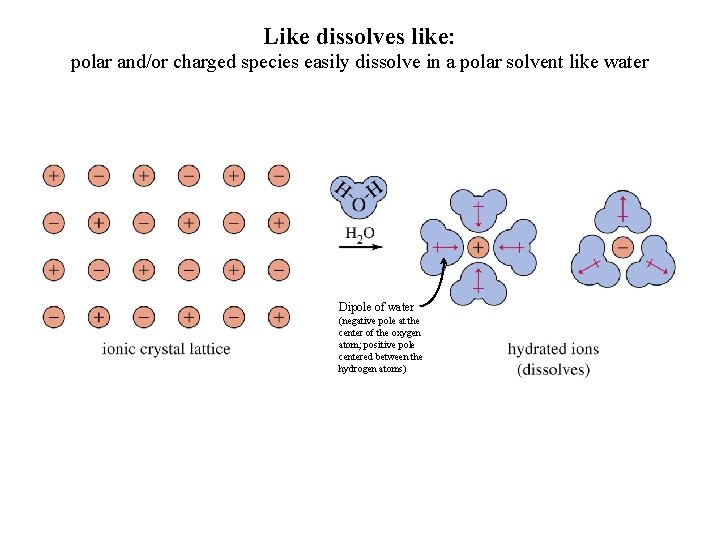
Like dissolves like: polar and/or charged species easily dissolve in a polar solvent like water Dipole of water (negative pole at the center of the oxygen atom; positive pole centered between the hydrogen atoms)

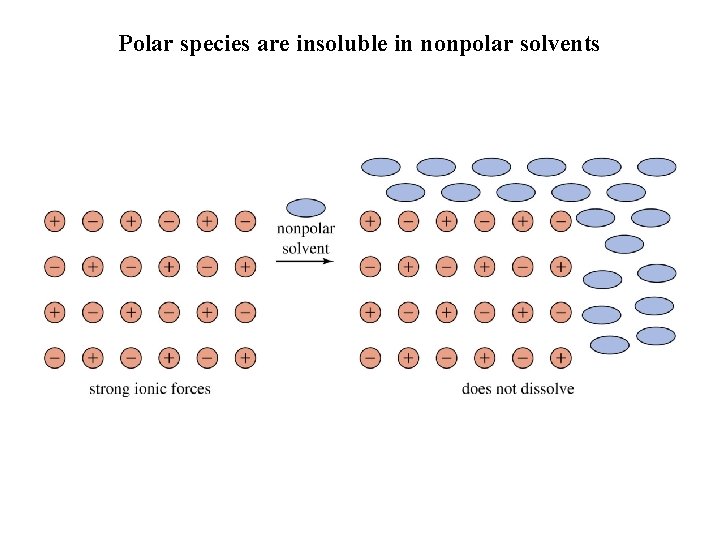
Polar species are insoluble in nonpolar solvents

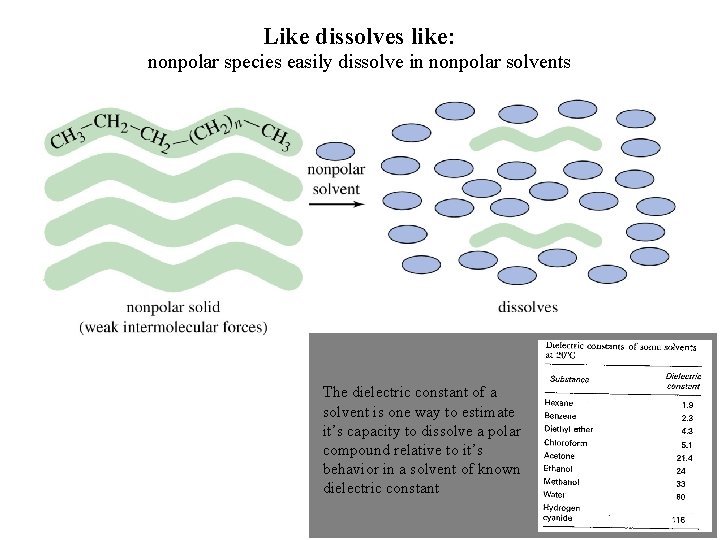
Like dissolves like: nonpolar species easily dissolve in nonpolar solvents The dielectric constant of a solvent is one way to estimate it’s capacity to dissolve a polar compound relative to it’s behavior in a solvent of known dielectric constant

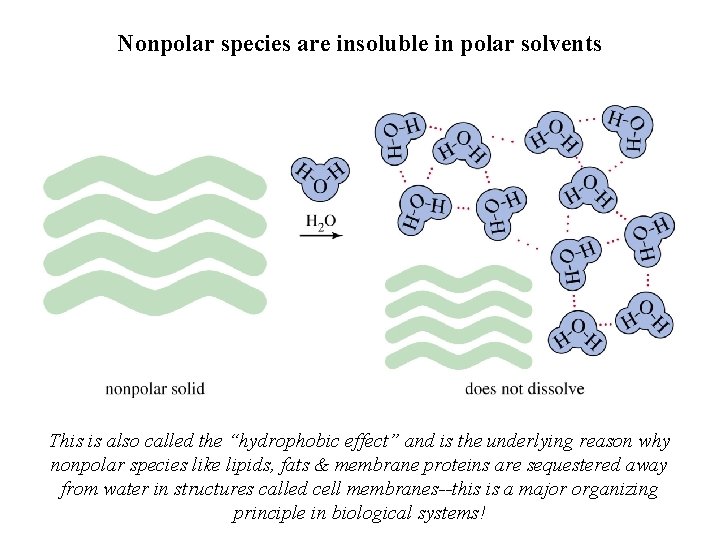
Nonpolar species are insoluble in polar solvents This is also called the “hydrophobic effect” and is the underlying reason why nonpolar species like lipids, fats & membrane proteins are sequestered away from water in structures called cell membranes--this is a major organizing principle in biological systems!

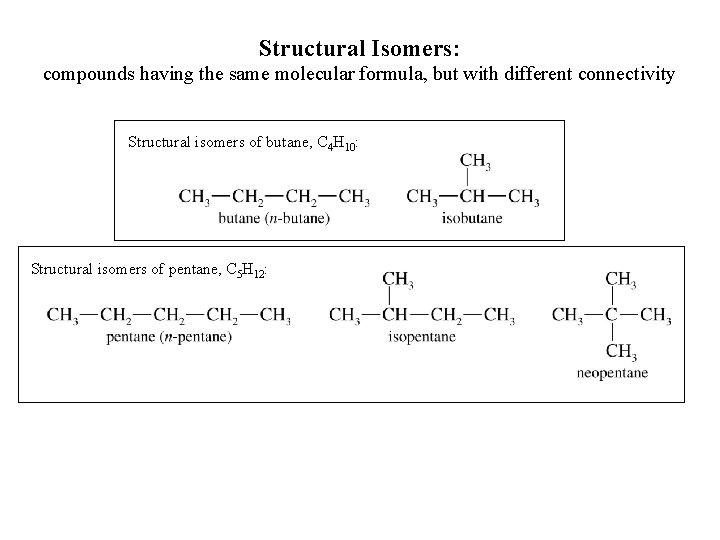
Structural Isomers: compounds having the same molecular formula, but with different connectivity Structural isomers of butane, C 4 H 10: Structural isomers of pentane, C 5 H 12:

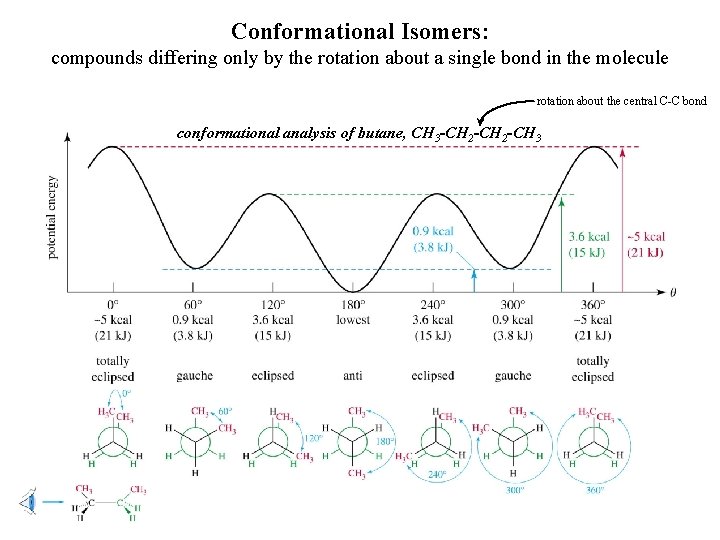
Conformational Isomers: compounds differing only by the rotation about a single bond in the molecule rotation about the central C-C bond conformational analysis of butane, CH 3 -CH 2 -CH 3

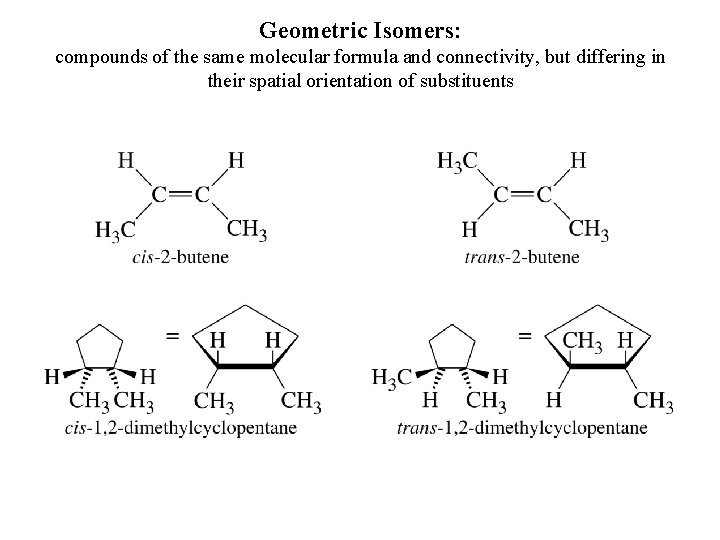
Geometric Isomers: compounds of the same molecular formula and connectivity, but differing in their spatial orientation of substituents

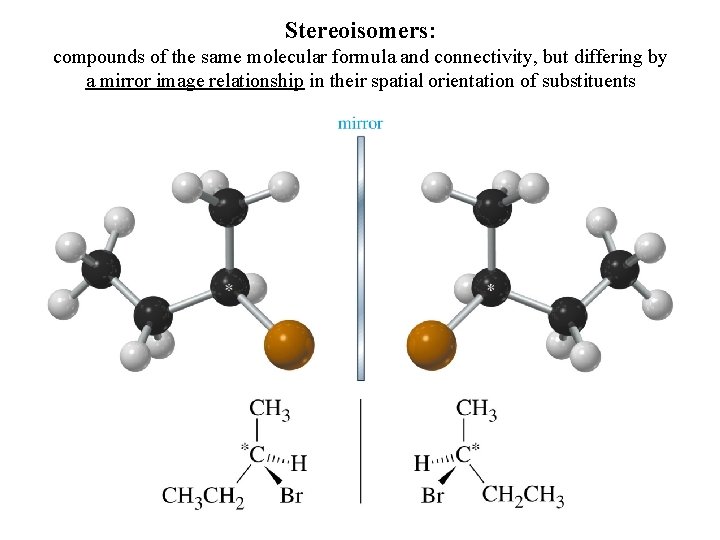
Stereoisomers: compounds of the same molecular formula and connectivity, but differing by a mirror image relationship in their spatial orientation of substituents

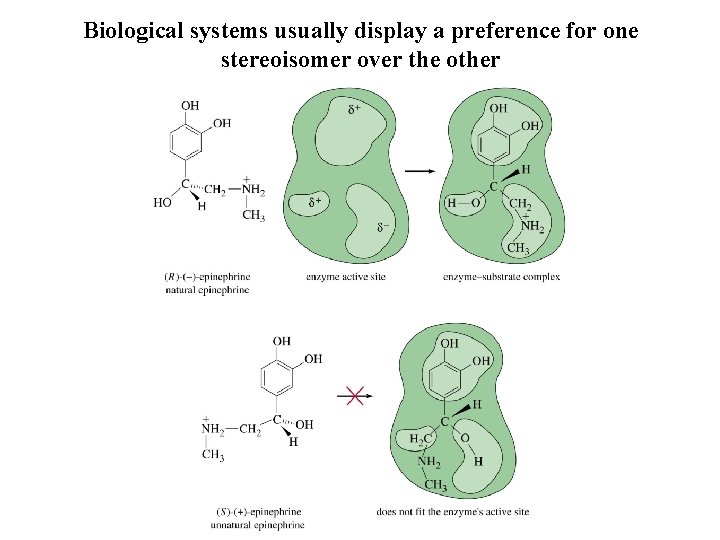
Biological systems usually display a preference for one stereoisomer over the other

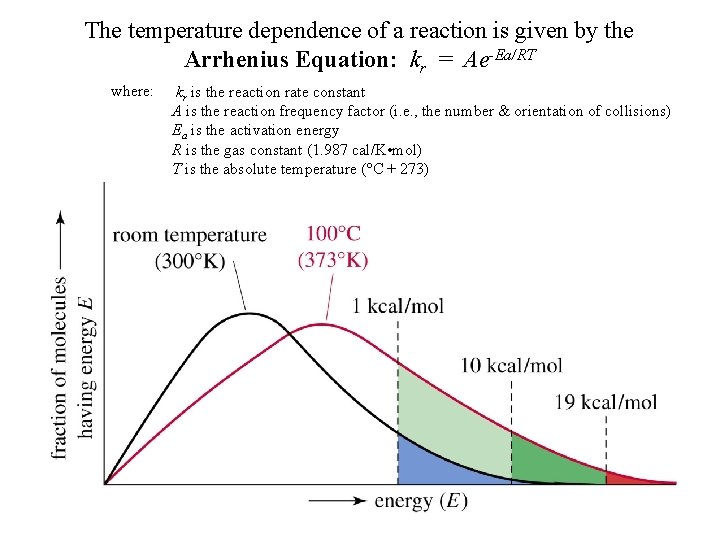
The temperature dependence of a reaction is given by the Arrhenius Equation: kr = Ae-Ea/RT where: kr is the reaction rate constant A is the reaction frequency factor (i. e. , the number & orientation of collisions) Ea is the activation energy R is the gas constant (1. 987 cal/K • mol) T is the absolute temperature (°C + 273)

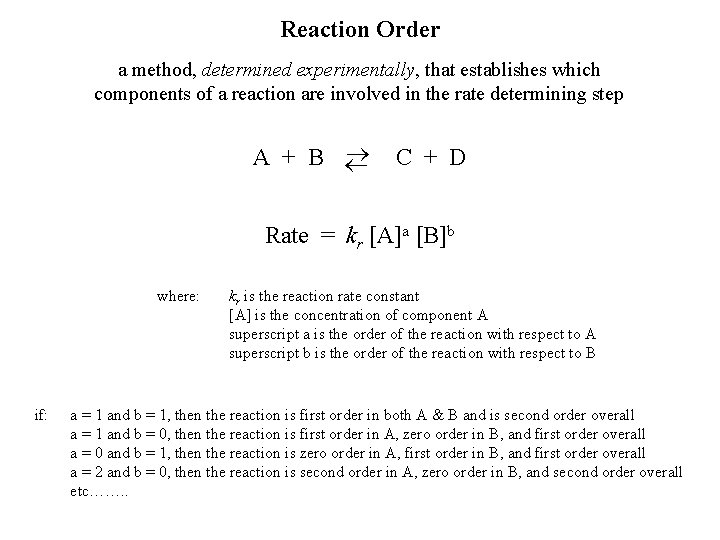
Reaction Order a method, determined experimentally, that establishes which components of a reaction are involved in the rate determining step A + B C + D Rate = kr [A]a [B]b where: if: kr is the reaction rate constant [A] is the concentration of component A superscript a is the order of the reaction with respect to A superscript b is the order of the reaction with respect to B a = 1 and b = 1, then the reaction is first order in both A & B and is second order overall a = 1 and b = 0, then the reaction is first order in A, zero order in B, and first order overall a = 0 and b = 1, then the reaction is zero order in A, first order in B, and first order overall a = 2 and b = 0, then the reaction is second order in A, zero order in B, and second order overall etc……. .

C: 8 e- Cl: 7 e- Cl: 8 e-

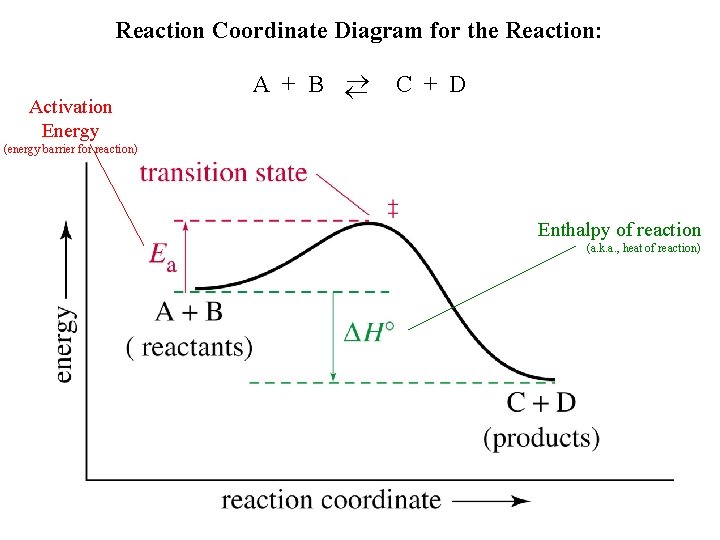
Reaction Coordinate Diagram for the Reaction: Activation Energy A + B C + D (energy barrier for reaction) Enthalpy of reaction (a. k. a. , heat of reaction)

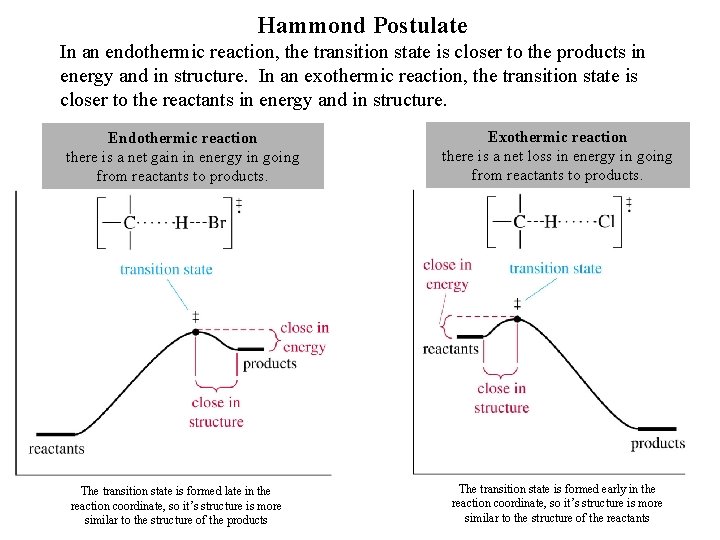
Hammond Postulate In an endothermic reaction, the transition state is closer to the products in energy and in structure. In an exothermic reaction, the transition state is closer to the reactants in energy and in structure. Endothermic reaction there is a net gain in energy in going from reactants to products. The transition state is formed late in the reaction coordinate, so it’s structure is more similar to the structure of the products Exothermic reaction there is a net loss in energy in going from reactants to products. The transition state is formed early in the reaction coordinate, so it’s structure is more similar to the structure of the reactants

Condensation Reactions These reactions involve coupling two molecules to form a larger molecule and a water molecule

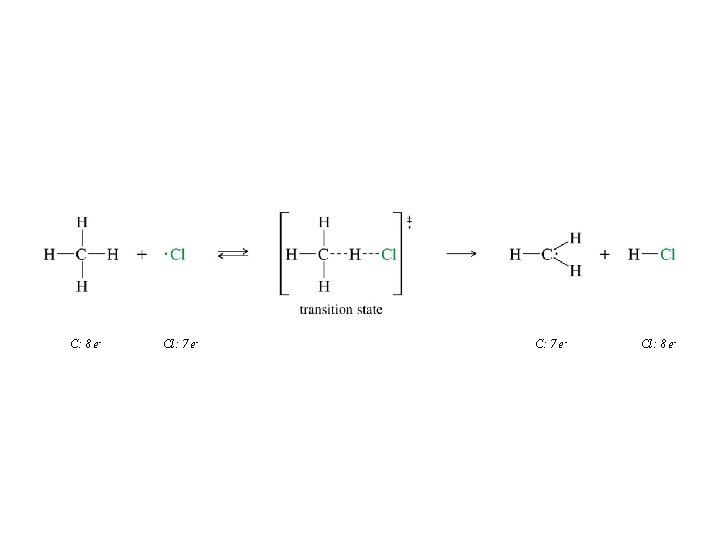
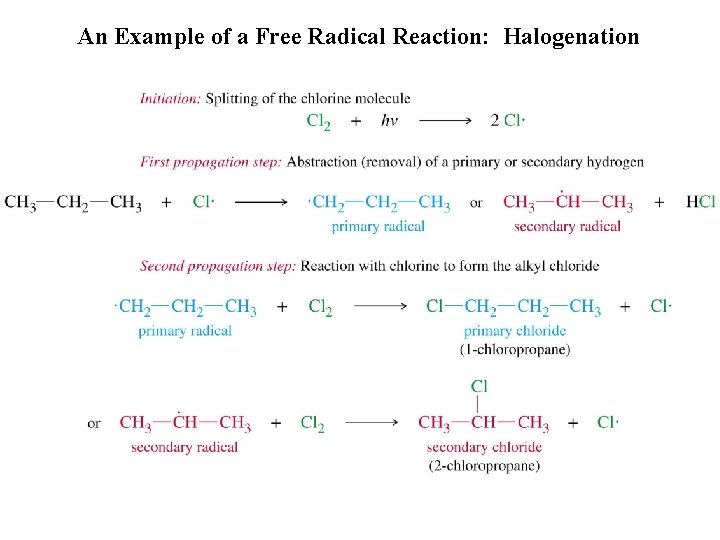
An Example of a Free Radical Reaction: Halogenation

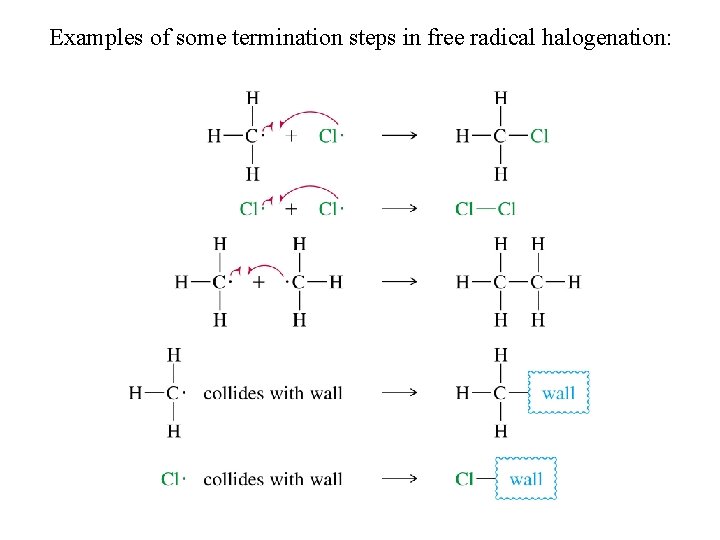
Examples of some termination steps in free radical halogenation:

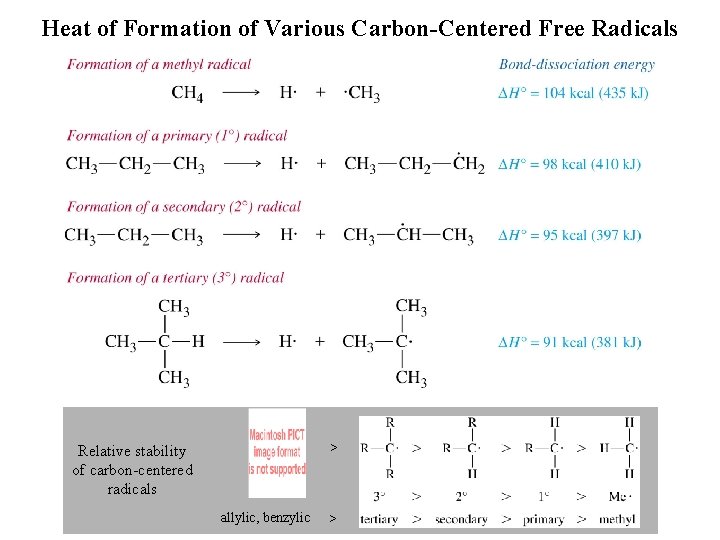
Heat of Formation of Various Carbon-Centered Free Radicals > Relative stability of carbon-centered radicals allylic, benzylic >

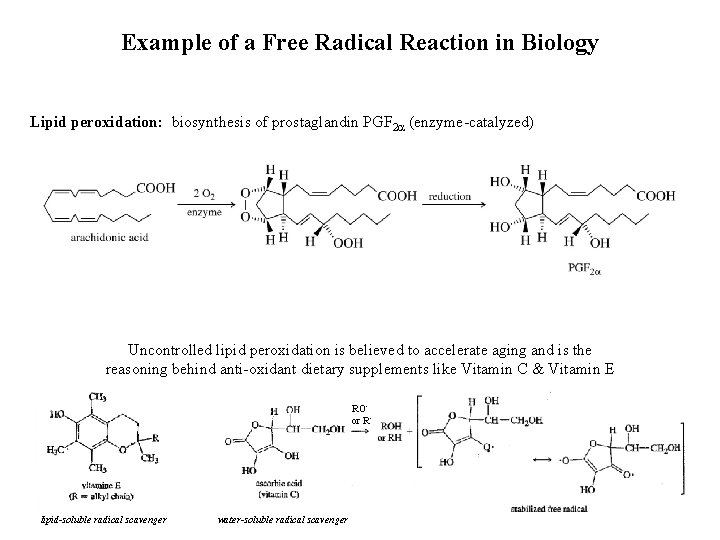
Example of a Free Radical Reaction in Biology Lipid peroxidation: biosynthesis of prostaglandin PGF 2 (enzyme-catalyzed) Uncontrolled lipid peroxidation is believed to accelerate aging and is the reasoning behind anti-oxidant dietary supplements like Vitamin C & Vitamin E RO • or R • lipid-soluble radical scavenger water-soluble radical scavenger

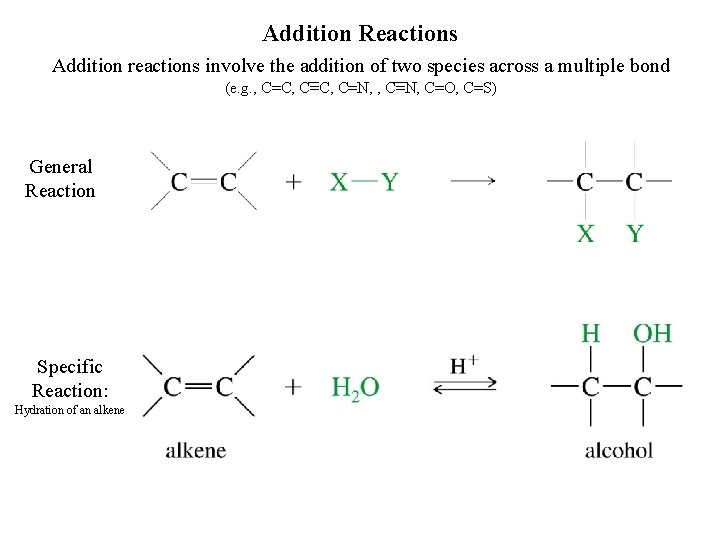
Addition Reactions Addition reactions involve the addition of two species across a multiple bond (e. g. , C=C, C=N, C=O, C=S) General Reaction Specific Reaction: Hydration of an alkene

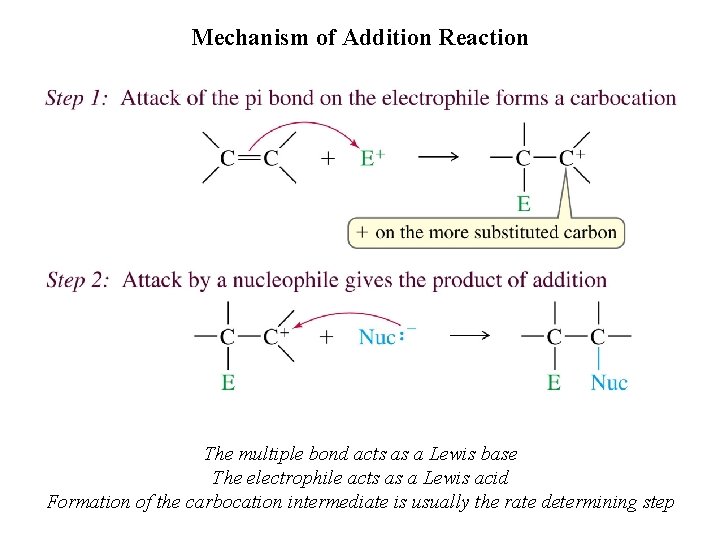
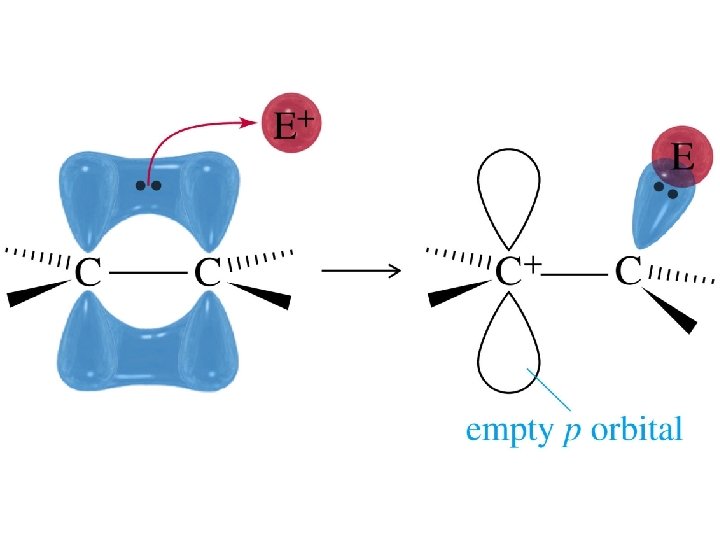
Mechanism of Addition Reaction The multiple bond acts as a Lewis base The electrophile acts as a Lewis acid Formation of the carbocation intermediate is usually the rate determining step


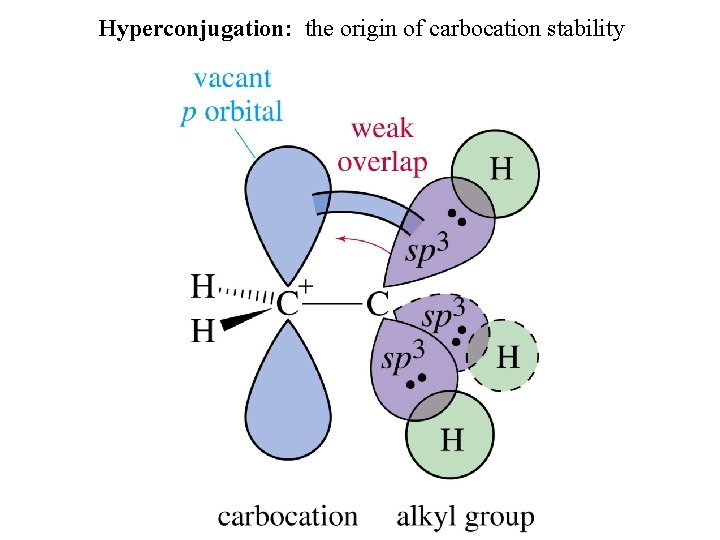
Hyperconjugation: the origin of carbocation stability

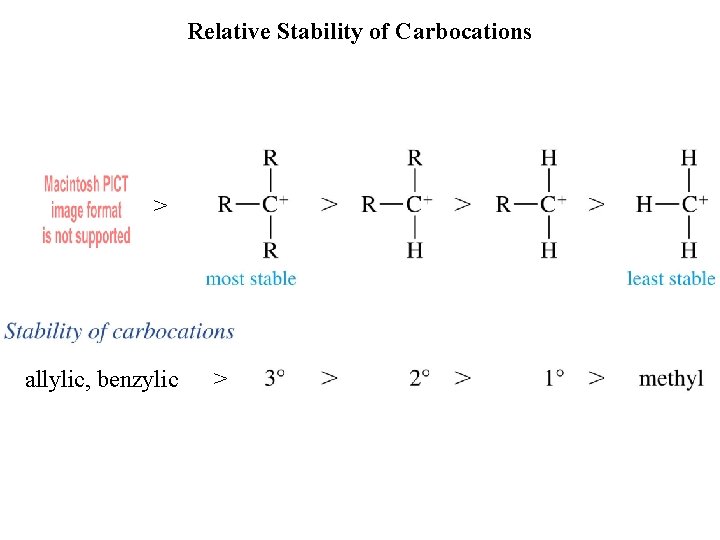
Relative Stability of Carbocations > allylic, benzylic >

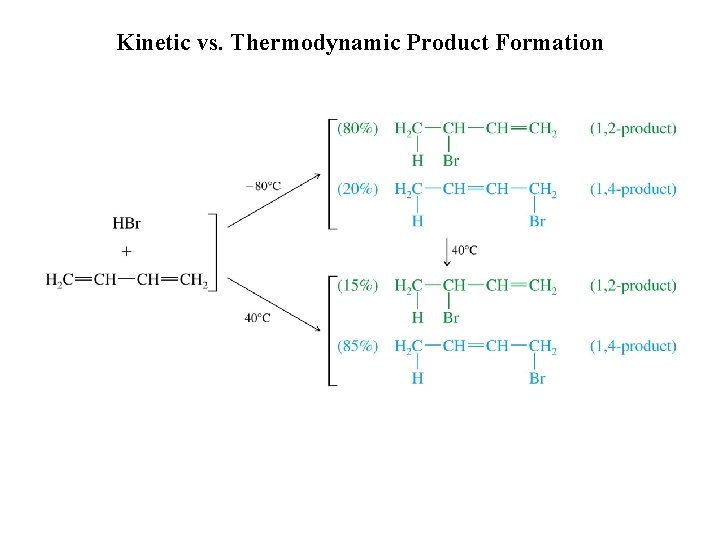
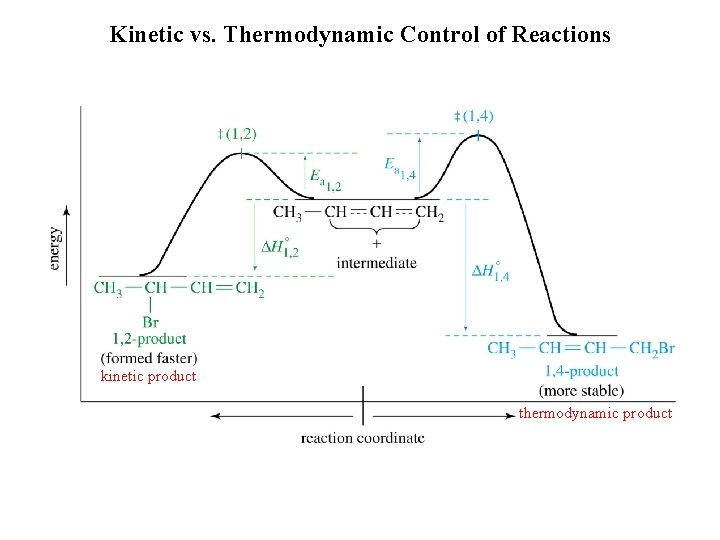
Kinetic vs. Thermodynamic Product Formation

Kinetic vs. Thermodynamic Control of Reactions kinetic product thermodynamic product

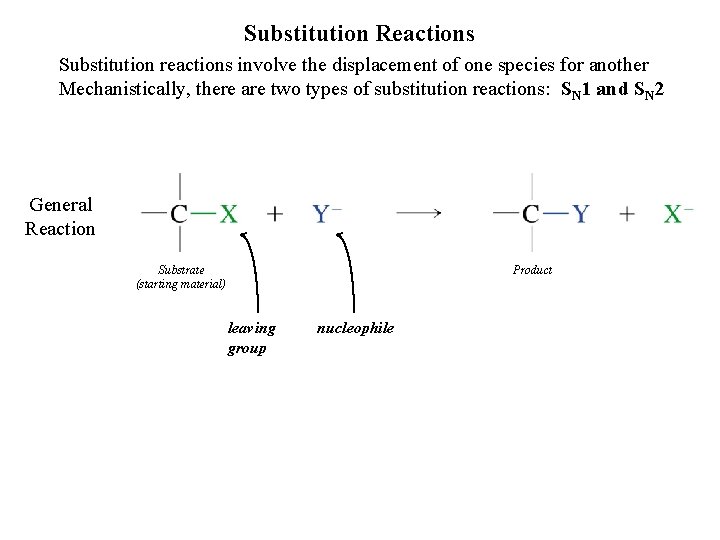
Substitution Reactions Substitution reactions involve the displacement of one species for another Mechanistically, there are two types of substitution reactions: SN 1 and SN 2 General Reaction Substrate (starting material) Product leaving group nucleophile

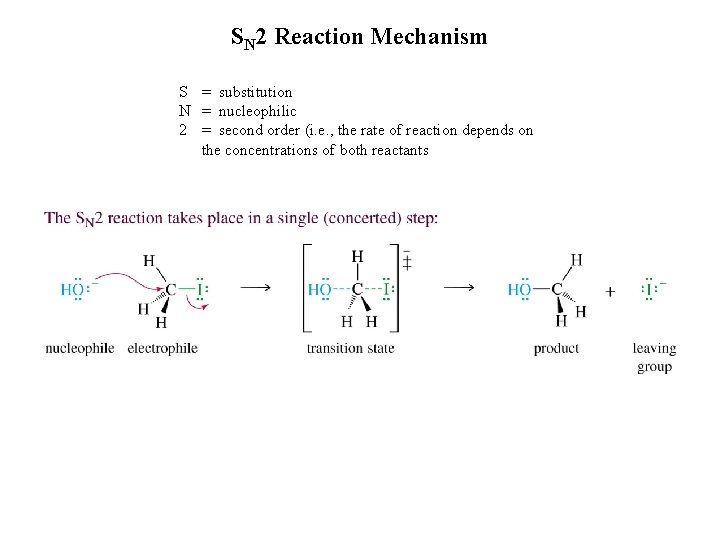
SN 2 Reaction Mechanism S = substitution N = nucleophilic 2 = second order (i. e. , the rate of reaction depends on the concentrations of both reactants

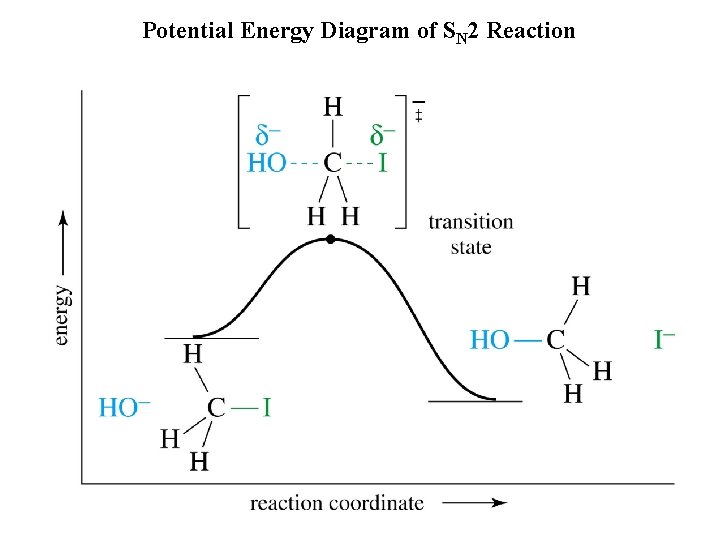
Potential Energy Diagram of SN 2 Reaction

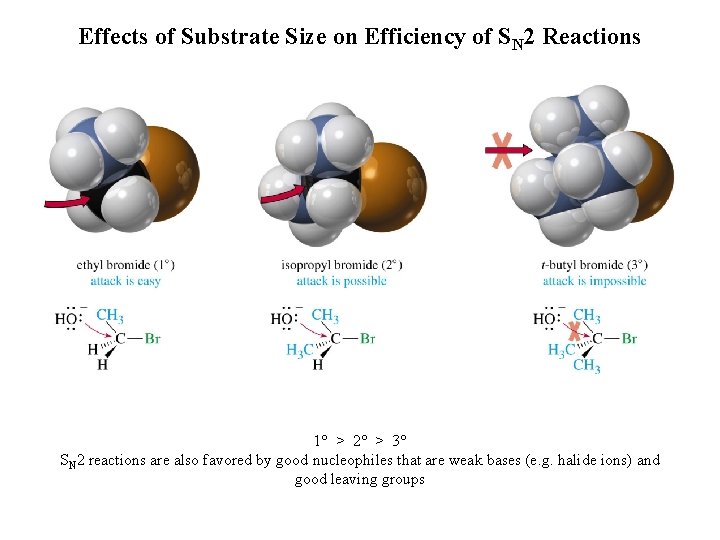
Effects of Substrate Size on Efficiency of SN 2 Reactions 1° > 2° > 3° SN 2 reactions are also favored by good nucleophiles that are weak bases (e. g. halide ions) and good leaving groups

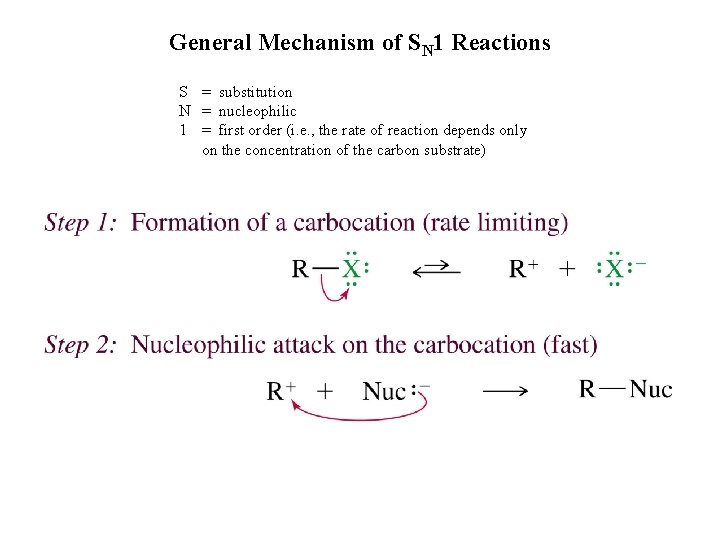
General Mechanism of SN 1 Reactions S = substitution N = nucleophilic 1 = first order (i. e. , the rate of reaction depends only on the concentration of the carbon substrate)

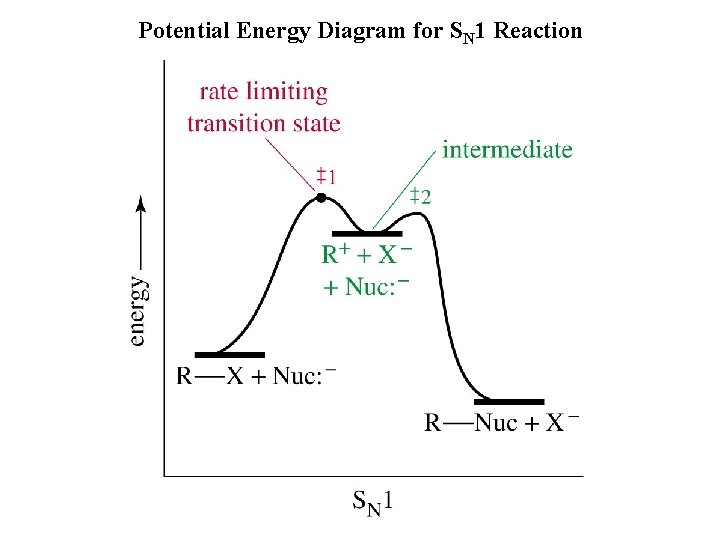
Potential Energy Diagram for SN 1 Reaction

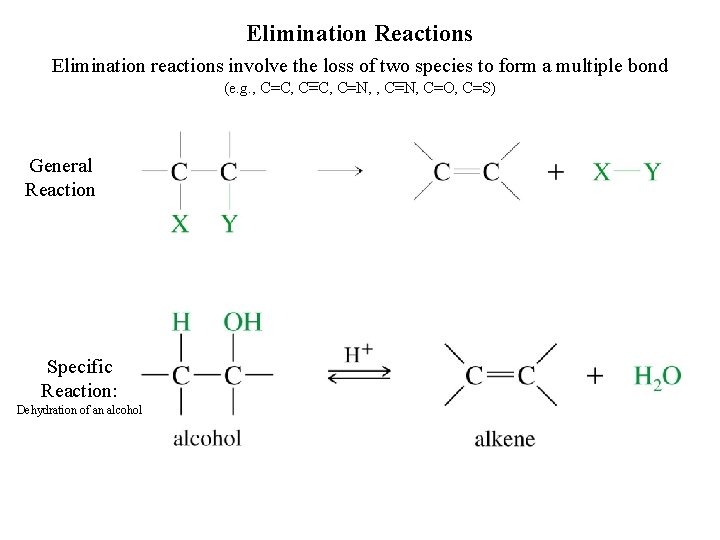
Elimination Reactions Elimination reactions involve the loss of two species to form a multiple bond (e. g. , C=C, C=N, C=O, C=S) General Reaction Specific Reaction: Dehydration of an alcohol

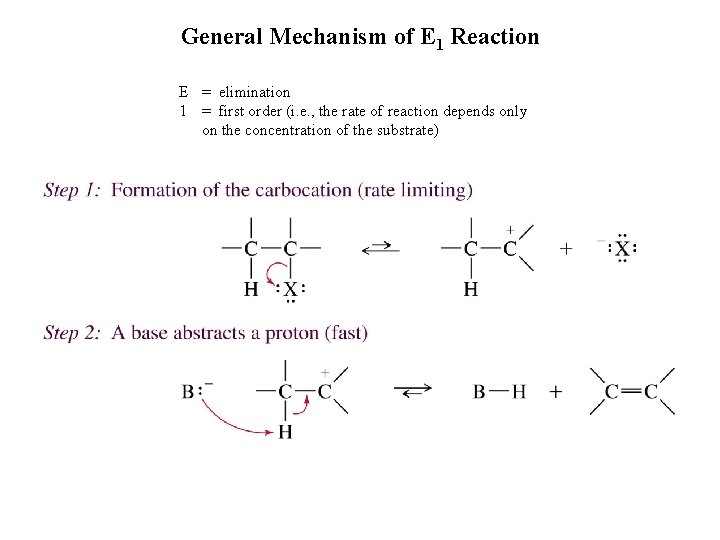
General Mechanism of E 1 Reaction E = elimination 1 = first order (i. e. , the rate of reaction depends only on the concentration of the substrate)

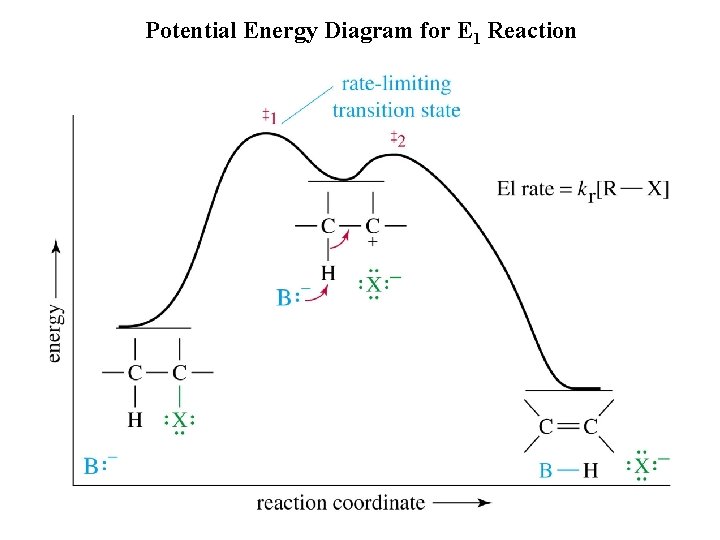
Potential Energy Diagram for E 1 Reaction

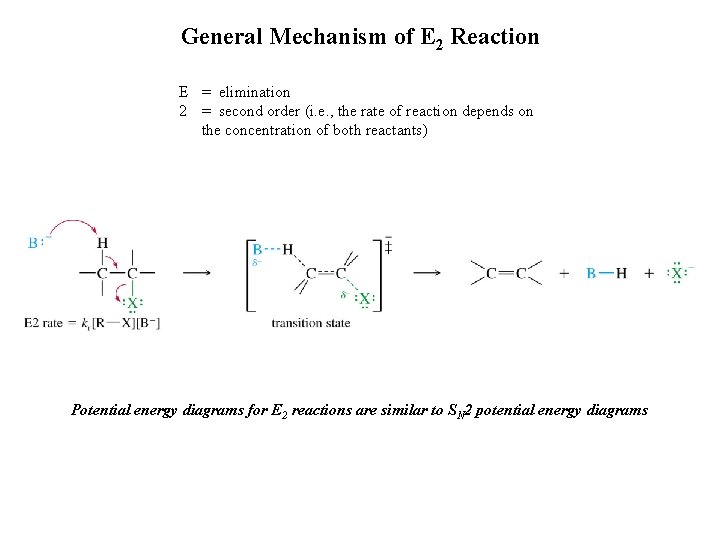
General Mechanism of E 2 Reaction E = elimination 2 = second order (i. e. , the rate of reaction depends on the concentration of both reactants) Potential energy diagrams for E 2 reactions are similar to SN 2 potential energy diagrams

In general, E 2 reactions compete with SN 2 reactions E 1 reactions compete with SN 1 reactions